Abstract
ATP-sensitive potassium (KATP) channels are well characterized in cardiac, pancreatic and many other muscle cells. In the present study, functional expression of the KATP channel was examined in non-pregnant murine longitudinal myometrium. Isometric contraction measurements and Western blot were used. KATP channel openers (KCOs), such as pinacidil, cromakalim, diazoxide and nicorandil, inhibited spontaneous myometrial contractions in a reversible and glibenclamide-sensitive manner. KCOs inhibited oxytocin (OXT)- and prostaglandin F2α (PGF2α)-induced phasic contractions in a glibenclamide-sensitive manner. SUR2B and Kir6.2 were detected by Western blot, whereas SUR1, SUR2A and Kir6.1 were not. These results show that pinacidl, cromakalim, diazoxide and nicorandil-sensitive KATP channels exist in murine myometrium, which are composed of SUR2B and Kir6.2. Based on the modulatory effects of the KATP channel on spontaneous contraction, OXT- and PGF2α-induced contractions, KATP channels seem to play an essential role in murine myometrial motility via activation of SUR2B and Kir6.2.
Keywords: ATP-sensitive potassium (KATP), Kir6.2, myometrium, relaxation, SUR2B
ATP-sensitive potassium (KATP) channels were first identified in cardiac cell, and their physiological functions, such as hypoxic and ischemic conditions, are well characterized, even in smooth muscle and pancreatic cells [3,4,5, 9, 10, 20]. Cellular metabolism is tightly linked to regulation of KATP channel activity, as the channels open in response to lower ATP levels and close in response to higher ATP levels. KCOs, such as pinacidil and diazoxide [3, 4, 16, 22], open KATP channels, and glibenclamide blocks opening of the channels [3, 4, 16, 24]. Basically, activated KATP channels hyperpolarize, which opposes muscle excitability and reduces calcium influx via voltage-dependent L-type Ca2+ channels (VDCCL) [3]. At molecular level, the KATP channel is composed of subunits from a combination of inwardly rectifying K+ channels (Kir6.1 or Kir 6.2) and sulfonylurea receptors (SUR1, 2A and 2B) [2, 3].
Antociban and β-adrenoceptor agonists have been used to reduce preterm myometrial contractions, but are unsatisfactory [5, 6]. Premature delivery remains a major risk factor for perinatal mortality [21, 25]. Myometrial contractions are quiescent during pregnancy, but are enhanced dramatically for labor at term [18]. The regulation of myometrial contractions is modulated by many endogenous factors, such as polypeptide hormones and neurotransmitters [26].
In addition, changes of ion channel activities in non-pregnant and pregnant myometra are also important for myometrial contractility [19]. Basically, activation of K+ channels opposes muscle excitability and relaxes the myometrium by regulating the resting membrane potential. Several types and/or different activities of K+ channels, such as Ca2+-activated K+ (KCa) channels and voltage-dependent K+ (KV) channels, are involved in the regulation of myometrial contractility in non-pregnant and pregnant myometria [10, 13,14,15, 23]. Recently, we also reported functional upregulation of TASK-2 channels in pregnant than that in non-pregnant mice [10]. Interestingly, KATP channels is also key contributors to labor at term [7]. However, the existence subtypes and role of KATP channels in myometrium is still not well defined. Therefore, we characterized the molecular isoforms and physiological functions of KATP channels in murine myometrium in this study.
MATERIALS AND METHODS
Tissue preparation for isometric contraction: All experiments were performed in accordance with the guidelines for animal care and use approved by Chungbuk National University and The Physiological Society of Shanghai Jiaotong University. Female non-pregnant ICR (age, 10–12 weeks) mice were anesthetized with fluoromethyl 2,2,2-trifluoro-1 (trifluoromethyl) ethyl ether (Sevoflurane; Maruishi Pharma., Osaka, Japan) and killed by cervical dislocation [10]. The uteri were cut open from the neck to the end of the uterine horns, rinsed in Krebs-Ringer bicarbonate (KRB) solution and pinned on a Sylgard plate [10]. Longitudinal muscle strips (1 × 5 mm) were mounted on vertical chambers in an isometric contractile measuring system. One end of the tissue was tied to a fixed holder, and the other side was linked to a force transducer (Harvard Instruments, Holliston, MA, U.S.A.). The PowerLab-Data Acquisition System and a personal computer running Charter v5.5 software (ADinstruments, Colorado Springs, CO, U.S.A.) were used. Each strip was stretched passively to resting tension for 1–2 hr after a 1.5 hr equilibration [10]. Contractile responses of the strip to high K+ (50 mM) were repeated two times.
