Abstract
Nasal lymphoma is the most common nasal tumor in cats and is generally a solitary and radiosensitive tumor. We retrospectively evaluated the response to radiation and survival time in relation to apoptosis and Ki-67 indices in feline nasal lymphomas treated with radiation therapy. The apoptotic and Ki-67 indices were evaluated with TUNEL and immunohistochemical staining in 30 biopsy tissues that were taken before any treatment. These two indices were compared, and differences between different treatment response groups were analyzed. The correlation between the median survival times (MST) and the indices was estimated using the Kaplan Meier method, and statistical differences between survival curves were analyzed using a log-rank method. With regard to apoptotic index, a statistical difference was observed between the samples taken from cats with complete response and stable disease (1.22% vs. 0.45%; P=0.045). The Ki-67 index in cats with both complete response and partial response was significantly higher than in cats with stable disease (44.4% and 39.6% vs. 16.3%; P<0.001 and P=0.008, respectively). The cats with a high level of apoptosis (>0.9%) nasal lymphoma were not significantly prolonged MSTs (P=0.202), however, high Ki-67-positive (>40%) cats experienced a statistically significant relationship with longer survival time (P=0.015). Our results indicate that spontaneous apoptotic and Ki-67 indices are strong predictors for response to radiation therapy in feline nasal lymphomas.
Keywords: apoptosis, feline nasal lymphoma, ki-67, radiation therapy, survival
Apoptosis is the process of programmed cell death, which eliminates old and damaged cells in tissue [1]. Apoptosis process is regulated by a number of proteins that involve the activation of caspases in cell. Biochemical changes lead to alterations in cell morphology, including membrane blebbing, cell shrinkage, chromatin condensation and DNA fragmentation [1, 29]. Apoptosis is thought to be one of consequence of radiation therapy; inducing cell death in tumors [35, 38]. A positive correlation between the rate of spontaneous apoptosis and radiation-induced apoptosis has previously been demonstrated [27, 29]. Tumor cell apoptosis is reported to correlate with the outcome of treatment in several human carcinomas [1, 25, 29]. These studies revealed that tumors with high spontaneous apoptotic rate could be related with good prognosis.
Ki-67 is a non-histone nuclear protein that is expressed in actively proliferating cells during all phases of cell cycle, except G0, resting phase. In previous studies, high Ki-67-positive tumors responded better to radiation therapy than tumors with lower level of Ki-67 in several human cancers [2, 7, 10, 18]. Therefore, a high Ki-67-positive tumor is believed to be associated with radiosensitivity, because it is likely to be radioresistant in the G0 phase. In veterinary medicine, a study also reported the relationship between high level of Ki-67 and longer disease-free intervals in feline squamous cell carcinomas that treated with electron beam radiation therapy [21].
Lymphomas are typically radiosensitive tumors in humans, dogs and cats [5, 6, 12, 36, 37]. Radiation therapy is useful to treat solitary lymphoma for local control, such as feline nasal lymphoma [12, 28]. A large retrospective study by Haney et al. of 97 cats with nasal lymphoma showed that the response rate for radiation therapy alone was 93% with the median survival tome (MST) of 456 days [12]. A few studies have shown the prognostic factors for feline nasal lymphomas. These factors include anemia, irradiated dose and cribriform plate destruction [12, 28].
This retrospective study was primarily aimed to evaluate the importance of apoptotic and proliferative activities in the pretreatment biopsy samples in cats with nasal lymphoma treated with radiation therapy. We hypothesized that tumors with high apoptotic and high Ki-67 index would be more effective to radiation therapy than that with low.
MATERIALS AND METHODS
Medical records were retrospectively reviewed for cats with nasal lymphoma treated with radiation therapy at the Rakuno Gakuen University Veterinary Teaching Hospital. The 30 cats included in this study were treated between April 2004 and December 2011. All cats had biopsies taken under general anesthesia and were diagnosed with lymphoma before any treatments. Pretreatment evaluation included complete blood counts, serum chemistry, three-view thoracic radiographs, abdominal ultrasounds, CT scans of head, chest and abdomen, and aspiration cytology of lymph nodes or organs. Follow-up information was obtained from the medical records or by contacting the referring veterinarians.
Radiation therapy was performed by use of an orthovoltage X-ray machine (TITAN-450S, GE, Tokyo, Japan) with a half-value layer of 4.8 mm of Cu at 450 kV and 10 mA. The exposure rate was 1.68 Gy/min with a filter of 1.0 mm of Al, 0.3 mm of Cu and 0.5 mm of Sn. The distance from the X-ray source to the skin was 60 cm. Radiation was delivered via single dorsal portal directed to the tip line of the nose to the top of the head. Eye and brain were included in the radiation field, if needed. The treated tumor volume had a 1 cm margin of normal tissue. Irradiation was 4 or 6 Gy for each fraction. Two fractions were carried out weekly (Monday/Friday schedule).
