Abstract
Bovine anaplasmosis is a vector-borne disease that results in substantial economic losses in other parts of the world but so far not in northern Europe. In August 2002, a fatal disease outbreak was reported in a large dairy herd in the Swiss canton of Grisons. Diseased animals experienced fever, anorexia, agalactia, and depression. Anemia, ectoparasite infestation, and, occasionally, hemoglobinuria were observed. To determine the roles of vector-borne pathogens and to characterize the disease, blood samples were collected from all 286 animals: 50% of the cows were anemic. Upon microscopic examination of red blood cells, Anaplasma marginale inclusion bodies were found in 47% of the cows. The infection was confirmed serologically and by molecular methods. Interestingly, we also found evidence of infections with Anaplasma phagocytophilum, large Babesia and Theileria spp., and Mycoplasma wenyonii. The last two species had not previously been described in Switzerland. Anemia was significantly associated with the presence of the infectious agents detected, with the exception of A. phagocytophilum. Remarkably, concurrent infections with up to five infectious vector-borne agents were detected in 90% of the ill animals tested by PCR. We concluded that A. marginale was the major cause of the hemolytic anemia, while coinfections with other agents exacerbated the disease. This was the first severe disease outbreak associated with concurrent infections with vector-borne pathogens in alpine Switzerland; it was presumably curtailed by culling of the entire herd. It remains to be seen whether similar disease outbreaks will have to be anticipated in northern Europe in the future.
Vector-borne diseases are of increasing importance to human populations, livestock, and pet animals (17, 42, 49). Bloodsucking vectors, such as ticks and mosquitoes, can transmit a variety of pathogens, including viruses, bacteria, and parasites. The present study investigates a fatal infectious disease outbreak of hemolytic anemia in a cattle herd in Switzerland. The disease was associated with the detection of multiple vector-borne pathogens.
The outbreak was unusual for two reasons: (i) the observation of organisms not seen in this area prior to this outbreak and (ii) the high percentage of ill animals apparently infected with several organisms. Animals had concurrent infections with as many as five vector-borne pathogens for which tests were conducted. One of these pathogens was Anaplasma marginale, which leads to extravascular hemolysis and anemia in infected cows (26, 27). Severe economic losses have been reported due to bovine anaplasmosis in other parts of the world (26). However, the clinical importance of anaplasmosis had been negligible in Switzerland so far; it had been reported once and just in solitary cases (7). Similarly, bovine babesiosis, which was also detected in the present study and which is characterized by hyperthermia, hemoglobinuria, and a decline in milk yield, has so far been observed only sporadically in cattle in this region (1, 8). Moreover, Theileria spp. and Mycoplasma wenyonii (18, 33) had not previously been reported at all in Switzerland. To our knowledge, the concurrent infections described here thus represent a novelty in northern Europe.
MATERIALS AND METHODS
Clinical history and sample collection.
The fatal outbreak affected a herd of mostly dairy cows located north of the Alps in the canton of Grisons, Switzerland. The animals were housed in three groups: two in free-range stables and one in tie-in stalls. The herd size of more than 300 cattle was large by Swiss measures. In addition, animals were traded frequently and introduced to the herd regularly without a quarantine period. During July and August 2002, 20 cows died of unknown causes. This event had been preceded by the introduction of 30 animals originating from the southern part of Switzerland (Brusio, Poschiavo, located south of the Alps near the Italian border). To our knowledge, no contact with or introduction of Italian cattle had occurred. Upon necropsy of the deceased cows, samples were tested for infectious bovine rhinotracheitis (IBR; Chekit Trachitest; Bommeli AG, Liebefeld, Switzerland) (40), malignant catarrhal fever (21), anthrax (51), and bovine viral diarrhea virus (46). The remaining cattle showed fever, weakness, depression, loss of appetite, and an up to 90% decrease in milk yield. In addition, very pale mucous membranes indicative of severe anemia and, occasionally, red urine as a sign of hemoglobinuria were observed. Blood samples were collected from all remaining 286 cows. In addition, lice (Haematopinus eurysternus) from five cows as well as six ticks (five Ixodes ricinus ticks and one tick whose species was not determined) and four flies (Musca domestica) found in the respective stables were collected. As a last measure to stop the rapid spread of the disease, the Swiss Federal authorities ruled that the entire herd had to be culled at the end of August 2002.
Hematology and blood chemistry.
Thin blood smears were performed with fresh blood in EDTA anticoagulant from 285 cows and Giemsa stained by using an AMES Hema Tek slide stainer (Bayer, Zürich, Switzerland). The packed cell volumes (PCVs) of 286 samples were determined. In addition, complete hematology was performed for 172 cows (Cell-Dyn 3500; Abbott, Baar, Switzerland). Biochemical parameters were assayed for 105 serum samples (Cobas-Integra 700; Roche Diagnostics, Rotkreuz, Switzerland) by standard procedures recommended by the International Federation of Clinical Chemistry, as compiled elsewhere (47).
PCR analysis and sequencing.