Solution and drugs: KRB solution (CO2/bicarbonate-buffered Tyrode) contained (in mM): NaCl 122, KCl 4.7, MgCl2 1, CaCl2 2, NaHCO3 15, KH2PO4 0.93 and glucose 11 (pH 7.3–7.4, bubbled with 5% CO2/95% O2). Equimolar concentration of Na+ was replaced by K+ to make high K+ (50 mM) solution. The external solution was changed by solutions which had previously been incubated (bubbled with 5% CO2/95% O2, 36°C) in water bath before the application. Pretreatment of various blockers was applied for 12–15 min before the application of KCOs. All drugs used in this study were purchased from Sigma (St. Louis, MO, U.S.A.).
Western blot: Tissues were fresh-frozen in liquid nitrogen and were homogenized in homogenation buffer containing 0.01% (v/v) protease inhibitor cocktail. Tissue homogenates were then centrifuged for 10 min, and proteins were measured by a Bradford protein assay using BSA as the standard. Equal amounts (20–40 µg) of soluble proteins were separated using 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) at 100V for 90 min and transferred to a PVDF membrane. The PVDF membranes were blocked with 5% skim-milk in TBS buffer solution, followed by incubation with Kir 6.1, Kir 6.2, SUR1, SUR2A and SUR2B (Santa Cruz (Dallas, TX, U.S.A.); Millipore (Darmstadt, Germany); and Streess Marq (Victoria, Canada); 1:500–1:200) antibodies. The membranes were incubated with secondary antibody (1:5,000) diluted in TBS for 1 hr, followed by 3 washes. β-actin (Abfrontier, 1:2,000) and Goat anti-rabbit IgG secondary antibody (Santa Cruz, 1:5,000) were also used.
Statistics: Data were expressed as means ± standard errors of the mean (means ± SEM). The ANOVA and Student’s t-test were used to measure the statistical significance. P-values less than 0.05 were regarded as statistically significant.
RESULTS
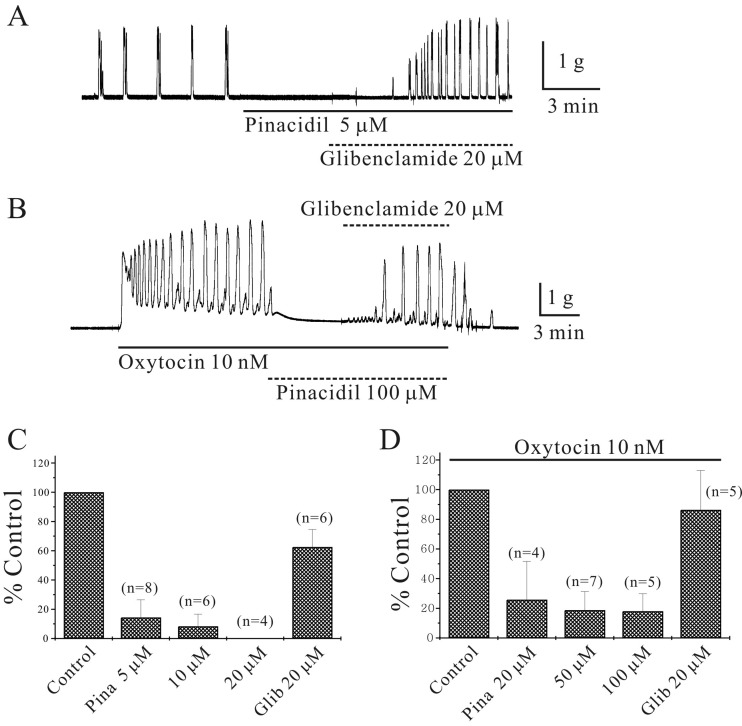
Inhibition of isometric contractions in murine myometrium by pinacidil: Spontaneous isometric contractions of 1.9 ± 0.4 g and 3 ± 2.1 cycles/min were recorded (n=60) (Figs. 1A, 2A, 2B and 4A). High K+ produced 0.4 ± 0.07 g contractions (n=60; data not shown). Pinacidil (5 µM), which opens KATP channels (KCOs), inhibited spontaneous myometrial contractions in a glibenclamide-sensitive manner (Fig. 1A). Pinacidil (5, 10 and 20 µM) inhibited phasic contractions of murine longitudinal myometrium to 14 ± 12.1%, 8 ± 8.3% and 0% of the control (n=8, 6 and 4, respectively; P<0.05; Fig. 1C). Glibenclamide, which blocks KATP channels, reversed the inhibition of spontaneous isometric contractions by pinacidil (5 µM) to 63 ± 12% of the control (n=6; P<0.05; Fig. 1C). Oxytocin (OXT, 10 nM) produced tri-phasic contractions that included initial contractions, followed by tonic contractions overlapped with phasic contractions (Fig. 1B). The phasic contractions produced by OXT (10 nM) were inhibited by pinacidil (20, 50 and 100 µM) to 26 ± 25.8%, 19 ± 12.5% and 18 ± 11.9% of the control (n=4, 7 and 5, respectively; P<0.05; Fig. 1D). Glibenclamide reversed the inhibition of spontaneous isometric contractions by pinacidil (50 µM) to 86 ± 26.5% of the control (n=5; P<0.05; Fig. 1D).