The response was assessed based on CT images at one month after finishing the radiation therapy. Complete response (CR) was determined, if the tumor disappeared from CT images. Partial response (PR) was defined as a decrease of 30% or greater in the sum of the longest diameters. Stable disease (SD) was defined as between a decrease of 30% and an increase of 20% in the sum of the longest diameter. Progressive disease (PD) was defined as an increase of 20% or greater in the sum of the longest diameters [32].
Apoptotic and proliferative activities in pretreatment biopsy samples in feline nasal lymphoma were detected. A terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay was carried out using an ApopTag In Situ Apoptosis Detection Kit (S7100, Chemicon Merck Millipore, Billerica, MA, U.S.A.) to detect apoptotic cells. All 3-µm sections from paraffin-embedded tissues that were taken before radiation therapy were cut. The samples were pretreated with proteinase K (20 µg/ml, Dako, Glostrup, Denmark). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. Slides were covered with equilibration buffer for a few sec. Working-strength TdT solution was then added to the slides at 37°C for 1 hr. The reaction was stopped by addition of working-strength wash/stop buffer for 10 min. Anti-digoxigenin peroxidase was applied, and the tissue sections were then incubated for 30 min at room temperature. 3-3′-Diaminobenzidine (Kanto Chemical Co., Tokyo, Japan) solution was used for color developing, and then, Mayer’s hematoxylin was used for counterstaining.
Immunohistochemical staining was used for detecting Ki-67-labeled cells on biopsied tumor samples. The antigen was retrieved by autoclaving at 121°C for 15 min with 0.1 M citrate buffer (pH 6.0). All sections were blocked for endogenous peroxidase activity and non-specific binding with 3% hydrogen peroxide and 1% horse serum in phosphate-buffered saline (PBS). Sections were then incubated with a mouse anti-Ki-67 monoclonal antibody (clone MIB-1, Dako) at a 1:100 dilution in PBS for 60 min at room temperature. The secondary antibody dilution and avidin-biotin complex solution (Vectastain ABC Kit, Vector, Burling, CA, U.S.A.) were applied to the sections and incubated for 30 min each at room temperature. 3-3′-Diaminobenzidine solution and Mayer’s hematoxylin were used as chromogen and counterstain, respectively.
The apoptotic index (AI) was determined as the percentage of apoptotic positive tumor cells that stained brown in pretreatment biopsy samples. Approximately 1,000 tumor cells were counted randomly by microscopic examination. A low AI was defined as an AI below 0.9%. High AI was defined as an AI above 0.9%. The Ki-67 labeling index, defined as the percentage of Ki-67-positive tumor cells, was counted in five random fields. The Ki-67 index was divided into two groups (high and low Ki-67) with the level of 40% as the cut point.
For statistical analysis, 30 samples were divided into two groups based on the clinical findings (age, sex, presence of cribriform plate destruction and response to radiation). We compared the two indices (apoptosis and Ki-67) and analyzed the differences between these groups using t-test for independent samples. The correlation between the AI and Ki-67 indices was analyzed according to Spearman’s correlation method. The cats that had a stage I disease (single organ affected) and treated with radiation alone were studied with survival time. Survival time of the patient was defined from the starting date of radiation therapy to the time of death. The correlation between the MSTs and the indices was estimated using the Kaplan Meier method, and statistically significant differences between survival curves were analyzed using a log-rank method. A P value of <0.05 (two-sides) was considered statistically significant. Commercial software StatMate III (ATMS Co., Ltd., Tokyo, Japan) was used to perform the statistical analysis.
RESULTS
Thirteen of the enrolled cats were neutered males, thirteen were spayed females, two were intact males, and two were intact females. The age of the cats that started treatment with radiation therapy ranged from 4 to 17 years with mean and median of 10.7 and 11 years, respectively. Mean weight was 4.2 kg (range of 2.5 to 8.7 kg, median of 4.7 kg). Only one pure breed of Persian cat was represented in this study, and the others were mixed-breed cats (n=29). The most common clinical sign at the time of diagnosis was purulent or mucous nasal discharge (73.3%) followed by facial deformity, nose bleeding, sneezing and exophthalmos. Fourteen cats (46.7%) were found to have cribriform plate destruction due to the tumor on pre-treatment CT images.