Blood samples (n = 58) and several potential vectors were analyzed by PCR. DNA was purified from 200 μl of EDTA-anticoagulated blood with a QIAamp Blood Mini kit (Qiagen, Basel, Switzerland) and eluted into 50 μL of AE elution buffer. Arthropods were mechanically disrupted (Mixer Mill MM 300; Retsch GmBH, Haan, Germany) and dissolved in 180 μl of ATL tissue lysis buffer for DNA extraction with the DNeasy tissue kit (Qiagen). DNA samples were analyzed by conventional PCR for A. marginale (MSP5) and Babesia spp. and by real-time TaqMan PCR for Borrelia burgdorferi and Anaplasma phagocytophilum (previously Ehrlichia phagocytophila), as described previously (29, 36, 38, 48). In addition, a 682-bp region of the 16S rRNA gene of Theileria spp. was amplified by using the following primers: forward primer 5′-CGTCAGTTTTTACGACTCC-3′ and reverse primer 5′-GTTCACAAAACTTCCCTAGAC-3′. Briefly, 5 μl of DNA solution was amplified with 2.5 U of Taq DNA polymerase (Sigma-Aldrich, Buchs, Switzerland), 1 μM each primer, 200 μM deoxynucleoside triphosphates, and 1.25 mM MgCl2 in a total volume of 25 μl. PCR cycling conditions were as follows: 5 min at 95°C and 40 cycles of 30 s at 95°C, 30 s at 62°C, and 1 min at 72°C, followed by 10 min at 72°C. Furthermore, the M. wenyonii-specific 16S rRNA gene was amplified by a method described for the amplification of Mycoplasma haemofelis (22). Comparison of the sequences of the feline and bovine strains showed that the primer sequences match the sequence of M. wenyonii (GenBank accession number AF016546).
PCR products from conventional PCR assays were analyzed on 2 or 3% agarose gels; representative amplicons were purified with a MinElute gel extraction kit (Qiagen) and sequenced from both sides. Briefly, cycle sequencing was performed with approximately 20 ng of DNA and 3.3 pmol of product-specific primers by using a BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Rotkreuz, Switzerland). Cycling conditions were as follows: 25 cycles at 96°C for 10 s, 50°C for 5 s, and 60°C for 3 min. The products were purified with a DyeEx Spin column (Qiagen) and analyzed on an ABI Prism 310 Genetic Analyzer (Applied Biosystems).
Serological assays.
Serum samples (n = 285) were tested for A. marginale-specific antibodies by a competitive enzyme-linked immunosorbent assay (cELISA; Veterinary Medical Research and Development Inc., Pullman, Wash.) (24). In addition, selected samples were tested for bovine ephemeral fever (52) and A. phagocytophilum (37).
Cell culture assays.
In order to detect retroviruses, mononuclear cell cultures were initiated from 10 cows by standard methods with concanavalin A and recombinant human interleukin-2 (a generous gift from Novartis, Basel, Switzerland); the supernatant was assayed for reverse transcriptase activity (39). Subsequently, the cells were used for cytospin preparations and were examined microscopically.
Statistics.
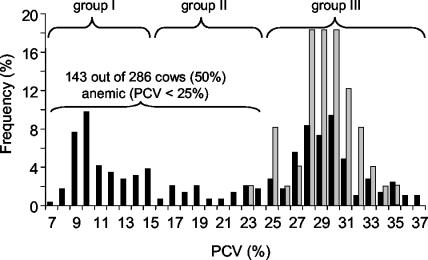
Data were compiled and analyzed with Excel software (Microsoft), Clinical Laboratory software (version 1.65; Analyse-it Software, Ltd., Leeds, United Kingdom), and Prism software (version 3.00; GraphPad Software, Inc., San Diego, Calif.). The chi-square statistic was used to test for associations between categorical variables; linear correlation and the nonparametric Spearman rank test were performed for continuous variables (41). Because anemia appeared to be the key sign of illness, animals were categorized into three groups according to their PCVs for further analysis: group I consisted of severely anemic cattle (PCVs < 15%); group II consisted of cattle that were anemic with intermediate PCVs (15 to <25%), and group III had normal PCVs (≥25%) (Fig. 1). Differences between groups I to III were tested for statistical significance by the nonparametric Kruskal-Wallis test with Dunn's multiple-comparison posttest (41). Calculation of the P value according to Kruskal-Wallis analysis values took the total number of comparisons into account. Differences were considered significant when P was <0.05 for the entire family of comparisons. Agreement between different methods was tested by using kappa statistics (4). Weighted kappa values (KW) >0.8 were interpreted as very good agreement.
FIG. 1.
Frequency distribution of PCVs of the 286 cattle sampled (black bars) in comparison to PCV reference values (gray bars) determined in the Clinical Laboratory, University of Zurich. Reference values were determined by identical methods with blood samples from 49 healthy lactating Swiss cows ages 3 to 11 years. The 5% quantile of the PCV reference range is 25%, the median is 29%, and the 95% quantile is 33%. Fifty percent of the cows of the affected herd were anemic (PCVs < 25%). For further analysis, animals were categorized into severely anemic animals (group I), cows with intermediate PCVs (group II), and cows with normal PCVs (group III).
RESULTS
Hematological and biochemical changes in diseased cattle.
