Abstract
A 12-month-old microminipig, weighing 12.6 kg, showed 3 repeated episodes of transient ST-segment elevation in 24 hr Holter electrocardiogram after placing an ameroid constrictor around the left anterior descending coronary artery. Ventricular fibrillation was noticed just after the cessation of the 24 hr Holter-electrocardiogram recording. Direct current defibrillations and cardiopulmonary resuscitation were performed; however, they were unsuccessful, leading to the animal’s death. Its heart was excised for macroscopic analysis, which indicated that lumen of the ameroid constrictor was not narrowed and that there was no dissection, embolus or thrombus in the coronary arteries, indirectly suggesting that coronary artery vasospasm may have caused the ischemic attacks. Thus, microminipig may possess some potential to have coronary vasospasm.
Keywords: ameroid constrictor, coronary vasospasm, sudden cardiac death
Microminipig is the smallest pig in the world, which has been optimized for life-science research by Fuji Micra Inc. (Shizuoka, Japan) [5, 6, 8]. Recently, we encountered one case of microminipig showing repeated episodes of transient ST-segment elevation in Holter electrocardiogram after placing an ameroid constrictor around its left anterior descending coronary artery. Since information regarding the onset mechanisms of coronary ischemic attack in swine is still limited, we precisely analyzed this case to better understand its pathophysiology. This is the first case report of microminipig describing reversible and repeated myocardial ischemic attacks possibly due to the coronary vasospasm.
A female microminipig was purchased from Fuji Micra Inc. The experiment was approved by the Animal Research Committee for Animal Experimentation of Toho University (No. 15-53-238) and performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Toho University. The microminipig at 12-month-old, weighing 12.6 kg, was pre-anesthetized by an intramuscular injection of ketamine (16 mg/kg; Ketalar®, Daiichi Sankyo Co., Ltd., Tokyo, Japan), xylazine (1.6 mg/kg; Celactal®, Bayer Health Care Co., Ltd., Osaka, Japan) and atropine sulfate (0.01 mg/kg; Mitsubishi Tanabe Pharma Co., Osaka, Japan). A cannula was introduced into a superficial auricular vein for anesthetic injection of 20 mg of propofol (Frensenius Kabi Co., Ltd., Tokyo, Japan). Endotracheal intubation was performed with the cuffed tube of 6 mm in diameter. Anesthesia was maintained with 1% isoflurane (Isoflu®, DS Pharma Animal Health Co., Ltd., Osaka, Japan) vaporized with 100% oxygen with volume-limited ventilator (SN-480-3; Shinano Manufacturing Co., Ltd., Tokyo, Japan). A tidal volume and respiratory rate were set at 10 ml/kg and 15 strokes/min, respectively. The microminipig was placed in a supine position. A-B lead electrocardiogram was obtained by using a polygraph system (RM-6000; Nihon Kohden Corporation, Tokyo, Japan). Heparin calcium in a dose of 100 IU/kg (Caprocin®, Sawai Pharmaceutical Co., Ltd., Osaka, Japan) was intravenously administered to prevent blood clotting. A 6F-size, catheter-sheath set (RADIOFOCUS®, RR-A60G07A; Terumo Corporation, Tokyo, Japan) was inserted into the left femoral vein. A thermodilution catheter (TC-504NH; Nihon Kohden Corporation) was positioned at the right side of the heart through the left femoral vein for evaluating of the cardiac function as the basal data before myocardial infarction, and it was removed immediately after evaluation.
Then, the animal was fixed in a recumbent position so that the left thorax was exposed. Left thoracotomy was performed between the fourth and fifth ribs. The pericardium was cut along the left anterior descending artery from the upper part of the left auricle. The distal portion of the first diagonal branch was exfoliated for approximately 5 mm. Then, left anterior descending coronary artery was made free for approximately 1 cm at the distal part after its bifurcation of the first diagonal branch. The distal portion of the first diagonal branch was completely ligated by 6-0 prolene (PROLENE®, Ethicon US, Somerville, NJ, U.S.A.) before the ameroid constrictor was placed. Thirty min later, ischemic preconditioning was made at the left anterior descending coronary artery, which was distal to the bifurcation of the first diagonal branch, by stretching retract-O-tape (RETRACT-O-TAPE®, Quest Medical, Inc., Allen, TX, U.S.A.). This stretching by the retract-O-tape was carefully performed for 5 min so as not to induce the lethal ventricular arrhythmia during mounting of the ameroid constrictor (COR-1.50-SS, Research Instruments SW, Escondidl, CA, U.S.A.). Thirty min later, an ameroid constrictor was placed on the left anterior descending coronary artery, and then, the chest was closed in the standard process. Fluid absorption by the constrictor causes gradual, predictable narrowing of the coronary lumen and possibly completes vessel occlusion [4].