The mean of the total skin surface radiation dose was 34.4 Gy (median of 36 Gy; range of 28 to 42 Gy). Sixteen cats were determined to have CR after the radiation therapy. Nine cats were found to have PR, and five cats had SD. No PD was observed in this study. The response rate of radiation therapy was 83.3% (25/30). Thirteen cats also received chemotherapy after radiation therapy, because of having remaining nasal mass or evidence of systemic disease. The cause of death was recorded in follow-up information. Fourteen cats died owing to distant disease (kidney, n=12; spleen, n=1; and brain, n=1), four cats died from local recurrence and brain involvement, three cats died from renal failure but no evidence of renal lymphoma, two cats died of causes other than tumor, three cats died of unknown causes, and four cats were still alive at the time of data analysis.
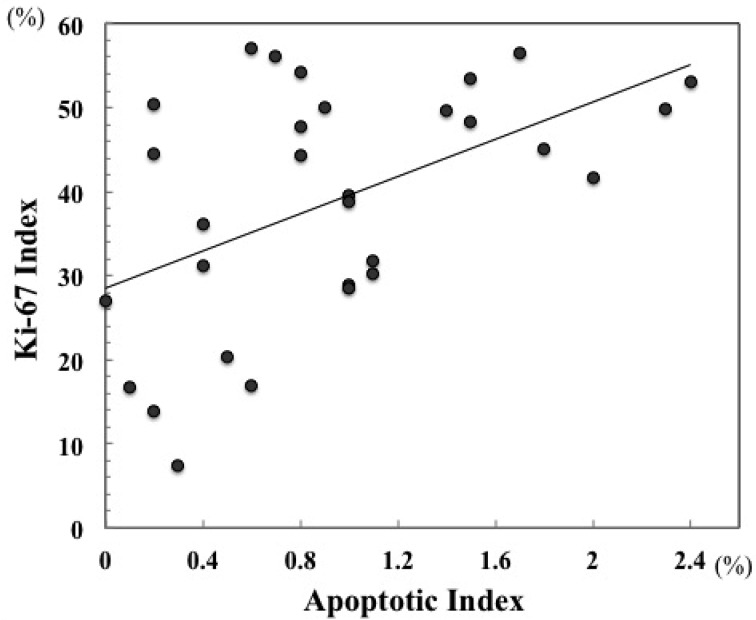
The AI and Ki-67-positive rates are shown in Table 1. The AI of all pre-treatment biopsy samples in feline nasal lymphoma ranged from 0 to 2.4%, with a median of 0.85% and mean of 0.94%. The mean AI for CR samples was 1.22%, and the mean AIs for the PR and SD samples were 0.77% and 0.46%, respectively. A statistically significant difference was observed between the samples from cats with CR and from cats with SD (P=0.045). The percentage of Ki-67-positive tumor cells in all samples ranged from 7.5 to 57.1% (median of 41.6% and mean of 38.9%). The samples from the cats with CR and PR had mean Ki-67 index of 44.4% and 39.6%. The mean Ki-67 index for the SD samples was 16.3%. The Ki-67 index in both the CR and PR samples was significantly higher than in the SD samples (P<0.001 and P=0.008). However, a significant difference was not observed between the samples from cats with CR and PR (P=0.334). In all samples of feline nasal lymphoma, a significant positive correlation was found between AI and Ki-67 index (r=0.509; P=0.005) (Fig. 1).
Table 1. Comparison of the apoptotic and Ki-67 indices according to clinical findings.
| Number of samples | Indices (Mean ± SD) (%) | |||
|---|---|---|---|---|
| AI | Ki-67 | |||
| Age | ||||
| ≤11 years | 16 | 0.92 ± 0.72 | 40.61 ± 13.22 | |
| >11 years | 14 | 0.98 ± 0.61 | 36.39 ± 15.46 | |
| Gender | ||||
| Female | 15 | 0.87 ± 0.70 | 35.58 ± 15.39 | |
| Male | 15 | 1.02 ± 0.63 | 41.37 ± 12.42 | |
| Destruction of cribriform plate | ||||
| Yes | 14 | 1.05 ± 0.70 | 39.84 ± 15.15 | |
| No | 16 | 0.87 ± 0.63 | 37.54 ± 13.89 | |
| Response to Treatment | ||||
| CR | 16 | 1.22 ± 0.77 | 44.43 ± 10.76 | |
| PR | 9 | 0.77 ± 0.24 | 39.64 ± 12.68 | |
| SD | 5 | 0.44 ± 0.43 | 16.30 ± 9.41 | |
Significant differences were calculated with the t-test. a) P<0.05.
Fig. 1.
Correlation between apoptotic and Ki-67 indices in cats with nasal lymphoma (r=0.509; P=0.005).