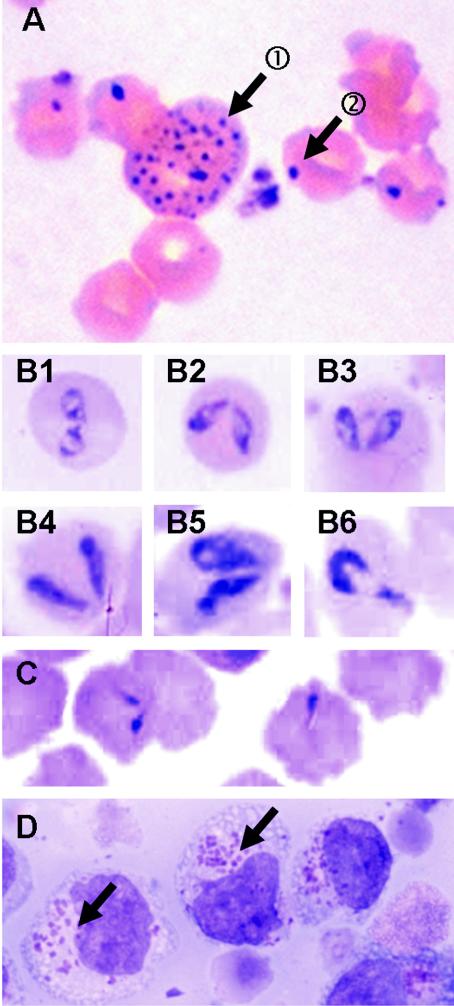
To characterize the disease, all animals of the affected herd were evaluated hematologically. Anemia, defined as PCVs <25%, according to the reference values for lactating cows, was found in 50% of the cattle (Fig. 1); 95 animals (33%) had very low PCVs (<15%). Thin blood smears of anemic cattle showed polychromatic red blood cells (RBCs) and anisocytosis. Howell-Jolly bodies and basophilic stippling of various degrees and sizes were evident (Fig. 2A, arrow 1); fine, intermediate, and large basophilic stippling was significantly more frequent in anemic animals than in cows with normal PCVs (Table 1). These changes as well as the nucleated RBCs observed in some blood smears are indicative of regenerative anemia. Anemic cattle had significantly higher (P < 0.0001 by Kruskal-Wallis analysis) total and direct serum bilirubin levels than animals with normal PCVs, indicative of extravascular hemolysis (data not shown).
FIG. 2.
Direct evidence of infectious agents: representative blood smears of anemic cows with inclusions in RBCs (A to C) and cytospin preparations of mononuclear cell culture (D). (A, arrow 1) Basophilic stippling in an RBC as a sign of regeneration of RBCs in the bone marrow; (A, arrow 2) basophilic, round, peripherally located inclusion bodies characteristic for A. marginale; (B) inclusions of large Babesia spp. as paired pyriforms (B1 to B5) and a single pyriform (B6); (C) example of small round inclusions with tails; (D) inclusions compatible with Theileria spp. schizonts in mononuclear cells after 6 weeks of cell culture first in the presence of and later in the absence of interleukin-2. The samples showing these inclusions in cell culture had been positive by the Theileria spp. PCR but negative by the A. phagocytophilum PCR.
TABLE 1.
Microscopy results for thin blood smears as differences between samples from cows with low, intermediate, or normal PCVa
| Microscopic finding | No. of positive animals/no. of animals tested (% of positive animals)
|
Pb | ||
|---|---|---|---|---|
| Group I | Group II | Group III | ||
| Basophilic stippling in RBCs | 74/95 (78) | 24/48 (50) | 1/142 (1) | <0.0001 |
| Fine stipples | 43/95 (45) | 9/48 (19) | 0/142 (0) | <0.0001 |
| Intermediate stipples | 35/95 (37) | 13/48 (27) | 1/142 (1) | <0.0001 |
| Large stipples | 39/95 (41) | 8/48 (17) | 0/142 (0) | <0.0001 |
| Inclusions in RBCs | ||||
| A. marginale | 94c/95 (99) | 35/48 (72) | 5/142 (4) | <0.0001 |
| Large Babesia spp. | 5/95 (5) | 0/48 (0) | 3/142 (2) | NS |
| Small ovoid | 12/95 (13) | 3/48 (6) | 0/142 (0) | 0.0001 |
| Small round with tail | 31/50 (62) | 14/20 (70) | 1/17 (6) | <0.0001 |
| A. phagocytophilum inclusions in WBCs | 2/95 (2) | 0/48 (0) | 0/142 (0) | NS |
Blood smears for 285 cattle were tested. No blood smear was available for one cow. Abbreviations: WBC, white blood cells; NS, not significant.
P values were determined by chi-square analysis.
Animal 96 tested negative for A. marginale by microscopy, serology, and PCR.
Direct evidence of infectious agents and association with disease.
A. marginale inclusion bodies, observed as small round basophilic inclusions of approximately 0.5 μm in diameter mainly located peripherally in the cytoplasms of RBCs (Fig. 2A, arrow 2), were found in 134 of 285 cows (47%); anemic cows had A. marginale inclusion bodies significantly more often than animals with normal PCVs (Table 1). The highest degree of parasitemia (34% of RBCs were parasitized) was found in a cow with a PCV of 14%. Merozoites of large Babesia spp. (Fig. 2B) were seen in RBCs of eight cows (3% of all cows; the merozoites had no correlation with anemia) (Table 1). In addition, we found several smaller inclusions of less well recognized appearance, such as comma- or tadpole-like shapes or small oval-round shapes that might be indicative of Theileria spp. inclusions or degenerated A. marginale inclusion bodies (Fig. 2C and Table 1). Furthermore, basophilic, intracytoplasmic A. phagocytophilum inclusion bodies were detected in the neutrophils of two cows (Table 1), and cytospin preparations of cultured mononuclear cells revealed inclusions compatible with schizonts of Theileria spp. (Koch's body-like structures; Fig. 2D).