After the chest was closed, Holter electrocardiogram was recorded from 16:07 for 24 hr. Then, the microminipig was transferred to the cage. During the whole operation, lethal ventricular arrhythmia or cardiohemodynamic collapse was not observed, except for a transit ST-segment elevation during the ischemic preconditioning. After finishing the recording of Holter electrocardiogram at 16:07 on the next day of the operation, we fed the microminipig at 17:00, and the animal suddenly fell down while eating bait. A-B lead electrocardiogram showed ventricular fibrillation. The direct current defibrillation of 360 J was applied 3 times, but could not terminate it. Cardiopulmonary resuscitation was performed, but it was unsuccessful. About 10 min later, its death was confirmed by electrocardiogram.
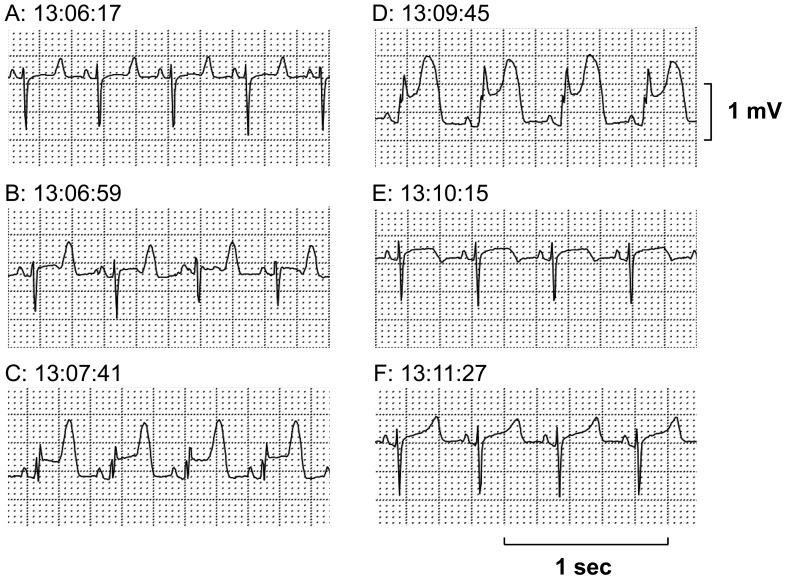
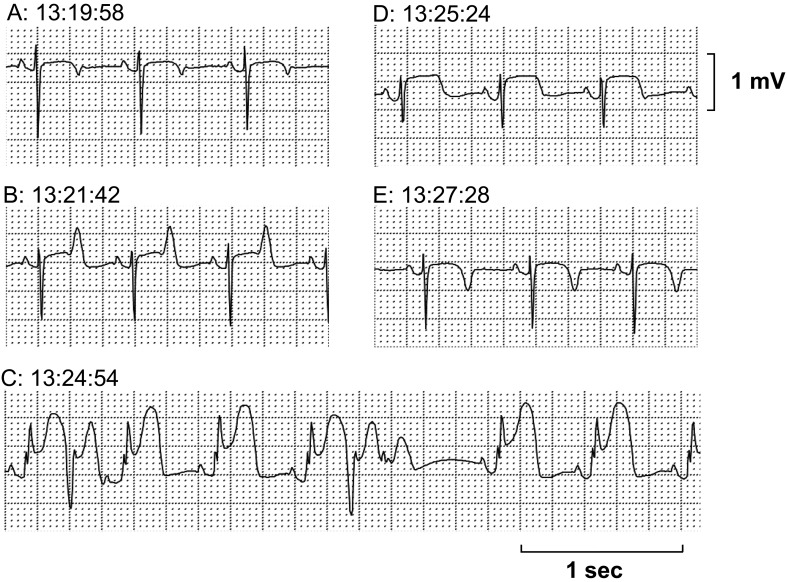
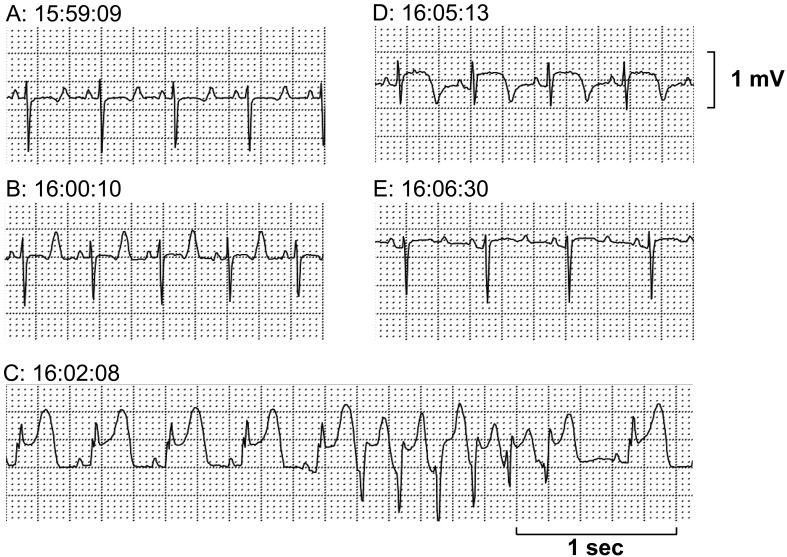
The Holter electrocardiogram was analyzed in the M-X leads. Three attacks of transient ST-segment elevation were recorded from 13:06, 13:19 and 15:59, which lasted for 5-8 min. In the first and second attacks, tall, symmetrical and peaked T waves were initially observed, which were followed by a progressive ST-segment elevation together with the onset of premature ventricular complexes. The first and second attacks lasted for 5 and 8 min, respectively (Figs. 1 and 2). In the third attack, the similar changes in the electrocardiogram were observed to those in the first and second attacks, which lasted for 7 min; moreover, nonsustained polymorphic ventricular tachycardia occurred at a period of peak ST-segment elevation (Fig. 3).
Fig. 1.
The first episode of the ST-segment elevation recorded in M-X lead by Holter electrocardiogram. (A) Control. (B) Initial phase with a tall T wave reflecting subendocardial ischemia. (C, D) Progressive ST-segment elevation. (E, F) Remission toward normal wave form. Total duration of the episode was about 5 min.