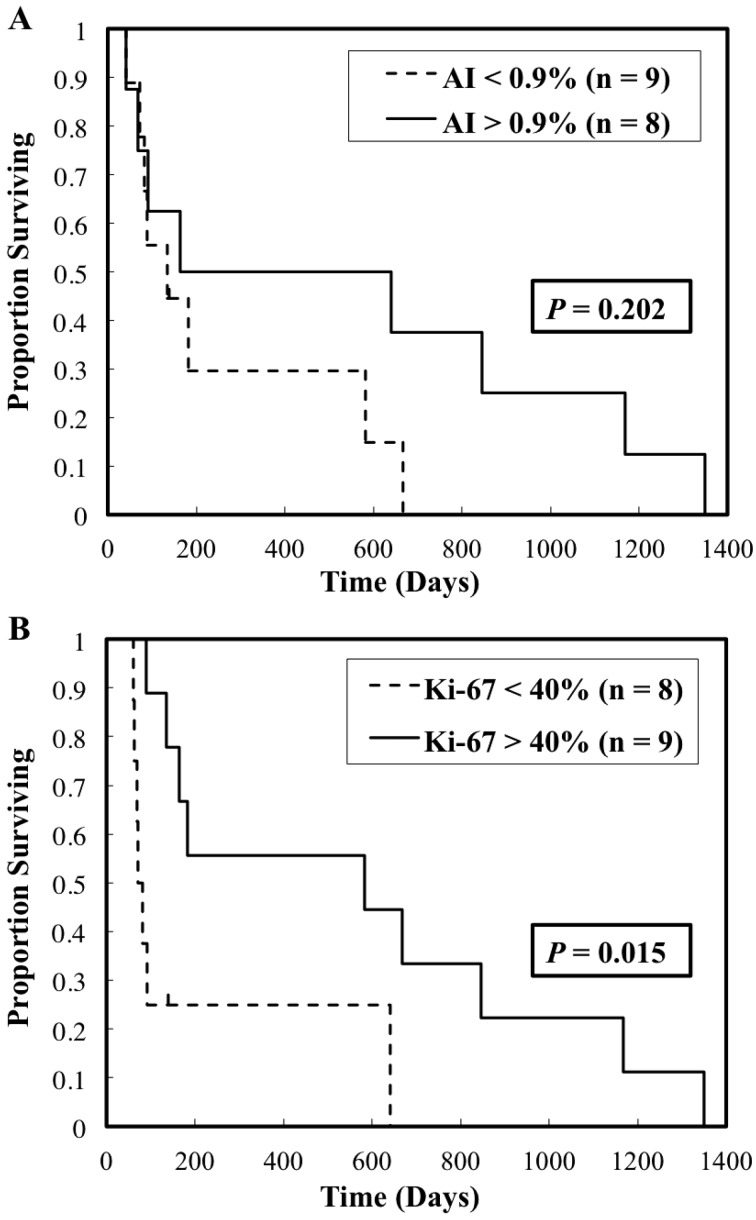
Seventeen cats diagnosed with stage I disease at first diagnosis day and only treated with radiation therapy were estimated with overall survival times. The cut-off levels of the apoptotic and Ki-67 indices were 0.9% and 40%, respectively. The respective MSTs for cats with high AI and low AI were 402.5 days and 135 days, respectively, but there was no statistical difference between the cats with high and low AI (P=0.202; Fig. 2A). On the other hand, the cats with a high Ki-67 index had significantly longer survival time compared with those with a low Ki-67 tumor (582 days vs. 77 days; P=0.015; Fig. 2B).
Fig. 2.
Kaplan–Meier curves for overall survival time in 17 cats comparing with different levels of apoptotic and Ki-67 indices. (A) The MST in cats with high AI and low AI was 402.5 days and 135 days, respectively (P=0.202). (B) The MST in cats with high Ki-67 and low Ki-67 was 582 days and 77 days, respectively (P=0.015).
DISCUSSION
Nasal lymphoma is the most common nasal tumor in cats [4, 13, 20, 23]. Chemotherapy should be considered as a top priority of treatment in feline nasal lymphoma, because it is generally a systemic disease. Previous larger studies of cats with nasal lymphoma that were treated with chemotherapy alone reported to have MSTs ranged from 98 to 456 days [12, 13, 30, 31, 33]. Owing to the nature of lymphomas being sensitive to radiation, radiation therapy is also thought to be treatment choice for localized form of lymphomas [12, 28, 37]. A large retrospective study by Haney et al. of 97 cats with nasal lymphoma showed that the MST for radiation therapy alone was 456 days [12]. Additionally, the nasal lymphoma cats treated with a combination of local radiation therapy and systemic chemotherapy had a MST from 174 to 955 days [12, 28].
