Abstract
The effects of group membership on brain responses to social exclusion have been investigated in adults, revealing greater anterior cingulate responses to exclusion by members of one’s in-group (e.g. same-gender). However, social exclusion is a critical aspect of peer relations in youth and reaches heightened salience during adolescence, a time when social anxiety disorders are also emergent. While the behavioral and neural correlates of social exclusion in adolescence have been extensively explored, the effects of group membership on peer rejection are less clear. The current study used functional magnetic resonance imaging (fMRI) to investigate the differential neural correlates of being excluded by peers of one’s same-versus opposite-gender during an online ball-toss game. Participants were a group of typically developing children and adolescents (7–17 years). As predicted, anterior cingulate cortex showed a main effect of social exclusion versus fair play. However, unlike a previous adult study, this region did not show increased activation to same-gender exclusion. Instead, several regions differentiating same-versus opposite-gender exclusion were exclusively more sensitive to exclusion by one’s opposite gender. These results are discussed in the context of adolescent socioemotional development.
Keywords: Social Exclusion, Group Membership, Anterior Cingulate Cortex, Gender, Development
INTRODUCTION
The ability of humans to discriminate males from females is an early emerging phenomenon, with evidence of this skill demonstrated in infants 6 to 8 months of age (Miller, 1983). By 31 months, children demonstrate the ability to categorize themselves as the correct gender (Weinraub et al., 1984), showing both verbal and non-verbal signs of gender identity. Beginning early in life, gender camaraderie is a powerful means of facilitating feelings of group membership. Same-gender groups are the preferred friendship and play networks beginning in preschool-aged children (Maccoby, 1988; Powlishta, Serbin, Doyle, & White, 1994). However, beginning in adolescence, opposite-gender friendships become increasingly frequent (Feiring, 1999). This finding is consistent with the report that out-group prejudice decreases with age from 5 to 13 years (Powlishta et al., 1994). Children and early adolescents were able to cite the immorality of social exclusion based on gender. However, adolescents were more willing to condone out-group exclusion if overall group-functioning was threatened by inclusion (Killen & Stangor, 2001). Thus, while a preference for same-gender peer groups prevails throughout life (Mehta & Strough, 2009), evidence suggests that the treatment of opposite-gender peers varies through development.
Irrespective of gender, peer rejection is especially salient in adolescence (Pharo, Gross, Richardson, & Hayne, 2011; Rudolph & Hammen, 1999; Steinberg & Morris, 2001). The social, emotional, and psychological effects of peer rejection have been researched extensively (for reviews see Blackhart, Nelson, Knowles & Baumeister, 2009; Gerber & Wheeler, 2009; Williams, 2007). Complementary neuroimaging research has identified a network of brain regions associated with the experience of social rejection, including anterior cingulate cortex (ACC), ventrolateral prefrontal cortex (vlPFC), and insula (Bolling et al., 2011a, 2011b; Eisenberger, Lieberman & Williams, 2003; Karremans, Heslenfeld, van Dillen & Van Lange, 2011; Krill & Platek, 2009; Masten et al., 2009, 2011a; Moor, van Leijenhorst, Rombouts, Crone & van der Molen, 2010; Onoda et al., 2009; Sebastian et al., 2011). These studies have demonstrated neural activation to rejection in adults, adolescents, and children as young as seven years of age. Activation in ACC has been shown to correlate positively with self-reported distress, suggesting a role for this region in the emotional response to peer exclusion (Eisenberger et al., 2003; Masten et al., 2009). In contrast, right vlPFC has shown a negative relationship with distress, implying its role in emotion regulation (Eisenberger et al., 2003; Masten et al., 2009).
To date, all neuroimaging work exploring the effects of group membership on neural responses to social exclusion has focused on adults, demonstrating relatively decreased ACC activation to exclusion by one’s out-group (Bolling et al., 2012; Krill & Platek, 2009). However, the psychological determinants of this neural difference are not fully understood. In adults, an investigation of the neural correlates of rejection by one’s racial out-group demonstrated that the extent to which an individual attributed the exclusion to discrimination was inversely related to ACC activation to social exclusion (Masten et al., 2011b). This finding led the authors to speculate that decreased neural responses to exclusion by one’s out-group may be a function of the ability to regulate emotions by attributing the exclusion to discrimination. Behavioral research in support of this theory has demonstrated that attributing negative behavior to gender discrimination decreased subsequent negative psychological outcomes (Crandall, Tsang, Harvey & Britt, 2000; Crocker, Voelkl, Testa & Major, 1991; Major, Kaiser & McCoy, 2003). With respect to gender group membership, being excluded by a group of opposite-gender peers dampened the negative psychological effects of rejection, compared to being excluded by a group of mixed-gendered peers (Wittenbaum, Shulman & Braz, 2010). Neural correlates of the buffering effects of attributing negative behavior to discrimination have been located in the right vlPFC, which shows an inverse relationship with distress to exclusion by members of an out-group (Bolling et al., 2012; Masten et al., 2011b).