Using PCR, we found a significant association between the presence of each of the four infectious agents, A. marginale, Theileria spp., M. wenyonii, and Babesia (spp., and anemia, but not between the presence of A. phagocytophilum and B. burgdorferi and anemia (Table 2). Concurrent infections were found in 37 of the 41 anemic cattle (90%) tested by PCR: five agents in the same sample were detected 2 times, four agents in the same sample were detected 8 times, three agents in the same sample were detected 13 times, and two agents in the same sample were detected 14 times. Very good agreement was found between positive results for A. marginale and microscopic detection of characteristic inclusions (Kw = 0.96); only 1 of 58 samples had a discrepant result (PCR positive and microscopy negative). The agreement between PCR and microscopy could not be tested either for Babesia spp., because of small numbers, or for other inclusions that could not be assigned with certainty to one of the infectious agents tested for by PCR. Several arthropods collected in the respective stables were also analyzed by PCR (Table 3). The majority (11 of 15) was found to have concurrent infections: four agents per arthropod were detected twice, three agents per arthropod were detected twice, and two agents per arthropod were detected seven times.
TABLE 2.
Differences in PCR results with DNA extracted from peripheral blood of selected cows with low, intermediate, normal PCVsa
| Agent(s) | No. of positive animals/no. of animals tested (% of positive animals)
|
Pb | ||
|---|---|---|---|---|
| Group I | Group II | Group III | ||
| A. marginale | 26c/27 (96) | 14/16 (88) | 1/15 (7) | <0.0001 |
| Theileria spp. | 23/25 (92) | 12/16 (75) | 1/15 (7) | <0.0001 |
| M. wenyonii | 21/27 (78) | 1/16 (6) | 0/15 (0) | <0.0001 |
| Babesia spp. | 12/25 (48) | 0/16 (0) | 0/15 (0) | <0.0001 |
| A. phagocytophilum | 1/27 (4) | 2/16 (13) | 0/15 (0) | NSd |
| B. burgdorferi | 0/27 (0) | 0/16 (0) | 0/15 (0) | NS |
A total of 58 blood samples were tested. Blood samples collected from 12 cows referred to the Animal Hospital in Zurich in 2004 for unrelated reasons, as well as 12 samples from healthy calves, tested negative by PCR for all agents with the exception of M. wenyonii (data not shown). Seven of 12 samples from sick adult cattle, but not from calves, were positive for M. wenyonii. The clinical relevance of this unexpected finding is not yet clear and is the topic of further investigations.
P values were determined by chi-square analysis.
See the data for animal 96 in Table 1.
NS, not significant.
TABLE 3.
PCR results with DNA extracted from potential arthropod vectorsa
| Agent(s) | No. of positive samples/no. of samples tested
|
||
|---|---|---|---|
| Ticks | Lice | Flies | |
| A. marginale | 3/6 | 3/5 | 3/4 |
| Theileria spp. | 6/6 | 0/5 | 2/4 |
| Babesia spp. | 5/6 | 1/5 | 2/4 |
| M. wenyonii | 0/6 | 5/5 | 4/4 |
| A. phagocytophilum | 2/6 | 0/5 | 0/4 |
| B. burgdorferi | 0/5 | NTb | NT |
Note that detection of a pathogen in a vector is not equivalent to transmission via this vector. DNA extracted from 20 ticks (I. ricinus, collected in 2000 for an unrelated Borrelia study) tested PCR negative for A. marginale and A. phagocytophilum, Theileria and Babesia spp., and M. wenyonii (data not shown).
NT, not tested.
To further substantiate the PCR results, representative products from conventional PCR assays were sequenced and confirmed to be A. marginale (633 bp of MSP5; >95% homology with the sequence with GenBank accession number M93392) and Theileria spp. (641 bp of the 16S rRNA gene; >99% homology with the Theileria buffeli, T. orientalis, and T. sergenti group). Sequencing for Babesia spp. was not achieved. When Mycoplasma PCR products were analyzed by electrophoresis, bands of two distinct lengths were found. The sequence of the longer PCR product was identical to that of M. wenyonii (193 bp of 16S rRNA gene; >99% homology with the sequence with GenBank accession number AF016546). Interestingly, the sequence of the shorter, unexpected amplicon (172 bp) was 95% homologous to the sequence of M. haemofelis (GenBank accession numbers U88563 and U95297). The results of detailed genetic analysis of all agents detected will be presented elsewhere.
A. marginale serology and agreement with microscopy and PCR analysis.
We aimed to further confirm the A. marginale infection by serology and to evaluate the use of the cELISA during this acute disease outbreak. Of the 285 serum samples tested, 158 (56%) were serologically positive, and seropositivity was strongly associated with anemia (P < 0.001 by chi-square analysis): 94 of 95 severely anemic cows (99%; group I) were positive, 39 of 48 cows with intermediate PCVs (81%; group II) were positive, and 25 of 142 animals with normal PCVs (18%; group III) were positive. Very good agreement was found between serology and microscopy (Kw = 0.82): 133 samples were positive by both tests, 125 samples were negative by both tests, 25 samples were serologically positive and microscopically negative, and 1 sample was serologically negative and microscopically positive. A similar distribution and very good agreement were also found between serology and PCR (Kw = 0.82). Agreement was even better when PCR and microscopy instead of antibody-detecting and antigen-detecting methods were compared (Kw = 0.96): only one sample with discrepant results (PCR positive and microscopy negative) was found.