A small number of studies showed prognostic indicators for feline nasal lymphomas, including anemia, irradiation dose, response and destruction of the cribriform plate [12, 28]. To our knowledge, this study is the first report to correlate the rate of apoptosis and cell proliferation in pretreatment biopsy samples, and the outcome of radiation therapy in feline nasal lymphoma. In veterinary medicine, immunohistochemistry has not been widely used to assess the response to irradiation at the cellular level and has not been used to indicate outcome to radiation [11, 21].
Ionizing radiation may cause DNA damage. The cells occur death when the damage of DNA is irreparable, which triggers the cellular pathways. Cell death induced by radiation includes mitotic death and interphase death. Mitotic death is the cells failing to mitosis completely and then death, whereas interphase cell death is the irradiated cells undergoing cell death through apoptosis before they enter mitosis, particularly soon after treatment [38]. Previous studies suggested that radiation-induced apoptosis closely correlates with radiosensitivity [1, 9, 22]. Spontaneous apoptotic rate also correlates with the induced apoptotic rate and radiosensitivity. The tumor with a high spontaneous apoptotic rate would occur apoptosis easily after irradiation [22]. The relationship between apoptosis and outcome in radiation has been studied in several human cancers. A high apoptotic rate is strongly associated with good prognosis in cancer patients treated with radiation therapy [1, 29, 35]. However, there were few studies focused on spontaneous apoptotic rate for a prognostic predictor in lymphomas. Apoptosis-induced death is considered major reason in lymphoid cells compared to other type cells after radiation [38]. Although it was unsignificant, the spontaneous apoptosis trended to be predictive to response to in vivo irradiation in a study of human non-Hodgkin’s lymphoma [9]. Moreover, one study of feline lymphoma cell lines also suggested that the more radioresistent cell line was prone to a lower ratio of spontaneous apoptosis [14].
The TUNEL method is a common technique used for detecting DNA fragmentation associated with apoptosis. TUNEL was used in this study, and the AI was considered as a response predictor for feline nasal lymphomas. The tumor achieving CR showed significantly higher rate of apoptosis than which with SD (mean of 1.22% vs. 0.46%; P=0.045). Although no significant difference was observed, the cats with a high spontaneous apoptosis rate in the pretreated biopsy sample trended to a longer survival time (MST of 402.5 vs. 135 days). Some proteins also have been linked to radiosensitivity, such as p53, Bcl-2 and survivin. They are some of the key regulators for the apoptotic pathways [25,26,27]. P53 gene contributed to the delay of induction of apoptosis in the more radioresistant human lymphoid cell lines [3]. Futher investigation is needed to clarify the linkage between apoptosis and radiosensitivity.
Cytological changes of feline lymphocytes after radiation were reported in a previous study. Cell nuclei were more likely to appear swelling and multi-nucleated after irradiation in the feline lymphocyte cell line that appeared to be resistant to radiation-induced apoptosis. These nucleic changes were similar to other mammalian cells undergoing mitotic catastrophe [14]. It is possible that some lymphoma cells may die via mitotic death after radiation.
Some human cancers have been shown to have higher Ki-67 tumors and were linked to better outcomes with radiation therapy [2, 7, 10, 18]. A study on feline squamous cell carcinoma also found that cats with higher Ki-67 had markedly favorable prognosis than cats with lower Ki-67 [21]. Nevertheless, no significant difference was observed in canine nasal carcinomas [11]. Ki-67 is not expressed at G0 phase in the cell cycle. Tumor cells are preferentially in G0 phase in a hypoxic environment. Cell cycle of G0 phase is also considered to be a radioresistant period in cells. Thus, a low level of Ki-67 in tumor cells is believed to reflect hypoxia and radioresistance [7, 16]. Ki-67 expression detects the proliferating cells, which has also been reported in feline and canine lymphomas [8, 17]. The rapidly proliferating tissues may have more radiosensitivity than those growing slowly. In our study, cats with a good response had a higher Ki-67 index than cats with a poor response, by a statistically significant margin (CR and PR vs. SD). Moreover, cats with a higher level of Ki-67 treated with radiation therapy had significantly longer MST than cats with a low Ki-67 (582 vs. 77 days, respectively). Despite the relation between proliferation and prognosis being suggested, the reason of this relationship is necessary to be clarified by a further study in lymphomas.
In our study, a significant positive correlation was observed between the rate of spontaneous apoptosis and Ki-67-positive cells in feline nasal lymphomas (r=0.509; P=0.005). High apoptotic factors have been associated with high proliferation indices in canine lymphomas and human non-Hodgkin’s lymphomas [19, 24]. Many of the genes that control cell death also regulate proteins involved in cell division, such as p53. Therefore, cell apoptosis and proliferation control appear to be closely linked with each other [15, 24, 34]. Faster proliferating tumors usually indicate better response to irradiation, and higher apoptosis levels may also reflect rapidly proliferating tumors. Our results show that the spontaneous apoptotic and Ki-67 indices are both strong predictors of response to radiation therapy in feline nasal lymphomas.