Fig. 1.
Inhibition of murine myometrial contractions by pinacidil. (A) Pinacidil (5 µM) inhibited spontaneous contractions of murine longitudinal myometrium in a glibenclamide-sensitive manner. (B) Oxytocin (OXT, 10 nM) produced tri-phasic contractions, such as initial contractions, followed by tonic contractions overlapped with phasic contractions. Pinacidil inhibited OXT-induced phasic contractions to 12% of the control. Data are summarized in (C) and (D).
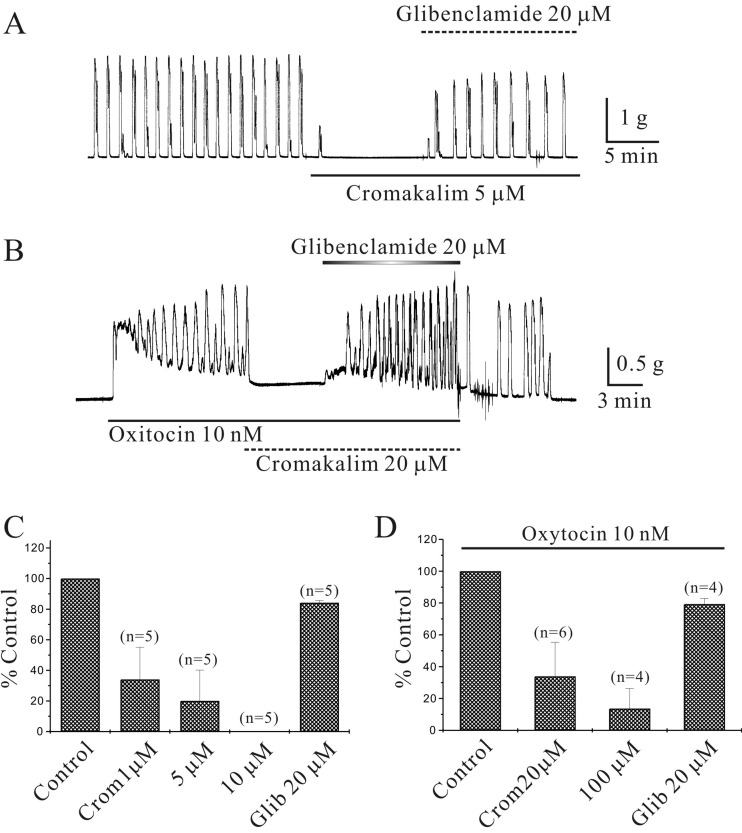
Inhibition of isometric contractions in murine myometrium by cromakalim: Cromakalim inhibited spontaneous contractions completely in a glibenclamide-sensitive manner. Cromakalim (5 µM) inhibited spontaneous contractions, which were recovered by glibenclamide (Fig. 2A and 2C). Cromakalim (1, 5 and 10 µM) inhibited phasic contractions to 34 ± 21.1%, 20.1 ± 20.0% and 0% of the control (n=5, respectively; P<0.05; Fig. 2C). Glibenclamide reversed the inhibition of spontaneous isometric contractions caused by cromakalim (5 µM) to 84 ± 1.5% of the control (n=5; P<0.05; Fig. 2C). Cromakalim (20 and 100 µM) also inhibited OXT (10 nM)-induced phasic contractions to 34 ± 21.4% and 14 ± 12.6% of the control (n=6 and 4, respectively; P<0.05; Fig. 2B and 2D). Glibenclamide reversed the inhibition of spontaneous isometric contractions by cromakalim (100 µM) to 79 ± 3.5% of the control (n=4; P<0.05;Fig. 2D). As shown in Fig. 2B, tonic contraction by OXT was also suppressed by cromakalim in a glibenclamide-sensitive manner.