The hypothesis that activation in vlPFC relates to affective responses to out-group exclusion is in line with work implicating this region in a more general role of emotion regulation (for review see Ochsner & Gross, 2005). However, recent work has suggested that this emotion regulation system does not reach maturity until adulthood (McRae et al., 2012). With this in mind, if a decreased ACC response to social exclusion by members of an out-group is dependent on emotion regulation processes of vlPFC, we would expect that unlike adults, children and adolescents would not show differential ACC responses to exclusion by members of the opposite gender. Conversely, a demonstration of differential ACC activation to exclusion based on group membership in youth allows for the possibility that some regulatory mechanisms are functionally mature in this age group.
The current study investigated the effects of gender group membership on brain responses to social exclusion in a group of male and female children and adolescents, as the effect of group membership on brain responses to social exclusion in youth remains uncharacterized. Social exclusion was elicited using an interactive ball-toss game called Cyberball (Williams, Cheung & Choi, 2000). Participants played two separate games of Cyberball, each with two ostensibly real peers, and were alternatingly included in or excluded from each game. Group membership was manipulated by changing the gender of the online players such that in one game peers appeared the same gender as the participant, while in the other game peers were members of the opposite gender.
We sought to identify brain regions that differentially responded to social exclusion based on the gender relationship of the excluders to the excluded participant, as past work characterizing brain responses to peer rejection in children has focused on either same-gender (Bolling et al., 2012) or mixed-gender excluders (Masten et al., 2011a; Moor et al., 2012). Based on previous work identifying differential brain responses to in-group and out-group exclusion in prefrontal regions that do not reach structural maturity until adulthood (Barnea-Goraly et al., 2005; Sowell, Trauner, Gamst, & Jernigan, 2002), we hypothesized that children and adolescents would fail to show adult characteristic decreased brain responses in ACC to out-group exclusion due to an inability to recruit aforementioned complex emotion regulation strategies.
Our knowledge of how contextual cues such as group-membership influence responses to social stressors is also relevant to our understanding of anxiety disorders. Anxiety disorders are characterized by abnormal responses to nonverbal social cues, namely increased attention to threatening stimuli (Gilboa-Schechtman & Shachar-Lavie, 2013). Research has found that even in healthy individuals, an increased attentional bias to threat magnifies subsequent anxiety in response to social exclusion (Heeren, Peschard, & Philippot, 2012). In addition, the emotional effects of social exclusion are more pronounced and more prolonged in individuals with higher levels of social anxiety (Zadro, Boland, & Richardson, 2006), and lead to prolonged impairment in emotion regulation compared to individuals with lower social anxiety (Oaten, Williams, Jones, & Zadro, 2008). Understanding how contextual factors such as group-membership influence responses to social exclusion in healthy populations might provide novel insight into situational factors which could magnify one’s sensitivity to social exclusion. These situations would presumably confer additional risk to individuals with high levels of social anxiety.
To date, our knowledge of how gender-group membership influences brain responses to social exclusion is limited to adult populations, while the average age of onset for social anxiety disorder is mid-adolescence (Schneider, Johnson, Hornig, Liebowitz, & Weissman, 1992). Thus, the current study conducted a novel, preliminary exploration of these neural effects in children and adolescents.
METHODS
Participants
Individuals were disqualified from participation if parents reported that the child had experienced brain injury, brain disease, or brain malformation. In addition, if the child ever experienced seizures, epilepsy, hearing or vision loss, motor impairment, or severe allergies he or she was excluded from participation. Children were also excluded from the current study if they had a diagnosis of an intellectual disability or a learning disability. Finally, children were not recruited for the current study if the parent had any concerns about possible signs of autism or developmental problems, or if a child had a sibling with an autism diagnosis.