Sick cattle without evidence of A. marginale infection.
Interestingly, one highly anemic cow (animal 96; PCV, 12%) was negative for A. marginale by serology, microscopy, and PCR (Tables 1 and 2). This animal tested positive for Babesia (microscopy) and Theileria (PCR). A second A. marginale-negative cow (animal 43) with a PCV of 18% tested positive for A. phagocytophilum and M. wenyonii (PCR).
Screening for other infectious agents.
We also aimed to determine the potential influence on the disease outbreak of other infectious agents not evident upon examination of blood smears. All samples collected upon necropsy and at the very beginning of the outbreak tested negative for IBR, malignant catarrhal fever, anthrax, bovine viral diarrhea virus, and bovine ephemeral fever. Two months later, however, almost 75% of the animals of the affected herd had seroconverted to positivity for IBR; this will be reported elsewhere. No evidence of retroviruses, such as the bovine leukemia or immunodeficiency virus, or of other hemolytic or cytolytic viruses was found in any of the cultivated peripheral blood samples collected from sick animals.
DISCUSSION
Sick animals died rapidly during an unexpected, severe infectious disease outbreak in a large cattle herd in the Swiss canton of Grisons in August 2002. Hematological and biochemical analyses of blood samples from diseased cows revealed signs of severe hemolytic regenerative anemia. In addition, considerable lice infestation and, occasionally, ticks were found. Thus, in the search for the etiology of this disease outbreak, we considered primarily infectious, possibly vector-borne agents that induce hemolytic regenerative anemia.
We found inclusion bodies characteristic of A. marginale in the RBCs of the majority of the diseased cows. A. marginale is a hemoparasite that leads to extravascular hemolysis in cattle (2, 27). The presence of the pathogen was subsequently ascertained by molecular techniques and serology. It was significantly associated with anemia. A. marginale was previously reported only once in Switzerland (7). A retrospective serological study initiated after the present outbreak, in which more than 500 serum samples collected from a representative sample of Swiss cattle in 1998 were analyzed, revealed a very low prevalence of A. marginale not exceeding the range of possibly false-positive results of the cELISA used (U. M. Dreher, unpublished data; details of this study will be presented elsewhere). Thus, prior to this outbreak, A. marginale infection played an insignificant role in Switzerland, and the extent and the severity of the present outbreak came unexpectedly. Concurrent infection with bovine retroviruses, which have occasionally been reported to be immunosuppressive (14, 54), seemed unlikely; no evidence of such a concurrent infection was found in 10 animals tested.
A. marginale infection can be fatal in susceptible adult cattle (3, 27) and might have been partially responsible for the high rate of mortality observed in the affected herd. The rapid spread of A. marginale could have been helped by the special husbandry conditions on the farm. A. marginale can be transmitted biologically via ticks, while infected blood can also be transferred mechanically via fomites and biting insects, such as lice (11, 44). To the best of our knowledge, iatrogenic transmission via fomites could be ruled out. Thus, the tick and lice infestation, the free roaming of the animals on limited space, and automatically rotating brushes that were accessible to and used by the majority of the cows in the free-range stables might have facilitated the rapid spread of the parasites from one animal to another. This assumption is also supported by the fact that no cows kept strictly in tie-in stalls had become sick.
Because A. marginale as the etiology of the anemia was unexpected by Swiss measures, the blood samples were also screened for numerous other infectious agents. We found conclusive evidence for the presence of Theileria and large Babesia spp., M. wenyonii, and A. phagocytophilum. The presence of the first three infectious agents was also significantly associated with anemia. This association can be either causal or accounted for by, e.g., identical modes of transmission. While A. marginale is spread biologically and mechanically (11, 44), Babesia and Theileria are usually transmitted biologically, but mechanical transmission has also been documented (23, 45). In the present study, all of the parasites evaluated might have been transmitted mechanically to some degree: we assume that the infectious pressure was very high and that interanimal transmission occurred very quickly, e.g., via the efficient transfer of lice from cow to cow, possibly exponentiated by the rotating scratching brushes. Nevertheless, we also found evidence for a causal link between the presence of anemia and parasites other than A. marginale: hemoglobinuria, an indicator of intravascular hemolysis, was observed in some of the diseased cows. Hemoglobinuria can be expected, e.g., in bovine babesiosis (12, 13) and has recently been documented in a T. buffeli-infected cow (10). However, it is not typically associated with bovine anaplasmosis, which leads to extravascular hemolysis. In addition, two highly anemic cows showed no evidence of A. marginale infection: one animal was positive for Theileria and Babesia spp.; the other tested positive for A. phagocytophilum and M. wenyonii. While A. phagocytophilum and Theileria spp. might have contributed to the observed disease via induction of immune suppression (28, 50), it is well recognized that infection with Babesia spp. may lead to hemolysis (20). The literature on the anemia-inducing potential of M. wenyonii in field studies is contradictory (43; R. E. Purnell, D. W. Brocklesby, and E. R. Young, Letter, Vet. Rec. 98:411, 1976). However, a recent experimental study documented the onset of severe anemia simultaneously with M. wenyonii parasitemia in an animal superinfected with A. marginale (34).
In the present study, we also found a second, not yet described Mycoplasma strain similar to, but distinct from, M. haemofelis, which causes feline infectious anemia (15, 19). Thus, the situation in cattle might be similar to that in cats, as recent molecular advances have made it possible to distinguish different strains of hemotrophic Mycoplasma spp. that have various pathogenic potentials (16, 22). Further characterization of the bovine Mycoplasma sp. observed is under way.