Fig. 2.
Inhibition of murine myometrial contractions by cromakalim. (A) Cromakalim (5 µM) completely inhibited spontaneous contractions of murine myometrium in a glibenclamide-sensitive manner. (B) Cromakalim (20 µM) completely inhibited OXT (10 nM)-induced phasic contractions in a glibenclamide-sensitive manner. Data are summarized in (C) and (D).
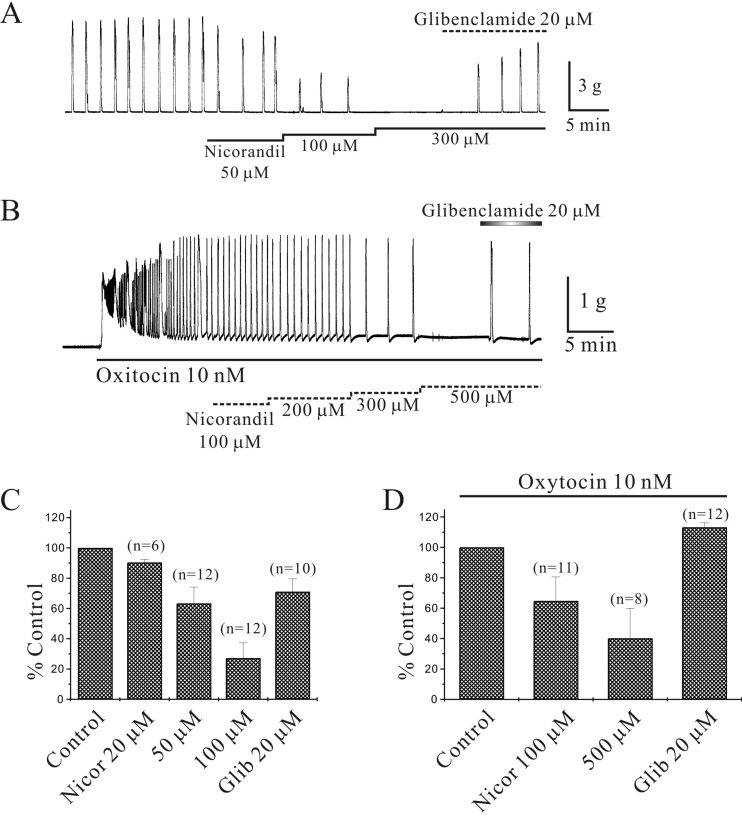
Inhibition of isometric contractions in murine myometrium by nicorandil: Nicorandil (20–100 µM) inhibited spontaneous myometrial contractions in a glibenclamide-sensitive manner (Fig. 3A). Nicorandil (20, 50, 100 and 300 µM) inhibited spontaneous myometrial contractions to 90 ± 2.0%, 63 ± 10.8%, 27 ± 10.2% and 0% of the control (n=6, 12, 12 and 1, respectively; P<0.05; Fig. 3C). OXT (10 nM)-induced phasic contractions were also inhibited by nicorandil (100–500 µM) in a glibenclamide-sensitive manner (Fig. 3B). OXT (10 nM)-induced phasic contractions were inhibited by nicorandil (100 and 500 µM) to 65 ± 15.7% and 40 ± 19.7% of the control (n=11 and 8, respectively; P<0.05; Fig. 3D). Glibenclamide reversed the inhibition of spontaneous and OXT-induced phasic contractions caused by nicorandil (100 µM and 500 µM) to 71 ± 8.6% and 113 ± 2.9% of the control (n=10 and 5, respectively; P<0.05; Fig. 3C and 3D).
Fig. 3.
Inhibition of murine myometrial contractions by nicorandil. (A) Nicorandil inhibited spontaneous contraction of murine longitudinal myometrium in a glibenclamide-sensitive manner. Nicorandil (100–300 µM) inhibited spontaneous contraction completely. (B) Oxytocin (OXT, 10 nM)-induced phasic contractions were inhibited to 40% of the control at Nicorandil (500 µM). Data are summarized in (C) and (D).