In accordance with the listed criteria, 25 typically-developing children and adolescents were recruited and participated in the current study specifically designed to assess the effects of gender group membership on brain responses to social exclusion (12 male, 12.4 ± 2.5 years). All participants played two rounds of Cyberball (same-gender and other-gender) in the same scanning session. The order of the two games was counterbalanced such that 14 participants played with their same gender first. For each participant, motion plots depicting movement in the three translations and three rotations were derived relative to head position at the first volume of acquisition. Participants who showed multiple or prolonged periods of excessive head movement within a scan (> 4mm in any direction or 4 degrees of rotation from initial head position) were excluded from further analyses (3 participants). An additional three participants who each showed an isolated period of excessive motion within a scan (> 4mm or degrees from initial head position) compromising less than half of the collected volumes remained in subsequent analyses, with the distinct period of maximum motion being excised from single-participant analyses. Of these 3 participants with isolated periods of excessive motion, one participant had 3 volumes removed from the other-gender Cyberball scan (volumes 71–73), one participant had the final 28 volumes removed from each of the two Cyberball scans (same- and other-gender), and the last participant had the final 4 blocks (2 fair play, 2 exclusion) and 10 second final fixation removed from the other-gender Cyberball scan. In addition, two participants were excluded from all analyses for failing to throw the ball on more than half of the trials in one of the two Cyberball games. After these exclusions, 20 participants (10 male, 12.61 ± 2.5 years) were included in subsequent group analyses. All 20 participants had IQ scores measured with the Differential Ability Scales (DAS-II; Elliott, 2007). The average standard scores for verbal reasoning, nonverbal reasoning, and general conceptual ability were 104.75 (±15), 104.65 (±18) and 105.5 (±15), respectively. In addition, 17 of the 20 participants had their social functioning assessed with the parent-report Social Responsiveness Scale (SRS; Constantino & Todd, 2003). The average normalized score was 50.3 (± 13). Higher scores on the SRS indicate more difficulty with social responsiveness. Normalized scores of 59 or less are considered within the normal range of social functioning (14 participants were in this range). Normalized scores between 60 and 75 indicate deficiencies in reciprocal social behavior in the mild to moderate range (2 participants were in this range). Normalized scores of 76 or higher indicate severe impairments in reciprocal social behavior. (1 participant was in this range). In the 20 participants, the average maximum amount of head motion during same-gender Cyberball was 1.51 mm or degrees (± 1.08, range 0.16–3.8), and during other-gender Cyberball was 1.65 mm or degrees (± 1.06, range 0.28–3.8). Age did not correlate with maximum head motion during same-gender Cyberball (r(18) = −0.11, p = 0.65) or other-gender Cyberball (r(18) = −.18, p = 0.45). From these 20 participants, 11 played with their same gender first. Sixteen of the 20 participants completed a social exclusion questionnaire (SE-Q) assessing exclusion-related distress.
Cyberball
Participants played two rounds of Cyberball (Williams et al., 2000), each lasting five minutes. A game of Cyberball began with a sham Google® screen, during which participants were told by the experimenter that they were being connected to the internet where they would play a ball-toss game with other children online. This was followed by a screen where participants chose the catching glove that would represent them in the game. Finally, participants were given visual and aural instructions and were asked to practice 16 throws to ensure that they understood the game. On completion of the practice, fMRI data acquisition began and participants played Cyberball continuously for 5 minutes in 30 second alternating blocks of social exclusion (participant receiving 0 out of 12 throws) and fair play (participant receiving 4 out of 8 throws from the other players). This whole sequence of events occurred twice during the participant’s scan session. In one round of Cyberball, participants played with two members of their own gender, while in the other round participants played with members of the opposite gender. Efforts were made to ensure that online players in both Cyberball games were matched by race to the participant. However, 4 participants did not play with race-matched players (though for these 4 participants, online players’ race was still held constant across the two rounds of Cyberball). To ensure that results were not unduly influenced by non-race matched sessions, significant findings from group-level contrasts of (1) condition and (2) gender group-membership were confirmed to be robust to removal of these 4 individuals (see Results). Participants were informed of the gender of the other players by pictures of the players’ faces next to each player’s corresponding catching glove. The order of these rounds (same- versus other-gender) was counter-balanced between all participants (11 played same-gender first), as well as within participant gender groups (male: 6 played same-gender first, female: 5 played same-gender first). Images of online players in the current study were comparable in age to the age range of the participants (7–17 years).