In conclusion, although our study was retrospective and the study was limited in population size, the response predictive power of measuring apoptosis and proliferation was strong. Assessment of apoptosis and proliferation before radiation therapy could be helpful for decision making to decide an appropriate protocol of treatment. Because there are many regulators of apoptosis and proliferation in tumor cells, and because radiosensitivity of cancer treatment is not clearly known, additional larger studies would be necessary in order to increase the benefit of treatment.
Acknowledgments
he authors sincerely thank Dr. Kazuko Hirayama, Dr. Hiroyuki Taniyama (Department of Veterinary Pathology, Rakuno Gakuen University, Ebetsu, Japan) and Dr. Yumiko Kagawa (North Lab, Sapporo, Japan) for cooperation in histopathology.
REFERENCES
- 1.Adell G. C., Zhang H., Evertsson S., Sun X. F., Stål O. H., Nordenskjöld B. A.2001. Apoptosis in rectal carcinoma: prognosis and recurrence after preoperative radiotherapy. Cancer 91: 1870–1875. doi: [DOI] [PubMed] [Google Scholar]
- 2.Ahmed W. A., Suzuki K., Imaeda Y., Horibe Y.2008. Ki-67, p53 and epidermal growth factor receptor expression in early glottic cancer involving the anterior commissure treated with radiotherapy. Auris Nasus Larynx 35: 213–219. doi: 10.1016/j.anl.2007.08.011 [DOI] [PubMed] [Google Scholar]
- 3.Aldridge D. R., Radford I. R.1998. Explaining differences in sensitivity to killing by ionizing radiation between human lymphoid cell lines. Cancer Res. 58: 2817–2824. [PubMed] [Google Scholar]
- 4.Allen H. S., Broussard J., Noone K.1999. Nasopharyngeal diseases in cats: a retrospective study of 53 cases (1991-1998). J. Am. Anim. Hosp. Assoc. 35: 457–461. doi: 10.5326/15473317-35-6-457 [DOI] [PubMed] [Google Scholar]
- 5.Axiak S. M., Carreras J. K., Hahn K. A., Endicott M. M., Parshley D. E., King G. K.2006. Hematologic changes associated with half-body irradiation in dogs with lymphoma. J. Vet. Intern. Med. 20: 1398–1401. doi: 10.1111/j.1939-1676.2006.tb00757.x [DOI] [PubMed] [Google Scholar]
- 6.Berlato D., Schrempp D., Van Den Steen N., Murphy S.2012. Radiotherapy in the management of localized mucocutaneous oral lymphoma in dogs: 14 cases. Vet. Comp. Oncol. 10: 16–23. doi: 10.1111/j.1476-5829.2011.00270.x [DOI] [PubMed] [Google Scholar]
- 7.Couture C., Raybaud-Diogène H., Têtu B., Bairati I., Murry D., Allard J., Fortin A.2002. p53 and Ki-67 as markers of radioresistance in head and neck carcinoma. Cancer 94: 713–722. doi: 10.1002/cncr.10232 [DOI] [PubMed] [Google Scholar]
- 8.Dank G., Lucroy M. D., Griffey S. M., Gandour-Edwards R., Madewell B. R.2002. bcl-2 and MIB-1 labeling indexes in cats with lymphoma. J. Vet. Intern. Med. 16: 720–725. [PubMed] [Google Scholar]
- 9.Dubray B., Breton C., Delic J., Klijanienko J., Maciorowski Z., Vielh P., Fourquet A., Dumont J., Magdelenat H., Cosset J. M.1998. In vitro radiation-induced apoptosis and early response to low-dose radiotherapy in non-Hodgkin’s lymphomas. Radiother. Oncol. 46: 185–191. doi: 10.1016/S0167-8140(97)00148-5 [DOI] [PubMed] [Google Scholar]
- 10.Freudlsperger C., Freier K., Hoffmann J., Engel M.2012. Ki-67 expression predicts radiosensitivity in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 41: 965–969. doi: 10.1016/j.ijom.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 11.Fu D. R., Kato D., Watabe A., Endo Y., Kadosawa T.2014. Prognostic utility of apoptosis index, Ki-67 and survivin expression in dogs with nasal carcinoma treated with orthovoltage radiation therapy. J. Vet. Med. Sci. 76: 1505–1512. doi: 10.1292/jvms.14-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haney S. M., Beaver L., Turrel J., Clifford C. A., Klein M. K., Crawford S., Poulson J. M., Azuma C.2009. Survival analysis of 97 cats with nasal lymphoma: a multi-institutional retrospective study (1986-2006). J. Vet. Intern. Med. 23: 287–294. doi: 10.1111/j.1939-1676.2008.0243.x [DOI] [PubMed] [Google Scholar]