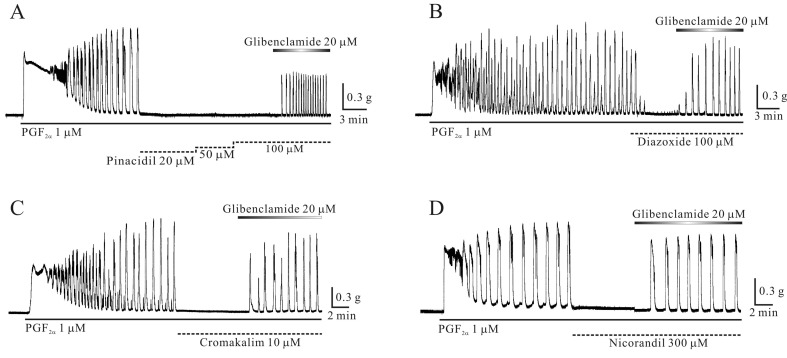
Inhibition of prostaglandin F2α (PGF2α)-induced phasic contractions in murine myometrium by KCOs: PGF2α produced tri-phasic contractions, such as initial contractions, followed by tonic contractions overlapped with phasic contractions in murine uterine smooth muscle (Fig. 4A–4D). PGF2α-induced phasic contractions were inhibited completely by pinacidil (10 µM), diazoxide (100 µM), cromakalim (10 µM) and nicorandil in a glibenclamide-sensitive manner (n=3, 3, 3 and 2, respectively; P<0.05; Fig. 4A–4D). In addition, all KCOs completely inhibited acetylcholine (ACh)-induced contractions in a glibenclamide-sensitive manner too (data not shown; see discussion).
Fig. 4.
Prostaglandin F2α (PGF2α)-induced contractions were inhibited by K+ channel opening agents (KCOs). (A–D) PGF2α produced tri-phasic contractions, such as initial contractions, followed by tonic contractions overlapped with phasic contractions. PGF2α-induced phasic contractions were inhibited by pinacidil, diazoxide, cromakalim and nicorandil.
Identity of the KATP channel subunits in murine myometrium: The identity of the KATP channel in murine myometrium was studied. SUR1, SUR2A and Kir6.1 were not detected by Western blot. However, proteins compatible with SUR2B (58K) and Kir6.2 (55K), which were composed of KATP channels, were detected in murine myometrium (Fig. 5A and 5B). The density of each SUR2B/β-actin and Kir6.2/β-actin was 383 ± 112.4 and 236 ± 58.8 (n=5 and 7, respectively).
Fig. 5.
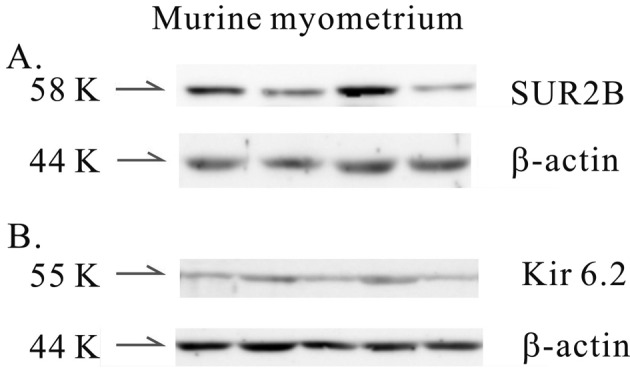
Identification of KATP channel subunits in murine longitudinal myometrium. The SUR and Kir subtypes were studied by Western blot. SUR1, SUR2A and Kir6.1 were not detected in murine myometrium. (A, B) However, the SUR2B and Kir6.2 subtypes were detected in murine myometrium by Western blot.
DISCUSSION
This is the first report that KATP channels composed or SUR2B and Kir6.2 produced relaxation of various contractions by diverse KCOs of murine myometrium.
Regulation of smooth muscle function via KATP channels is dependent on these types of Kir and/or SUR isoforms [3, 22]. KATP channels are octomeric proteins formed with inwardly rectifying potassium (Kir) channel subunits along with SUR receptor subunits, and there are several types of KATP channel combinations [2, 3]. In structure, Kir is a pore forming structure, and SUR provides binding sites for ATP and KCOs [2, 3]. The combination of Kir6.1/SUR2B (and/or Kir6.2/SUR2B) in vascular smooth muscle [3] and Kir6.2/SUR2B in guinea-pig stomach was identified [22]. KATP channels in atrial and ventricular myocytes are composed differently, as atrial and ventricular myocytes have the same Kir 6.2 subunit but different SUR subtypes called SUR1 and SUR2A, respectively [3]. The physiological functions of KATP channels are diverse in various tissues, including regulation of resting membrane potential modulation of calcium influx, and regulating the force and frequency of gastrointestinal tract contractions [3, 22]. Therefore, their function cannot be determined without isolating the isoforms from each tissue.