Upon completion of both rounds of Cyberball, participants were prompted to answer 12 questions on a 1 to 5 Likert scale, relating to their experience of social exclusion by both the same and other gender. Eight of these questions were identical to the social exclusion questionnaire previously used in adults to assess general distress to social exclusion; an additional four items were modified to relate specifically to exclusion by each gender (i.e. “I felt like the female players were interacting with me a lot”). Questions were originally adapted from the Need Threat Questionnaire previously designed to assess exclusion-related distress (van Beest & Williams, 2006). Items included statements like “I felt like an outsider.” Questions were delivered visually and aurally immediately after the completion of the second round of Cyberball, while the participant remained in the magnet (in the absence of fMRI data acquisition).
Imaging protocol
Images were collected on a Siemens 3T Tim Trio scanner located in the Yale University Magnetic Resonance Research Center. Whole brain T1-weighted anatomical images were acquired using an MPRAGE sequence (TR=1900 ms; TE=2.96 ms; flip angle=9°; FOV=256 mm; image matrix 2562; voxel size =1×1×1 mm; 160 slices; NEX=1). Whole-brain functional images were acquired using a single-shot, gradient-recalled echo planar pulse sequence (TR=2000 ms; TE=25 ms; flip angle=60°; FOV=220 mm; image matrix=642; voxel size=3.4×3.4×4 mm; 34 slices) sensitive to BOLD contrast. Each Cyberball game (same-gender and other-gender) constituted a separate scan, consisting of 160 functional volume acquisitions.
Data Analysis
fMRI
Single-Participant Level
Each participant’s data was preprocessed and analyzed using the BrainVoyager QX 2.0.8 software package (Brain Innovation, Maastricht, the Netherlands). Preprocessing of functional data from each Cyberball game included slice time correction (using sinc interpolation), 3-dimensional rigid-body motion correction (using trilinear-sinc interpolation), spatial smoothing with a 4mm Gaussian kernel, and temporal high-pass filtering (fast Fourier basis set, 2 cycles per time course). From each Cyberball game, the first 5 volumes of acquisition (fixation) were discarded to allow for scanner equilibrium. Processed functional data were coregistered to within-session anatomical images, which were subsequently normalized to Talairach space.
Preceding group-level analyses, confounding activation associated with ball throws (which occurred during fair play and not social exclusion) was modeled in each game and regressed out on a single-participant level prior to task-based general linear model (GLM) analyses. Regressors for the ball throw analysis were defined as boxcar functions peaking during the period beginning when the participant received the ball and ending with the participant’s throw response, convolved with a double-gamma hemodynamic response function (HRF). Following this regression, single-participant task-based activation to each experimental condition (social exclusion and fair play) was modeled with a boxcar function (defined as 1 during the condition and 0 otherwise) convolved with a double-gamma HRF. To further account for head motion, time series of the previously described motion plots were also included in each single subject model as predictors of no interest.
Group Level
All group level whole-brain analyses were restricted to voxels within the extent of the MNI template brain normalized to Talairach space. Group level, random-effects GLM analyses were conducted with functional data from every participant in both Cyberball games together, and from each gender condition (same and other) separately. For the group analysis collapsed across gender condition (40 functional data sets), results were thresholded at p < .01. This whole-brain analysis was corrected for multiple comparisons at the cluster level, with a cluster threshold of 20 functional voxels, determined to correspond to a corrected threshold of α < .05. Thresholds were calculated using the cluster threshold estimator plug-in for BrainVoyager, which performs 1000 Monte Carlo simulations to estimate the desired false positive rate (Xiong, Gao, Lancaster & Fox, 1995). For group analyses assessing activation within gender conditions (same- or other-gender; 20 functional data sets each), results were thresholded at p < .05, corrected with a cluster threshold of 35 functional voxels, calculated to correspond to α < .05.