- 13.Henderson S. M., Bradley K., Day M. J., Tasker S., Caney S. M., Hotston Moore A., Gruffydd-Jones T. J.2004. Investigation of nasal disease in the cat—a retrospective study of 77 cases. J. Feline Med. Surg. 6: 245–257. doi: 10.1016/j.jfms.2003.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakizaki T., Hamada N., Wada S., Funayama T., Sakashita T., Hohdatsu T., Sano T., Natsuhori M., Kobayashi Y., Ito N.2006. Distinct modes of cell death by ionizing radiation observed in two lines of feline T-lymphocytes. J. Radiat. Res. (Tokyo) 47: 237–243. doi: 10.1269/jrr.0618 [DOI] [PubMed] [Google Scholar]
- 15.Kastan M. B., Canman C. E., Leonard C. J.1995. P53, cell cycle control and apoptosis: implications for cancer. Cancer Metastasis Rev. 14: 3–15. doi: 10.1007/BF00690207 [DOI] [PubMed] [Google Scholar]
- 16.Kennedy A. S., Raleigh J. A., Perez G. M., Calkins D. P., Thrall D. E., Novotny D. B., Varia M. A.1997. Proliferation and hypoxia in human squamous cell carcinoma of the cervix: first report of combined immunohistochemical assays. Int. J. Radiat. Oncol. Biol. Phys. 37: 897–905. doi: 10.1016/S0360-3016(96)00539-1 [DOI] [PubMed] [Google Scholar]
- 17.Kiupel M., Teske E., Bostock D.1999. Prognostic factors for treated canine malignant lymphoma. Vet. Pathol. 36: 292–300. doi: 10.1354/vp.36-4-292 [DOI] [PubMed] [Google Scholar]
- 18.Kropveld A., Slootweg P. J., Blankenstein M. A., Terhaard C. H., Hordijk G. J.1998. Ki-67 and p53 in T2 laryngeal cancer. Laryngoscope 108: 1548–1552. doi: 10.1097/00005537-199810000-00023 [DOI] [PubMed] [Google Scholar]
- 19.Leoncini L., Del Vecchio M. T., Megha T., Barbini P., Galieni P., Pileri S., Sabattini E., Gherlinzoni F., Tosi P., Kraft R., et al. 1993. Correlations between apoptotic and proliferative indices in malignant non-Hodgkin’s lymphomas. Am. J. Pathol. 142: 755–763. [PMC free article] [PubMed] [Google Scholar]
- 20.Little L., Patel R., Goldschmidt M.2007. Nasal and nasopharyngeal lymphoma in cats: 50 cases (1989-2005). Vet. Pathol. 44: 885–892. doi: 10.1354/vp.44-6-885 [DOI] [PubMed] [Google Scholar]
- 21.Melzer K., Guscetti F., Rohrer Bley C., Sumova A., Roos M., Kaser-Hotz B.2006. Ki67 reactivity in nasal and periocular squamous cell carcinomas in cats treated with electron beam radiation therapy. J. Vet. Intern. Med. 20: 676–681. doi: 10.1111/j.1939-1676.2006.tb02914.x [DOI] [PubMed] [Google Scholar]
- 22.Meyn R. E., Stephens L. C., Ang K. K., Hunter N. R., Brock W. A., Milas L., Peters L. J.1993. Heterogeneity in the development of apoptosis in irradiated murine tumours of different histologies. Int. J. Radiat. Biol. 64: 583–591. doi: 10.1080/09553009314551801 [DOI] [PubMed] [Google Scholar]
- 23.Mukaratirwa S., van der Linde-Sipman J. S., Gruys E.2001. Feline nasal and paranasal sinus tumours: clinicopathological study, histomorphological description and diagnostic immunohistochemistry of 123 cases. J. Feline Med. Surg. 3: 235–245. doi: 10.1053/jfms.2001.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips B. S., Kass P. H., Naydan D. K., Winthrop M. D., Griffey S. M., Madewell B. R.2000. Apoptotic and proliferation indexes in canine lymphoma. J. Vet. Diagn. Invest. 12: 111–117. doi: 10.1177/104063870001200202 [DOI] [PubMed] [Google Scholar]
- 25.Rödel C., Grabenbauer G. G., Rödel F., Birkenhake S., Kühn R., Martus P., Zörcher T., Fürsich D., Papadopoulos T., Dunst J., Schrott K. M., Sauer R.2000. Apoptosis, p53, bcl-2, and Ki-67 in invasive bladder carcinoma: possible predictors for response to radiochemotherapy and successful bladder preservation. Int. J. Radiat. Oncol. Biol. Phys. 46: 1213–1221. doi: 10.1016/S0360-3016(99)00544-1 [DOI] [PubMed] [Google Scholar]