In myometrium, Kir6.1, Ki6.2, SUR1 and SUR2B isoforms have been identified in human myometrium [7]. Recently, expression of SUR2A subtype was also reported [9]. However, isoforms of Kir6.1 and SUR2B only have been identified as functional KATP channel in rat and human [9, 20]. In our study, as shown in results, we detected Kir6.2 and SUR2B subtypes in murine myometrium by Western blot (Fig. 5). Other subtypes were not detected in Western blot. At this moment, unfortunately, we cannot explain the different expression of KATP channels in each myometrium. Functionally, pinacidil and diazoxide are representative activators of KATP channels in mammals by relaxing human uterine arterial and human pregnant uterine smooth muscle [17, 20]. As shown in Figs. 1 and 4A, pinacidil relaxed murine longitudinal uterine muscle. Although data not shown, diazoxide (100, 200 and 300 µM) inhibited spontaneous contractions to 63 ± 11.1%, 26 ± 16.3% and 0% of the control (n=9, 5 and 4, respectively; P<0.05) in a glibenclamide-sensitive manner (recovered to 56 ± 7.1% of the control; P<0.05; Fig. 2C). Diazoxide (100 and 300 µM) also inhibited OXT-induced phasic contractions to 51 ± 15.0% and 14 ± 13.5% of the control (n=12 and 6, respectively; P<0.05) in a glibenclamide-sensitive manner (recovered to 40 ± 18.4% of the control; P<0.05). It also inhibited PGF2α–induced contraction (Fig. 4B). In addition, cromakalim and nicorandil suppressed myometrial contractions in a reversible and glibenclamide-sensitive manner (Figs. 2, 3, 4C and 4D). Nicorandil and diazoxide have been used to treat ischemic heart disease and hypertension [11]. Nicorandil was as effective as nifedipine for tocolysis during preterm labor in a randomized-controlled study [11]. We found every four KCOs produced glibenclamide-sensitive relaxation of diverse isometric contractions of murine myometrium. Therefore, we believed that combination of Kir6.2 and SUR2B in murine myometrium might be target for every four KCOs and play a crucial role for myometrial relaxation.
KATP channel expression levels in myometrium may be associated with the stage of pregnancy [9], and KATP channels in myometrium are down regulated during late pregnancy. It has been suggested that decreased KATP channel expression at term of pregnancy is responsible for enhanced myometrial contractility at labor. It has also been suggested that upregulation of KATP channels in parturients >35-year-of-age might be responsible for the increased rate of birth complication in these women [9]. As strong contractions are produced from several hours to even days during labor, energy metabolism is very complex at this time. In addition, we found that substituting D-mannitol for glucose inhibited the frequency of PGF2α-induced contractions from 1.8 ± 0.17 to 0.5 ± 0.14 (n=2; data not shown). Thus, KATP channels may be one of a target for regulation of myometrial contractions. At molecular level, Kir6.2 is required for protection against myocardiac ischemia/reperfusion (I/R) injury and skeletal muscle weakness [8, 12]. In addition, it was also reported that vasodilation by metabolic inhibition, such as oxygen and glucose deprivation (OGD)-mediated inhibition, was attenuated in SUR2 null arteries [1].
Uterine smooth muscle spasms via vascular contracture and/or local contraction of vessels [17] through production of PGF2α might be responsible for dysmenorrhea. Unfortunately, no medications can effectively reduce myometrial spasms factory. We only found the molecular and physiological functions of KATP channels in this study, and activation of KATP channels (Kir6.2/SUR2B) in the murine uterus produced reversible relaxation (Fig. 5). KATP channel regulator agents modulate contractility of human and rat smooth muscle. We also observed that KCOs inhibited PGF2α-induced contraction in murine myometrium in a reversible- and glibenclamide-sensitive manner (Fig. 4). Generally, the regulation of myometrial contraction is modulated by many endogenous factors [26]. Finally, pinacidil (10 µM) inhibited ACh-induced phasic contractions to 17% of the control (n=11; P<0.05) in a glibenclamide-sensitive manner (recovered to 73% of the control; n=8; P<0.05). Diazoxide (100 µM), cromakalim (10 µM) and nicorandil (100 µM) also inhibited it to 43%, 8.6% and 40% of the control in a glibenclamide-sensitive manner (n=6, 5 and 8, respectively; P<0.05; data not shown). Therefore, KATP channels may be a key factor in menstrual pain, which needs to be studied in the future.