Two region of interest (ROI) analyses were carried out to address region-specific a priori hypotheses. The first ROI analysis was conducted with a structurally-defined area of the ACC derived from the Talairach atlas (Lancaster et al., 1997, 2000). We further divided this ACC region into a ventral and a dorsal portion by segmenting it along the plane z = 9, roughly corresponding to the genu of the corpus callosum. Based on previous work in adults showing differential activation in ACC to same-versus other-gender exclusion (Bolling et al., 2012) and differential activation in ACC to same-versus other-race exclusion (Krill & Platek, 2009), we used our structurally-defined regions of ACC to investigate whether activation differed based on the gender of the excluders in our present sample of children and adolescents.
To investigate whether activation to social exclusion in dorsal or ventral ACC correlated with age, Pearson correlations between age and activation to Exclusion – Fair Play were conducted for (1) same-gender Cyberball, (2) other-gender Cyberball and (3) the within-participant difference between same- and other-gender Cyberball. Results of these correlations were not corrected for multiple comparisons due to the exploratory nature of the age analyses.
The second ROI analysis was conducted with a structurally delineated area of right ventrolateral prefrontal cortex, defined by combining Talairach-defined regions of right inferior and middle frontal gyri (Lancaster et al., 1997, 2000), then subsequently restricting the combined region to the extent of the MNI brain normalized to Talairach space. Activation to other-gender exclusion versus fair play in this region was previously shown to correlate negatively with self-reported distress in adults (Bolling et al., 2012). This ROI analysis sought to replicate this finding in a younger participant group.
RESULTS
Distress questionnaire
Sixteen of the 20 participants completed the SE-Q measuring exclusion-related distress following the completion of both Cyberball games in the scanner. Each of the 12 items was rated on a 1–5 Likert scale (1 = “not at all”, 5 = “extremely”), making the possible scores range from 12 to 60. The average overall score on the questionnaire was 34.19 (± 7.73; average item score of 2.85). Statements receiving the highest average item scores were the following: “I felt powerful” (reverse-scored; 3.9), “I felt like the other players decided everything” (3.4), “I felt like the male players were interacting with me a lot” (reverse-scored; 3.4) and “I felt like the female players were interacting with me a lot” (reverse-scored; 3.1). Sub-scores relating specifically to distress caused by same-gender or other-gender exclusion derived from the 2 items relating to each category were calculated for each person (possible sub-score range: 2–10). The average score on the same-gender exclusion sub-scale was 5.81 (± 1.72). The average score on the other-gender exclusion sub-scale was 5.94 (± 1.91). A within-subject t-test did not reveal a significant difference between self-reported distress to same- versus other-gender exclusion (p > .05).
fMRI
Social Exclusion versus Fair Play
In the contrast of social exclusion versus fair play collapsed across gender conditions, a group of brain regions which have been considered part of the neural network for processing rejection were significantly active (Table 1, Figure 1), including ventral anterior cingulate cortex (vACC), right insula, bilateral hippocampus, and posterior cingulate cortex (PCC). In addition, regions of right precentral gyrus, bilateral occipital cortex, left temporal pole, left superior frontal gyrus and left ventrolateral prefrontal cortex (vlPFC) showed significantly greater activation during exclusion compared to fair play. Right middle frontal gyrus, right precuneus, left cerebellum and right temporoparietal junction all showed significant activation during fair play compared to exclusion. All of these regions still remained significant (p < 0.01) with removal of the 4 participants who did not play with race-matched players.
Table 1.
Activation to social exclusion > fair play collapsed across gender conditions. Regions identified in a whole brain contrast of social exclusion (same- and other-gender) versus fair play (same- and other-gender). Talairach coordinates and statistics refer to the voxel of maximum signal difference in each region. Region size is reported in structural voxels (1 mm3). Abbreviations: anterior cingulate cortex (ACC), ventrolateral prefrontal cortex (vlPFC), medial prefrontal cortex (mPFC).