In conclusion, this report demonstrates the presence of A. marginale, A. phagocytophilum, Babesia, Theileria, and hemotrophic Mycoplasma spp. in a Swiss dairy herd that had been killed as a last measure to circumvent the further spread and perpetuation of the disease. A. marginale infections had been observed only rarely in Swiss cattle (7) and never to the extent described here. Moreover, this study is the first to demonstrate Theileria spp. and hemotrophic Mycoplasma spp. in Swiss cattle and extends the known range of these parasites. We present evidence for a causal link of several of these infectious agents with the disease. We hypothesize that A. marginale was the most important agent as the cause of the anemia, but coinfections with other agents may have aggravated disease development. The origin of the pathogens is not yet clear. In countries with warmer climates, such as Italy south of the Alps, all of the vector-borne pathogens mentioned are common (5, 9, 11), and mixed infections with several of these infectious agents have also been described in other parts of the world (25, 32). It is well documented that climatic changes influence species distributions (35, 53), and it had been speculated that with the global warming trend (31) species such as ticks can follow the warmer climate (6, 30). Whether this could have been the case in the present study and whether similar outbreaks should be expected more frequently in the future are not clear.
Acknowledgments
We thank D. Knowles (Washington State University, Pullman) for helpful discussion; E. Rogg, E. Rhiner, Y. Bosshart, C. Brönnimann, U. Egger, B. Glaus, E. Grässli, M. Huder, S. Keo, B. Lange, T. Meili Prodan, M. Nussbaumer, J. Wälchli, B. Weibel, C. Wolfensberger, and B. Wenger for excellent technical assistance; H. Yadin, Kimron Veterinary Institute (Bet Dagan, Israel) for bovine ephemeral fever testing; and R. Perl, G. Regi, and M. Mehli (Chur, Switzerland) for efficient cooperation.
This work was supported in part by the Swiss Federal Veterinary Office, Bern, and the Schweizerische Vereinigung für Wiederkäuermedizin.
REFERENCES
- 1.Aeschlimann, A., and B. Horning. 1972. History of piroplasmosis research in Switzerland. Schweiz. Arch. Tierheilkd. 114:392-394. [PubMed] [Google Scholar]
- 2.Ajayi, S. A., A. J. Wilson, and R. S. Campbell. 1978. Experimental bovine anaplasmosis: clinico-pathological and nutritional studies. Res. Vet. Sci. 25:76-81. [PubMed] [Google Scholar]
- 3.Allen, P. C., K. L. Kuttler, and T. E. Amerault. 1981. Clinical chemistry of anaplasmosis: blood chemical changes in infected mature cows. Am. J. Vet. Res. 42:322-325. [PubMed] [Google Scholar]
- 4.Altman, D. G. 1991. Practical statistics for medical research. CRC Press, Inc., Boca Raton, Fla.
- 5.Baumgartner, W., G. Schlerka, M. Fumicz, J. Stoger, M. Awad-Masalmeh, W. Schuller, and P. Weber. 1992. Seroprevalence survey for Anaplasma marginale-infection of Austrian cattle. Zentbl. Veterinarmed. B 39:97-104. [DOI] [PubMed] [Google Scholar]
- 6.Bernasconi, M. V., S. Casati, O. Peter, and J. C. Piffaretti. 2002. Rhipicephalus ticks infected with Rickettsia and Coxiella in southern Switzerland (Canton Ticino). Infect. Genet. Evol. 2:111-120. [DOI] [PubMed] [Google Scholar]
- 7.Braun, U., G. Winkler, P. Wild, and R. Eicher. 1987. Anaplasmosis in cattle in Switzerland. Schweiz. Arch. Tierheilkd. 129:113-124. [PubMed] [Google Scholar]
- 8.Brossard, M., and A. Aeschlimann. 1975. Cattle piroplasmoses in the Italian part of Switzerland (notes on latent infections). Schweiz. Arch. Tierheilkd. 117:287-292. [PubMed] [Google Scholar]
- 9.Ceci, L., F. Jongejan, G. Carelli, P. Tassi, and O. Sparagano. 1999. Identification of Theileria buffeli/orientalis and Babesia bigemina in Apulian cattle using molecular techniques and study of changes in blood parameters. Parassitologia 41(Suppl. 1):31-32. [PubMed] [Google Scholar]
- 10.Cossio-Bayugar, R., R. Pillars, J. Schlater, and P. J. Holman. 2002. Theileria buffeli infection of a Michigan cow confirmed by small subunit ribosomal RNA gene analysis. Vet. Parasitol. 105:105-110. [DOI] [PubMed] [Google Scholar]
- 11.Cringoli, G., D. Otranto, G. Testini, V. Buono, G. Di Giulio, D. Traversa, R. Lia, L. Rinaldi, V. Veneziano, and V. Puccini. 2002. Epidemiology of bovine tick-borne diseases in southern Italy. Vet. Res. 33:421-428. [DOI] [PubMed] [Google Scholar]
- 12.De Vico, G., B. Macri, C. Sammartino, and G. R. Loria. 1999. Bovine babesiosis in Sicily: preliminary study on pathology. Parassitologia 41(Suppl. 1):37-38. [PubMed] [Google Scholar]