CONFLICTS OF INTERESTS
No conflicts of interest exist for this study.
Acknowledgments
Seung Hwa Hong, Kyu-Sang Kyeong and Chan Hyung Kim equally contributed to this work. This work was supported by the research grant of the Chungbuk National University in 2013. We give special thanks for experimental assistance to Hee Sun Park.
REFERENCES
- 1.Adebiyi A., McNally E. M., Jaggar J. H.2011. Vasodilation induced by oxygen/glucose deprivation is attenuated in cerebral arteries of SUR2 null mice. Am. J. Physiol. Heart Circ. Physiol. 301: H1360–H1368. doi: 10.1152/ajpheart.00406.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryan J., Muñoz A., Zhang X., Düfer M., Drews G., Krippeit-Drews P., Aguilar-Bryan L.2007. ABCC8 and ABCC9: ABC transporters that regulate K+ channels. Pflugers Arch. 453: 703–718. doi: 10.1007/s00424-006-0116-z [DOI] [PubMed] [Google Scholar]
- 3.Burke M. A., Mutharasan R. K., Ardehali H.2008. The sulfonylurea receptor, an atypical ATP-binding cassette protein, and its regulation of the KATP channel. Circ. Res. 102: 164–176. doi: 10.1161/CIRCRESAHA.107.165324 [DOI] [PubMed] [Google Scholar]
- 4.Cook D. L., Hales C. N.1984. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature 311: 271–273. doi: 10.1038/311271a0 [DOI] [PubMed] [Google Scholar]
- 5.Creasy R. K., Resnik R., Iams J. D.2009. Ch28 pathogenesis of spontaneous preterm labor. pp. 521–543. In: Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice, 6th ed., Saunders/Elsevier, Philadelphia. [Google Scholar]
- 6.Cunningham F. G., MacDonald P. C., Gant N. F., Leveno K. J., Gilstrap L. C., Hankins G. D. V.1997. Williams Obstetrics. 20th ed., p. 817. Appleton and Lange, Norwalk. [Google Scholar]
- 7.Curley M., Cairns M. T., Friel A. M., McMeel O. M., Morrison J. J., Smith T. J.2002. Expression of mRNA transcripts for ATP-sensitive potassium channels in human myometrium. Mol. Hum. Reprod. 8: 941–945. doi: 10.1093/molehr/8.10.941 [DOI] [PubMed] [Google Scholar]
- 8.Du R. H., Dai T., Cao W. J., Lu M., Ding J. H., Hu G.2014. Kir6.2-containing ATP-sensitive K(+) channel is required for cardioprotection of resveratrol in mice. Cardiovasc. Diabetol. 13: 35–43. doi: 10.1186/1475-2840-13-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Q., Jovanović S., Tulić L., Sljivančanin D., Jack D. W., Zižić V., Abdul K. S. M., Tulić I., Jovanović A.2013. KATP channels are up-regulated with increasing age in human myometrium. Mech. Ageing Dev. 134: 98–102. doi: 10.1016/j.mad.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 10.Hong S. H., Sung R., Kim Y. C., Suzuki H., Choi W., Park Y. J., Ji I. W., Kim C. H., Myung S. C., Lee M. Y., Kang T. M., You R. Y., Lee K. J., Lim S. W., Yun H. Y., Song Y. J., Xu W. X., Kim H. S., Lee S. J.2013. Mechanism of relaxation via TASK-2 channels in uterine circular muscle of mouse. Korean J. Physiol. Pharmacol. 17: 359–365. doi: 10.4196/kjpp.2013.17.4.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii H., Ichimiya S., Kanashiro M., Amano T., Ogawa Y., Mitsuhashi H., Sakai S., Uetani T., Murakami R., Naruse K., Murohara T., Matsubara T.2007. Effect of intravenous nicorandil and preexisting angina pectoris on short- and long-term outcomes in patients with a first ST-segment elevation acute myocardial infarction. Am. J. Cardiol. 99: 1203–1207. doi: 10.1016/j.amjcard.2006.12.034 [DOI] [PubMed] [Google Scholar]