| Brain Region | X | Y | Z | size | t | p |
|---|---|---|---|---|---|---|
| Exclusion > Fair Play | ||||||
| Right precentral gyrus | 30 | −25 | 71 | 2506 | 4.77 | 0.000133 |
| Right posterior insula | 42 | −22 | 19 | 818 | 4.93 | 0.000092 |
| Right middle insula | 42 | −10 | 19 | 834 | 5.35 | 0.000036 |
| Right hippocampus | 33 | −37 | −5 | 8890 | 8.41 | < 0.0001 |
| Left hippocampus / retrosplenial cortex | −27 | −40 | −8 | 15379 | 7.19 | 0.000001 |
| Right occipital cortex | 15 | −82 | 22 | 614 | 4.65 | 0.000174 |
| Left occipital cortex | −36 | −82 | 22 | 5325 | 4.96 | 0.000087 |
| Ventral ACC / mPFC | −9 | 41 | −5 | 6985 | 5.15 | 0.000057 |
| Posterior cingulate cortex | −3 | −40 | 31 | 1131 | 4.58 | 0.000205 |
| Left superior frontal gyrus | −12 | 32 | 46 | 1031 | 5.01 | 0.000077 |
| Left vlPFC | −30 | 32 | −5 | 2713 | 6.72 | 0.000002 |
| Left temporal pole | −42 | 8 | −23 | 2971 | 5.4 | 0.000033 |
| Fair Play > Exclusion | ||||||
| Right middle frontal gyrus | 39 | 29 | 43 | 733 | −6.32 | 0.000005 |
| Right precuneus | 9 | −61 | 46 | 3307 | −5.68 | 0.000018 |
| Left cerebellum | −36 | −34 | −32 | 784 | −4.33 | 0.000361 |
| Right temporoparietal junction | 45 | −46 | 37 | 3027 | −4.55 | 0.000217 |
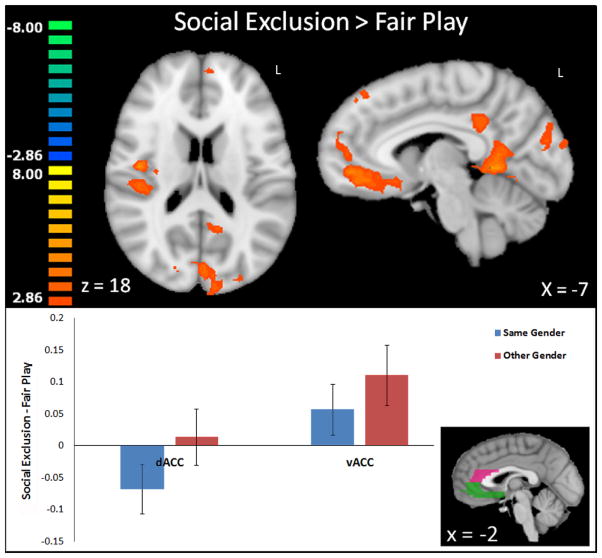
Figure 1.
Top Panel: Activation to social exclusion versus fair play collapsed across gender conditions. Regions in orange showed greater activation to social exclusion, while regions in blue showed greater activation to fair play (none visible). Results were assessed at a threshold of p < .01, k = 20. Activations are interpolated to 1mm3 resolution and depicted in radiologic convention. Bottom Panel: Bar graph depicts average activation to same-gender and other-gender social exclusion versus fair play within structural regions of dorsal and ventral anterior cingulate cortex (dACC and vACC, respectively). Error bars depict standard error of the mean.
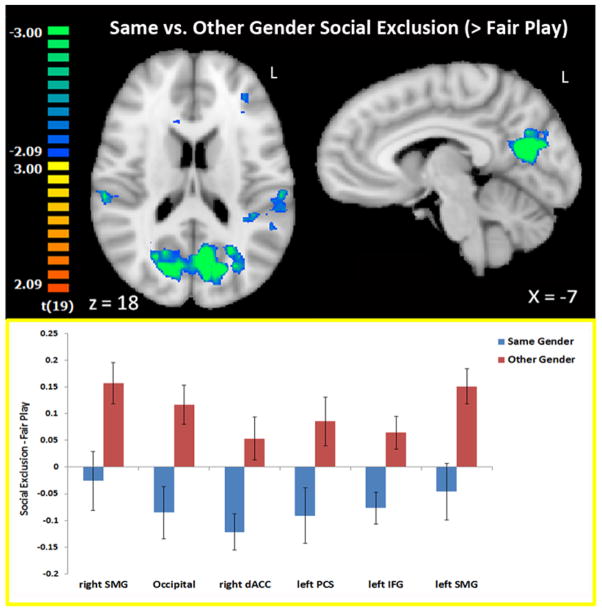
Using a within-participant design, we compared activation to social exclusion (versus fair play) by one’s same-gender versus one’s opposite-gender (Table 2, Figure 2). No regions showed significantly greater activation to exclusion by one’s own gender. Regions showing significantly greater activation to exclusion by one’s opposite gender included bilateral supramarginal gyrus, bilateral occipital cortex, right dorsal anterior cingulate cortex (dACC), left postcentral sulcus, and left inferior frontal gyrus. All of these regions remained significant (p < 0.05) with removal of the 4 participants who did not play with race-matched players. Additionally, all of these regions remained significant when controlling for game order (same-gender first or other-gender first) as a between-participants variable. To determine which gender condition was driving these differences, we extracted beta values from each region and compared activation to social exclusion versus fair play in each Cyberball game separately. Bilateral supramarginal gyrus and occipital cortex showed significant activation to social exclusion by the opposite (but not same) gender players. Dorsal ACC showed significant activation to fair play (versus exclusion) with same-gender (but not other-gender) players. Left inferior frontal gyrus showed significant activation to fair play by the same gender and significant activation to exclusion by the opposite gender. Left postcentral gyrus did not show significantly different activation to exclusion versus fair play in either gender condition independently.
