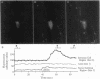
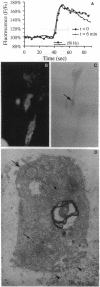
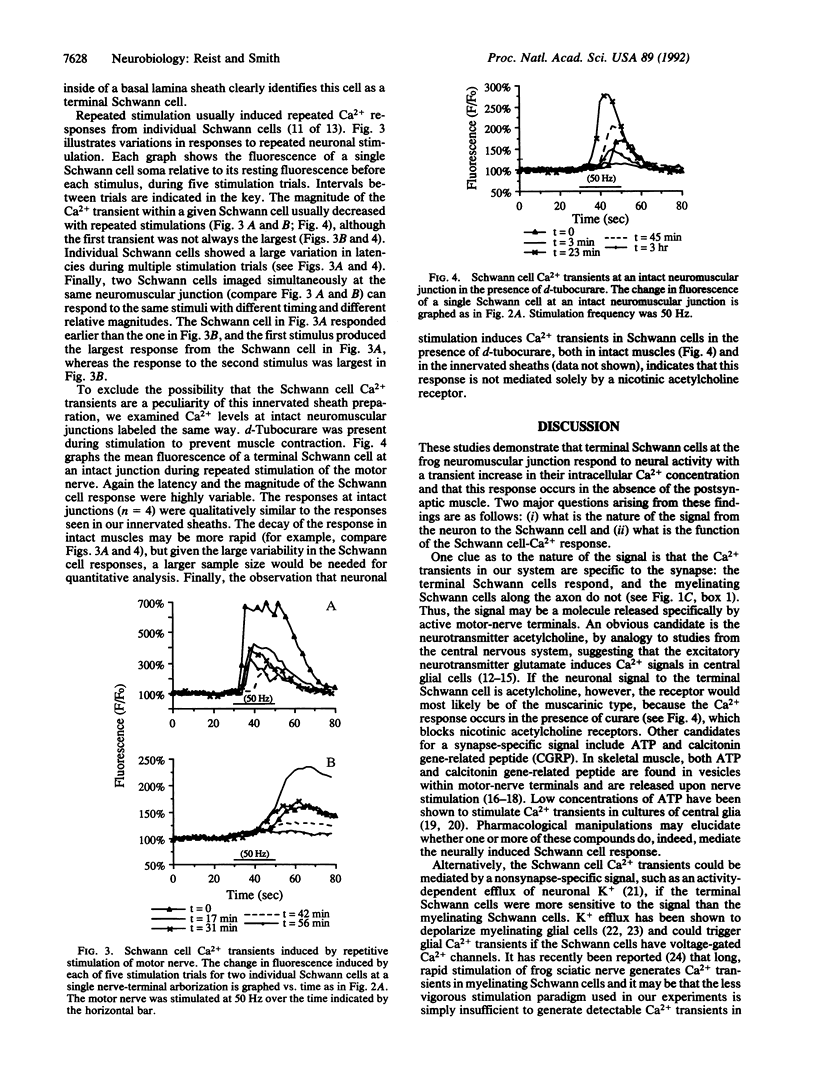
Abstract
We examined the effects of motor-nerve stimulation on the intracellular Ca2+ levels of Schwann cells, the glial cells at the frog neuromuscular junction. Schwann cells, which were loaded with the fluorescent Ca2+ indicator fluo-3 and examined by confocal microscopy, showed a transient increase in free Ca2+ within a few seconds of the onset of tetanic stimulation of the motor nerve. The Ca2+ response was specific to the synapse in that it was found in the terminal Schwann cells at the junction but not in the myelinating Schwann cells along the axon. The Ca2+ transients occurred in the presence of d-tubocurare, indicating that they were not mediated by nicotinic acetylcholine receptors and recurred when the stimulus was repeated. The Ca2+ response persisted after degeneration of the postsynaptic muscle fiber, demonstrating that the terminal Schwann cell was stimulated directly by presynaptic activity. The finding that terminal Schwann cells at the neuromuscular junction respond to presynaptic activity suggests that glial-cell function is modulated by synaptic transmission.