- 26.Rödel C., Grabenbauer G. G., Papadopoulos T., Bigalke M., Günther K., Schick C., Peters A., Sauer R., Rödel F.2002. Apoptosis as a cellular predictor for histopathologic response to neoadjuvant radiochemotherapy in patients with rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 52: 294–303. doi: 10.1016/S0360-3016(01)02643-8 [DOI] [PubMed] [Google Scholar]
- 27.Rödel C., Haas J., Groth A., Grabenbauer G. G., Sauer R., Rödel F.2003. Spontaneous and radiation-induced apoptosis in colorectal carcinoma cells with different intrinsic radiosensitivities: survivin as a radioresistance factor. Int. J. Radiat. Oncol. Biol. Phys. 55: 1341–1347. doi: 10.1016/S0360-3016(02)04618-7 [DOI] [PubMed] [Google Scholar]
- 28.Sfiligoi G., Théon A. P., Kent M. S.2007. Response of nineteen cats with nasal lymphoma to radiation therapy and chemotherapy. Vet. Radiol. Ultrasound 48: 388–393. doi: 10.1111/j.1740-8261.2007.00262.x [DOI] [PubMed] [Google Scholar]
- 29.Sheridan M. T., Cooper R. A., West C. M.1999. A high ratio of apoptosis to proliferation correlates with improved survival after radiotherapy for cervical adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 44: 507–512. doi: 10.1016/S0360-3016(99)00081-4 [DOI] [PubMed] [Google Scholar]
- 30.Taylor S. S., Goodfellow M. R., Browne W. J., Walding B., Murphy S., Tzannes S., Gerou-Ferriani M., Schwartz A., Dobson J. M.2009. Feline extranodal lymphoma: response to chemotherapy and survival in 110 cats. J. Small Anim. Pract. 50: 584–592. doi: 10.1111/j.1748-5827.2009.00813.x [DOI] [PubMed] [Google Scholar]
- 31.Teske E., van Straten G., van Noort R., Rutteman G. R.2002. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol. J. Vet. Intern. Med. 16: 179–186. doi: 10.1111/j.1939-1676.2002.tb02352.x [DOI] [PubMed] [Google Scholar]
- 32.Therasse P., Arbuck S. G., Eisenhauer E. A., Wanders J., Kaplan R. S., Rubinstein L., Verweij J., Van Glabbeke M., van Oosterom A. T., Christian M. C., Gwyther S. G.2000. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 92: 205–216. doi: 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 33.Vail D. M., Moore A. S., Ogilvie G. K., Volk L. M.1998. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J. Vet. Intern. Med. 12: 349–354. doi: 10.1111/j.1939-1676.1998.tb02134.x [DOI] [PubMed] [Google Scholar]
- 34.Wellmann A., Krenacs L., Fest T., Scherf U., Pastan I., Raffeld M., Brinkmann U.1997. Localization of the cell proliferation and apoptosis-associated CAS protein in lymphoid neoplasms. Am. J. Pathol. 150: 25–30. [PMC free article] [PubMed] [Google Scholar]
- 35.Wheeler J. A., Stephens L. C., Tornos C., Eifel P. J., Ang K. K., Milas L., Allen P. K., Meyn R. E., Jr1995. ASTRO Research Fellowship: apoptosis as a predictor of tumor response to radiation in stage IB cervical carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 32: 1487–1493. doi: 10.1016/0360-3016(95)00156-S [DOI] [PubMed] [Google Scholar]
- 36.Wilder R. B., Rodriguez M. A., Tucker S. L., Ha C. S., Hess M. A., Cabanillas F. F., Cox J. D.2001. Radiation therapy after a partial response to CHOP chemotherapy for aggressive lymphomas. Int. J. Radiat. Oncol. Biol. Phys. 50: 743–749. doi: 10.1016/S0360-3016(01)01503-6 [DOI] [PubMed] [Google Scholar]
- 37.Williams L. E., Pruitt A. F., Thrall D. E.2010. Chemotherapy followed by abdominal cavity irradiation for feline lymphoblastic lymphoma. Vet. Radiol. Ultrasound 51: 681–687. doi: 10.1111/j.1740-8261.2010.01723.x [DOI] [PubMed] [Google Scholar]
- 38.Wouters B. G.2009. Cell death after irradiation: how, when and why cells die. pp. 27–40. In: Basic Clinical Radiobiology. 4th ed. (Joiner, M. and van der Kogel, A. eds.), Hodder Arnold, London. [Google Scholar]