Fig. 2.
The second episode of the ST-segment elevation recorded in M-X lead by Holter electrocardiogram. (A) Control. (B) Initial phase with a tall T wave reflecting subendocardial ischemia. (C) Progressive ST-segment elevation with premature ventricular complex at peak ST-segment elevation. (D, E) Remission toward normal wave form. Total duration of the episode was about 8 min.
Fig. 3.
The third episode of the ST-segment elevation recorded in M-X lead by Holter electrocardiogram. (A) Control. (B) Initial phase with a tall T wave reflecting subendocardial ischemia. (C) Progressive ST-segment elevation with a short run of ventricular tachycardia. (D, E) Remission toward normal wave form. Total duration of the episode was about 7 min.
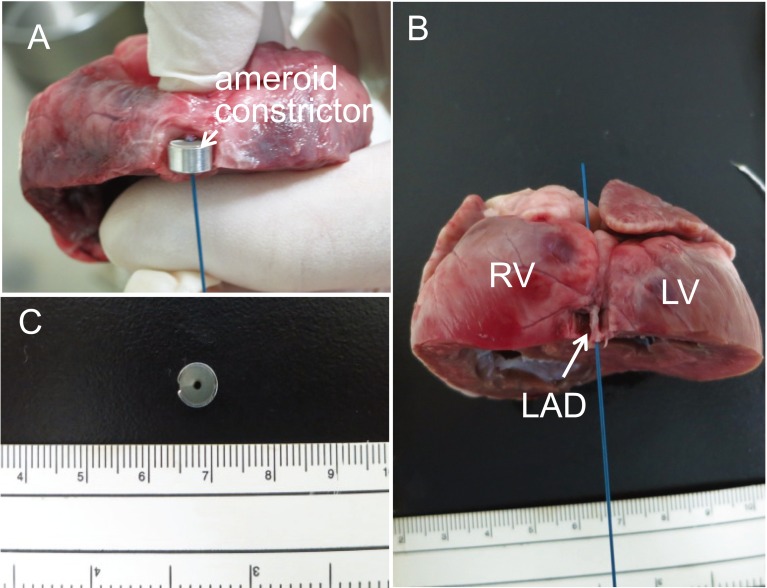
An autopsy was performed, and the heart was excised. Macroscopic analysis indicated that there was no dissection, embolus or thrombus in the right or left coronary artery. In addition, a guidewire in a diameter of 0.035 inch was easily passed through the left anterior descending coronary artery (Fig. 4A), indicating the lumen of ameroid constrictor was not narrowed. Microscopic pathological observation was not performed, because of lack of abnormal macroscopic findings in coronary arteries and ventricular walls.
Fig. 4.
Photos of excised heart. (A) The heart was cut just distal to the mounting position of ameroid constrictor. A guidewire in a diameter of 0.035 inch was placed in the left anterior descending coronary artery, which was mounted by ameroid constrictor. (B) A guidewire in a diameter of 0.035 inch was placed in the left anterior descending coronary artery. The passage of the guidewire indicates the patency of the left anterior descending coronary artery. (C) Lumen of ameroid constrictor was not narrowed. RV: right ventricle; LV: left ventricle; and LAD: left anterior descending artery.