Full text
PDF
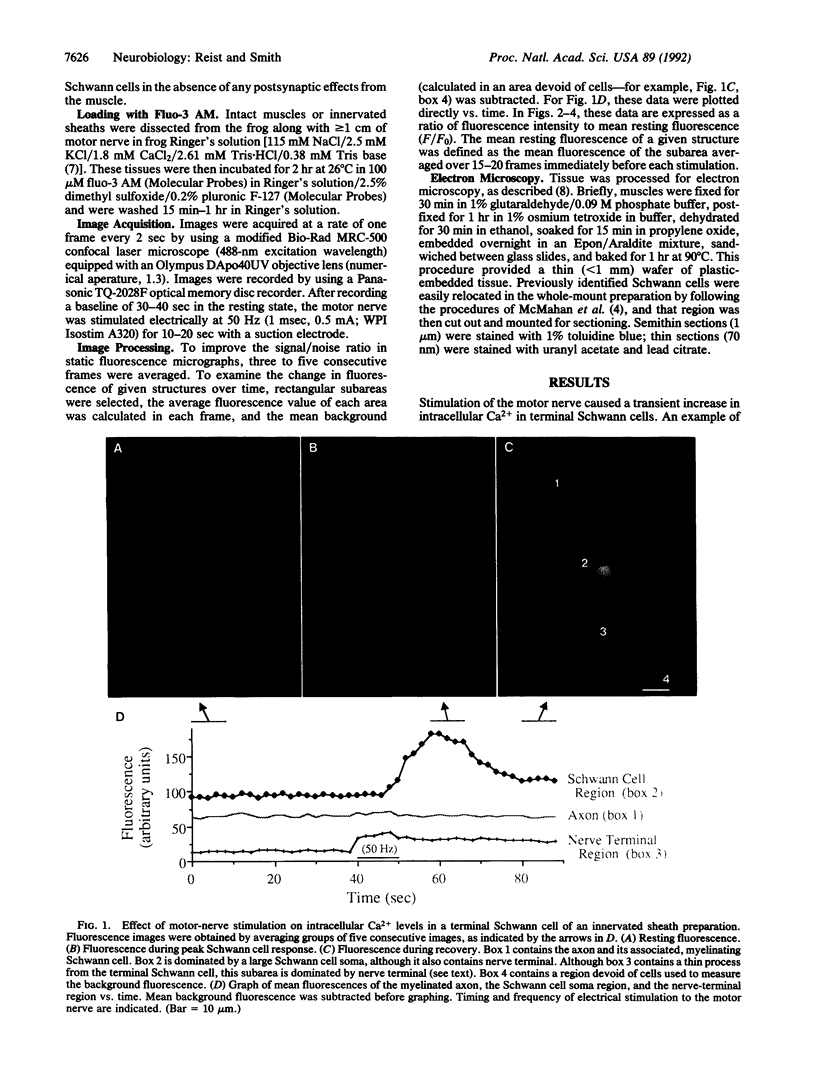
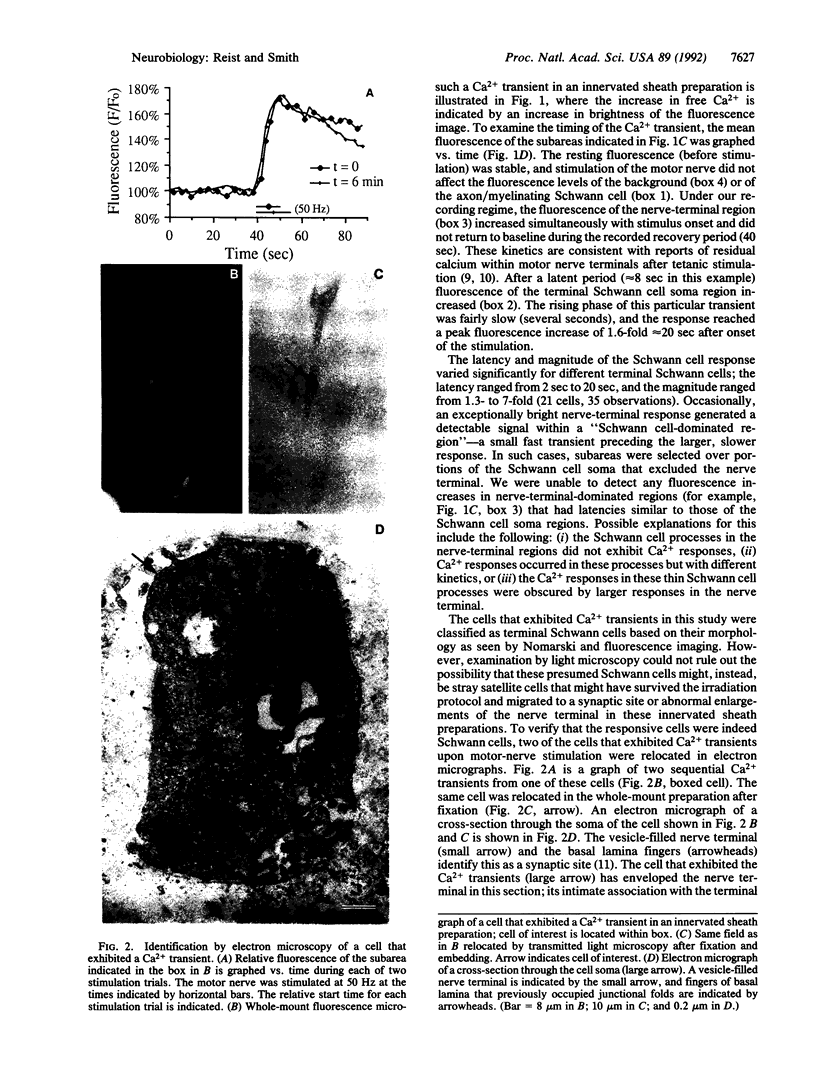

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRKS R., KATZ B., MILEDI R. Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol. 1960 Jan;150:145–168. doi: 10.1113/jphysiol.1960.sp006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B. A. New roles for glia. J Neurosci. 1991 Dec;11(12):3685–3694. doi: 10.1523/JNEUROSCI.11-12-03685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles A. C., Merrill J. E., Dirksen E. R., Sanderson M. J. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991 Jun;6(6):983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell A. H., Finkbeiner S. M., Cooper M. S., Smith S. J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990 Jan 26;247(4941):470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Dani J. W., Chernjavsky A., Smith S. J. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992 Mar;8(3):429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- Delaney K. R., Zucker R. S., Tank D. W. Calcium in motor nerve terminals associated with posttetanic potentiation. J Neurosci. 1989 Oct;9(10):3558–3567. doi: 10.1523/JNEUROSCI.09-10-03558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkvist M. O., Holopainen I., Akerman K. E. Glutamate receptor-linked changes in membrane potential and intracellular Ca2+ in primary rat astrocytes. Glia. 1989;2(6):397–402. doi: 10.1002/glia.440020602. [DOI] [PubMed] [Google Scholar]
- GALAMBOS R. A glia-neural theory of brain function. Proc Natl Acad Sci U S A. 1961 Jan 15;47:129–136. doi: 10.1073/pnas.47.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A. M., Chiu S. Y. Fluorescence measurement of changes in intracellular calcium induced by excitatory amino acids in cultured cortical astrocytes. J Neurosci. 1990 Apr;10(4):1165–1175. doi: 10.1523/JNEUROSCI.10-04-01165.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. An endplate potential due to potassium released by the motor nerve impulse. Proc R Soc Lond B Biol Sci. 1982 Nov 22;216(1205):497–507. doi: 10.1098/rspb.1982.0088. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Ram V., Grinvald A. Ca2+- and K+-dependent communication between central nervous system myelinated axons and oligodendrocytes revealed by voltage-sensitive dyes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6651–6655. doi: 10.1073/pnas.83.17.