- 13.Donnelly, J., P. J. Crossman, and M. D. McKendrick. 1970. An outbreak of redwater on a farm in Sussex. Vet. Rec. 87:729. [DOI] [PubMed] [Google Scholar]
- 14.Flaming, K. P., D. E. Frank, S. Carpenter, and J. A. Roth. 1997. Longitudinal studies of immune function in cattle experimentally infected with bovine immunodeficiency-like virus and/or bovine leukemia virus. Vet. Immunol. Immunopathol. 56:27-38. [DOI] [PubMed] [Google Scholar]
- 15.Flint, J. C., M. H. Roepke, and R. Jensen. 1958. Feline infectious anaemia. I. Clinical aspects. Am. J. Vet. Res. 19:164-168. [PubMed] [Google Scholar]
- 16.Foley, J. E., S. Harrus, A. Poland, B. Chomel, and N. C. Pedersen. 1998. Molecular, clinical, and pathologic comparison of two distinct strains of Haemobartonella felis in domestic cats. Am. J. Vet. Res. 59:1581-1588. [PubMed] [Google Scholar]
- 17.Gratz, N. G. 1999. Emerging and resurging vector-borne diseases. Annu. Rev. Entomol. 44:51-75. [DOI] [PubMed] [Google Scholar]
- 18.Gubbels, M. J., H. Yin, Q. Bai, G. Liu, I. J. Nijman, and F. Jongejan. 2002. The phylogenetic position of the Theileria buffeli group in relation to other Theileria species. Parasitol. Res. 88:S28-S32. [DOI] [PubMed] [Google Scholar]
- 19.Hayes, H. M., and W. A. Priester. 1973. Feline infectious anaemia: risk by age, sex and breed; prior disease; seasonal occurrence; mortality. J. Small Anim. Pract. 14:797-804. [DOI] [PubMed] [Google Scholar]
- 20.Homer, M. J., I. Aguilar-Delfin, S. R. Telford III, P. J. Krause, and D. H. Persing. 2000. Babesiosis. Clin. Microbiol. Rev. 13:451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussy, D., N. Stauber, C. M. Leutenegger, S. Rieder, and M. Ackermann. 2001. Quantitative fluorogenic PCR assay for measuring ovine herpesvirus 2 replication in sheep. Clin. Diagn. Lab. Immunol. 8:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen, W. A., M. R. Lappin, S. Kamkar, and W. J. Reagan. 2001. Use of a polymerase chain reaction assay to detect and differentiate two strains of Haemobartonella felis in naturally infected cats. Am. J. Vet. Res. 62:604-608. [DOI] [PubMed] [Google Scholar]
- 23.Kimber, C. D., and A. S. Young. 1977. Serological studies on strains of Theileria mutans isolated in East Africa using the indirect fluorescent antibody technique. Ann. Trop. Med. Parasitol. 71:1-10. [DOI] [PubMed] [Google Scholar]
- 24.Knowles, D., S. Torioni de Echaide, G. Palmer, T. McGuire, D. Stiller, and T. McElwain. 1996. Antibody against an Anaplasma marginale MSP5 epitope common to tick and erythrocyte stages identifies persistently infected cattle. J. Clin. Microbiol. 34:2225-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knowles, R. T., M. Montrose, T. M. Craig, G. G. Wagner, and R. F. Long. 1982. Clinical and serological evidence of bovine babesiosis and anaplasmosis in St. Lucia. Vet. Parasitol. 10:307-311. [DOI] [PubMed] [Google Scholar]
- 26.Kocan, K. M., E. F. Blouin, and A. F. Barbet. 2000. Anaplasmosis control. Past, present, and future. Ann. N. Y. Acad. Sci. 916:501-509. [DOI] [PubMed] [Google Scholar]
- 27.Kuttler, K. L. 1984. Anaplasma infections in wild and domestic ruminants: a review. J. Wildl. Dis. 20:12-20. [DOI] [PubMed] [Google Scholar]
- 28.Larsen, H. J., G. Overnes, H. Waldeland, and G. M. Johansen. 1994. Immunosuppression in sheep experimentally infected with Ehrlichia phagocytophila. Res. Vet. Sci. 56:216-224. [DOI] [PubMed] [Google Scholar]
- 29.Leutenegger, C. M., N. Pusterla, C. N. Mislin, R. Weber, and H. Lutz. 1999. Molecular evidence of coinfection of ticks with Borrelia burgdorferi sensu lato and the human granulocytic ehrlichiosis agent in Switzerland. J. Clin. Microbiol. 37:3390-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindgren, E., L. Talleklint, and T. Polfeldt. 2000. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ. Health Perspect. 108:119-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luterbacher, J., D. Dietrich, E. Xoplaki, M. Grosjean, and H. Wanner. 2004. European seasonal and annual temerature variability, trends, and extremes since 1500. Science 303:1499-1503. [DOI] [PubMed] [Google Scholar]
- 32.Magona, J. W., and J. S. Mayende. 2002. Occurrence of concurrent trypanosomosis, theileriosis, anaplasmosis and helminthosis in Friesian, Zebu and Sahiwal cattle in Uganda. Onderstepoort. J. Vet. Res. 69:133-140. [PubMed] [Google Scholar]
- 33.Neimark, H., K. E. Johansson, Y. Rikihisa, and J. G. Tully. 2002. Revision of haemotrophic Mycoplasma species names. Int. J. Syst. Evol. Microbiol. 52:683. [DOI] [PubMed] [Google Scholar]