- 12.Jovanović S., Du Q., Mukhopadhyay S., Swingler R., Buckley R., McEachen J., Jovanović A.2008. A patient suffering from hypokalemic periodic paralysis is deficient in skeletal muscle ATP-sensitive K+ channels. Clin. Transl. Sci. 1: 71–74. doi: 10.1111/j.1752-8062.2008.00007.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan R. N., Smith S. K., Morrison J. J., Ashford M. L.1997. Ca2+ dependence and pharmacology of large-conductance K+ channels in nonlabor and labor human uterine myocytes. Am. J. Physiol. 273: C1721–C1731. [DOI] [PubMed] [Google Scholar]
- 14.Kostrzewska A., Laudański T., Batra S.1996. Inhibition of contractile responses of human myometrium and intramyometrial arteries by potassium channel openers. Acta Obstet. Gynecol. Scand. 75: 886–891. doi: 10.3109/00016349609055022 [DOI] [PubMed] [Google Scholar]
- 15.Monaghan K., Baker S. A., Dwyer L., Hatton W. C., Sik Park K., Sanders K. M., Koh S. D.2011. The stretch-dependent potassium channel TREK-1 and its function in murine myometrium. J. Physiol. 589: 1221–1233. doi: 10.1113/jphysiol.2010.203869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noma A.1983. ATP-regulated K+ channels in cardiac muscle. Nature 305: 147–148. doi: 10.1038/305147a0 [DOI] [PubMed] [Google Scholar]
- 17.Novakovic R., Milovanovic S., Protic D., Djokic J., Heinle H., Gojkovic-Bukarica L.2007. The effect of potassium channel opener pinacidil on the non-pregnant rat uterus. Basic Clin. Pharmacol. Toxicol. 101: 181–186. doi: 10.1111/j.1742-7843.2007.00096.x [DOI] [PubMed] [Google Scholar]
- 18.Pinto R. M., Lerner U., Pontelli H.1967. The effect of progesterone on oxytocin-induced contraction of the three separate layers of human gestational myometrium in the uterine body and lower segment. Am. J. Obstet. Gynecol. 98: 547–554. [DOI] [PubMed] [Google Scholar]
- 19.Sanborn B. M.1995. Ion channels and the control of myometrial electrical activity. Semin. Perinatol. 19: 31–40. doi: 10.1016/S0146-0005(95)80045-X [DOI] [PubMed] [Google Scholar]
- 20.Sawada K., Morishige K., Hashimoto K., Tasaka K., Kurachi H., Murata Y., Kurachi Y.2005. Gestational change of K+ channel opener effect is correlated with the expression of uterine KATP channel subunits. Eur. J. Obstet. Gynecol. Reprod. Biol. 122: 49–56. doi: 10.1016/j.ejogrb.2004.11.026 [DOI] [PubMed] [Google Scholar]
- 21.Schwarz M. K., Page P.2003. Preterm labour: an overview of current and emerging therapeutics. Curr. Med. Chem. 10: 1441–1468. doi: 10.2174/0929867033457331 [DOI] [PubMed] [Google Scholar]
- 22.Sim J. H., Yang D. K., Kim Y. C., Park S. J., Kang T. M., So I., Kim K. W.2002. ATP-sensitive K(+) channels composed of Kir6.1 and SUR2B subunits in guinea pig gastric myocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 282: G137–G144. [DOI] [PubMed] [Google Scholar]
- 23.Smith R. C., McClure M. C., Smith M. A., Abel P. W., Bradley M. E.2007. The role of voltage-gated potassium channels in the regulation of mouse uterine contractility. Reprod. Biol. Endocrinol. 5: 41–52. doi: 10.1186/1477-7827-5-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T.1989. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science 245: 177–180. doi: 10.1126/science.2501869 [DOI] [PubMed] [Google Scholar]
- 25.Wray S.2015. Insights from physiology into myometrial function and dysfunction. Exp. Physol.100: 1468–1476. [DOI] [PubMed] [Google Scholar]
- 26.Wray S., Noble K.2008. Sex hormones and excitation-contraction coupling in the uterus: the effects of oestrous and hormones. J. Neuroendocrinol. 20: 451–461. doi: 10.1111/j.1365-2826.2008.01665.x [DOI] [PubMed] [Google Scholar]