In this case, nonsustained ST-segment elevations were repeated 3 times within a few min, suggesting that events of transient and reversible transmural ischemia were induced. Several etiologies have been described that can explain the onset of transmural ischemia, i.e. coronary artery occlusion by embolism, dissection, thrombus and/or coronary vasospasm, etc. [1, 2, 7]. In this case, the lumen of ameroid constrictor was not narrowed, whereas embolism, dissection or thrombus-induced coronary artery occlusion was not identified by macroscopic pathological observation of the heart, suggesting a high possibility that the coronary vasospasm, rather than its morphological occlusions of the coronary artery caused the ischemic attacks. The pathophysiology of coronary artery vasospasm includes an increased vasomotor tone with regional sympathetic dysinnervation, endothelial dysfunction and/or increased platelet activation [3]. In this study, the mounting procedure of ameroid constrictor around the coronary artery might have provoked the endothelial dysfunction. Thus, microminipig may possess some potential to have coronary vasospasm. Experiment is now ongoing to demonstrate the occurrence of coronary vasospasm by using the coronary angiography with pharmacological and pathological interventions.
CONFLICT OF INTEREST. The authors declare no conflict of interest.
Acknowledgments
This study was supported in part by JSPS KAKENHI (#25460344), MEXT KAKENHI (#S1101016), AMED Grant (#AS2116907E) and Project Research Grant of Toho University School of Medicine (No. 27-20). We thank Ms. Misako Nakatani, Mr. Takuya Kishie, Miss Mayuko Okamura and Miss Azajargal Enkhsakhan for their technical assistance.
REFERENCES
- 1.Bloor C. M., White F. C., Roth D. M.1992. The pig as a model for myocardial ischemia and gradual coronary arterial occlusion. pp. 162-175. In: Swine as Models in Biomedical Research, 1st ed (M. M. Swindle E. ed.), Iowa State University Press, Ames. [Google Scholar]
- 2.Camaro C., Aengevaeren W. R. M.2009. Acute myocardial infarction due to coronary artery embolism in a patient with atrial fibrillation. Neth. Heart J. 17: 297–299. doi: 10.1007/BF03086271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Luna A. B., Cygankiewicz I., Baranchuk A., Fiol M., Birnbaum Y., Nikus K., Goldwasser D., Garcia-Niebla J., Sclarovsky S., Wellens H., Breithardt G.2014. Prinzmetal angina: ECG changes and clinical considerations: a consensus paper. Ann. Noninvasive Electrocardiol. 19: 442–453. doi: 10.1111/anec.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elzinga W. E.1969. Ameroid constrictor: uniform closure rates and a calibration procedure. J. Appl. Physiol. 27: 419–421. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchi H., Miyoshi N., Miura N., Fujiki M., Horiuchi M., Izumi Y., Miyajima H., Nagata R., Misumi K., Takeuchi T., Tanimoto A., Yoshida H.2011. Microminipig, a non-rodent experimental animal optimized for life science research:novel atherosclerosis model induced by high fat and cholesterol diet. J. Pharmacol. Sci. 115: 115–121. doi: 10.1254/jphs.10R17FM [DOI] [PubMed] [Google Scholar]
- 6.Miyoshi N., Horiuchi M., Inokuchi Y., Miyamoto Y., Miura N., Tokunaga S., Fujiki M., Izumi Y., Miyajima H., Nagata R., Misumi K., Takeuchi T., Tanimoto A., Yasuda N., Yoshida H., Kawaguchi H.2010. Novel microminipig model of atherosclerosis by high fat and high cholesterol diet, established in Japan. In Vivo 24: 671–680. [PubMed] [Google Scholar]
- 7.Munz M. R., Faria M. A., Monteiro J. R., Aguas A. P., Amorim M. J.2011. Surgical porcine myocardial infarction model through permanent coronary occlusion. Comp. Med. 61: 445–452. [PMC free article] [PubMed] [Google Scholar]
- 8.Sugiyama A., Nakamura Y., Akie Y., Saito H., Izumi Y., Yamazaki H., Kaneko N., Itoh K.2011. Microminipig, a non-rodent experimental animal optimized for life science research: in vivo proarrhythmia models of drug-induced long QT syndrome: development of chronic atrioventricular block model of microminipig. J. Pharmacol. Sci. 115: 122–126. doi: 10.1254/jphs.10R21FM [DOI] [PubMed] [Google Scholar]