- 34.Neimark, H., and K. M. Kocan. 1997. The cell wall-less rickettsia Eperythrozoon wenyonii is a mycoplasma. FEMS Microbiol. Lett. 156:287-291. [DOI] [PubMed] [Google Scholar]
- 35.Parmesan, C., and G. Yohe. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37-42. [DOI] [PubMed] [Google Scholar]
- 36.Persing, D. H., D. Mathiesen, W. F. Marshall, S. R. Telford, A. Spielman, J. W. Thomford, and P. A. Conrad. 1992. Detection of Babesia microti by polymerase chain reaction. J. Clin. Microbiol. 30:2097-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pusterla, N., U. Braun, C. Wolfensberger, and H. Lutz. 1997. Intrauterine infection with Ehrlichia phagocytophila in a cow. Vet. Rec. 141:101-102. [DOI] [PubMed] [Google Scholar]
- 38.Pusterla, N., J. B. Huder, C. M. Leutenegger, U. Braun, J. E. Madigan, and H. Lutz. 1999. Quantitative real-time PCR for detection of members of the Ehrlichia phagocytophila genogroup in host animals and Ixodes ricinus ticks. J. Clin. Microbiol. 37:1329-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pyra, H., J. Boni, and J. Schupbach. 1994. Ultrasensitive retrovirus detection by a reverse transcriptase assay based on product enhancement. Proc. Natl. Acad. Sci. USA 91:1544-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosskopf, M., E. Staub, and M. Ackermann. 1994. Comparison of two ELISA systems for the detection of antibodies against IBR/IPV and against enzootic bovine leukemia virus. Schweiz. Arch. Tierheilkd. 136:58-67. [PubMed] [Google Scholar]
- 41.Siegel, S., and N. J. J. Castellan. 1988. Non-parametric statistics for the behavioral sciences. McGraw-Hill Book Co., New York, N.Y.
- 42.Singh-Behl, D., S. P. La Rosa, and K. J. Tomecki. 2003. Tick-borne infections. Dermatol. Clin. 21:237-244. [DOI] [PubMed] [Google Scholar]
- 43.Smith, J. A., M. A. Thrall, J. L. Smith, M. D. Salman, S. V. Ching, and J. K. Collins. 1990. Eperythrozoon wenyonii infection in dairy cattle. J. Am. Vet. Med. Assoc. 196:1244-1250. [PubMed] [Google Scholar]
- 44.Stiller, D., and M. E. Coan. 1995. Recent developments in elucidating tick vector relationships for anaplasmosis and equine piroplasmosis. Vet. Parasitol. 57:97-108. [DOI] [PubMed] [Google Scholar]
- 45.Thomas, S. E., and T. E. Mason. 1981. Isolation and transmission of an unidentified Babesia sp. infective for cattle. Onderstepoort. J. Vet. Res. 48:155-158. [PubMed] [Google Scholar]
- 46.Thür, B., P. Caplazi, M. Hilbe, K. Zlinszky, M. Strasser, L. Corboz, and F. Ehrensperger. 1998. Pestivirus as causative agent of abortion and perinatal mortality in cattle and sheep in Switzerland. Dtsch. Tierarztl. Wochenschr. 105:145-148. [PubMed] [Google Scholar]
- 47.Tieze, N. W. 1995. Clinical guide to laboratory tests, 3rd ed. The W. B. Saunders Company, Philadelphia, Pa.
- 48.Torioni de Echaide, S., D. P. Knowles, T. C. McGuire, G. H. Palmer, C. E. Suarez, and T. F. McElwain. 1998. Detection of cattle naturally infected with Anaplasma marginale in a region of endemicity by nested PCR and a competitive enzyme-linked immunosorbent assay using recombinant major surface protein 5. J. Clin. Microbiol. 36:777-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trotz-Williams, L. A., and A. J. Trees. 2003. Systematic review of the distribution of the major vector-borne parasitic infections in dogs and cats in Europe. Vet. Rec. 152:97-105. [DOI] [PubMed] [Google Scholar]
- 50.Tuomi, J. 1967. Experimental studies on bovine tick-borne fever. 1. Clinical and haematological data, some properties of the causative agent, and homologous immunity. Acta Pathol. Microbiol. Scand. 70:429-445. [PubMed] [Google Scholar]
- 51.Turnbull, P. C. 1996. Bacteriology. 15. Bacillus. In S. Baron (ed.), Medical microbiology, 4th ed. The University of Texas Medical Branch at Galveston. [PubMed]
- 52.Uren, M. F., T. D. St. George, and G. M. Murphy. 1992. Studies on the pathogenesis of bovine ephemeral fever in experimental cattle. III. Virological and biochemical data. Vet. Microbiol. 30:297-307. [DOI] [PubMed] [Google Scholar]
- 53.Walther, G. R., E. Post, P. Convey, A. Menzel, C. Parmesan, T. J. Beebee, J. M. Fromentin, O. Hoegh-Guldberg, and F. Bairlein. 2002. Ecological responses to recent climate change. Nature 416:389-395. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, S., C. Wood, W. Xue, S. M. Krukenberg, Q. Chen, and H. C. Minocha. 1997. Immune suppression in calves with bovine immunodeficiency virus. Clin. Diagn. Lab. Immunol. 4:232-235. [DOI] [PMC free article] [PubMed] [Google Scholar]