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli M., Haimann C., Torri-Tarelli F., Polak J. M., Ceccarelli B., De Camilli P. Differential effect of alpha-latrotoxin on exocytosis from small synaptic vesicles and from large dense-core vesicles containing calcitonin gene-related peptide at the frog neuromuscular junction. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7366–7370. doi: 10.1073/pnas.85.19.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Slater C. R. The influence of basal lamina on the accumulation of acetylcholine receptors at synaptic sites in regenerating muscle. J Cell Biol. 1984 Apr;98(4):1453–1473. doi: 10.1083/jcb.98.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary J. T., van Breemen C., Forster E., Norenberg L. O., Norenberg M. D. ATP stimulates calcium influx in primary astrocyte cultures. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1410–1416. doi: 10.1016/s0006-291x(88)81032-5. [DOI] [PubMed] [Google Scholar]
- Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Pearce B., Murphy S., Jeremy J., Morrow C., Dandona P. ATP-evoked Ca2+ mobilisation and prostanoid release from astrocytes: P2-purinergic receptors linked to phosphoinositide hydrolysis. J Neurochem. 1989 Mar;52(3):971–977. doi: 10.1111/j.1471-4159.1989.tb02549.x. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Marshall L. M., McMahan U. J. Reinnervation of muscle fiber basal lamina after removal of myofibers. Differentiation of regenerating axons at original synaptic sites. J Cell Biol. 1978 Jul;78(1):176–198. doi: 10.1083/jcb.78.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Hubbard J. I. Thermal synthesis of amino acids from a simulated primitive atmosphere. Nature. 1973 Jun 15;243(5407):404–405. doi: 10.1038/243404a0. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I. Glial glutamate receptors: likely actors in brain signaling. FASEB J. 1991 Dec;5(15):3086–3091. doi: 10.1096/fasebj.5.15.1660422. [DOI] [PubMed] [Google Scholar]
- Uchida S., Yamamoto H., Iio S., Matsumoto N., Wang X. B., Yonehara N., Imai Y., Inoki R., Yoshida H. Release of calcitonin gene-related peptide-like immunoreactive substance from neuromuscular junction by nerve excitation and its action on striated muscle. J Neurochem. 1990 Mar;54(3):1000–1003. doi: 10.1111/j.1471-4159.1990.tb02349.x. [DOI] [PubMed] [Google Scholar]
- Valdiosera R., Clausen C., Eisenberg R. S. Impedance of frog skeletal muscle fibers in various solutions. J Gen Physiol. 1974 Apr;63(4):460–491. doi: 10.1085/jgp.63.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]