Table 2.
Differential activation to social exclusion by one’s same-versus other-gender. Regions identified in a whole-brain within-participant contrast of same-gendersocial exclusion - fair play versus other-gendersocial exclusion - fair play. Talairach coordinates and statistics refer to the voxel of maximum signal difference in each region. Region size is reported in structural voxels (1 mm3). Significance of the contrast of exclusion > fair play is also reported for each gender condition separately (rightmost columns). Abbreviations: anterior cingulate cortex (ACC).
| Brain Region | X | Y | Z | size | t | p | Same | Other |
|---|---|---|---|---|---|---|---|---|
| Exclusion > Fair Play | ||||||||
| same-gender vs. other-gender | ||||||||
| right supramarginal gyrus | 57 | −25 | 22 | 2609 | −4.22 | 0.000459 | - | p < 0.001 |
| left supramarginal gyrus | −51 | −28 | 13 | 2977 | −4.07 | 0.00065 | - | p < 0.001 |
| bilateral occipital cortex | −6 | −64 | 16 | 16467 | −6.32 | 0.000005 | - | p < 0.01 |
| right dorsal ACC | 15 | 41 | 7 | 1030 | −3.42 | 0.002896 | p < 0.01 | - |
| left postcentral sulcus | −24 | −37 | 52 | 2390 | −4 | 0.000769 | - | - |
| left inferior frontal gyrus | −21 | 26 | 7 | 1042 | −3.77 | 0.00129 | p < 0.05 | p < 0.05 |
Figure 2.
Differential activation to social exclusion by one’s same-versus other-gender. Regions in blue showed greater activation to other-gender (versus same-gender) social exclusion – fair play. Results were assessed at a threshold of p < .05, k = 35. Activations are interpolated to 1mm3 resolution and depicted in radiologic convention. Bar graphs depict average activation to same-gender and other-gender social exclusion - fair play within each significant region. Error bars depict standard error of the mean (Abbreviations: supramarginal gyrus (SMG), dorsal anterior cingulate cortex (dACC), postcentral sulcus (PSC), inferior frontal gyrus (IFG).
While the current study demonstrated a main effect of social exclusion (versus fair play) in the ventral ACC (Figure 1) when combining same- and other-gender games, a whole-brain voxel-wise within-participants comparison of same-versus other-gender exclusion failed to find greater activation to in-group exclusion in the ACC region, a difference that was previously demonstrated in adults (Bolling et al., 2012). To further investigate this result for potential sub-threshold trend-level differences, activation in each Cyberball game was assessed in the aforementioned structurally-defined regions of ACC (ventral and dorsal).
In a within-participant comparison, ventral ACC activation to exclusion > fair play did not differ between same- and other-gender Cyberball (t(19) = 0.97, p = 0.35; Figure 1). This result did not change with removal of the 4 non-race mated participants. Activation in vACC was significantly greater to exclusion (versus fair play) during the other-gender but not same-gender game (same-gender: t(19) = 1.4, p = 0.18; other-gender: t(19) = 2.3, p = .03, Figure 1).
Likewise, dorsal ACC activation to exclusion > fair play also did not differ between same- and other-gender Cyberball (t(19) = 1.58, p = 0.13; Figure 1). This result did not change with removal of the 4 non-race mated participants. Activation in dACC was not significantly greater to exclusion (versus fair play) during same- or other-gender games (same-gender: t(19) = −1.76, p = 0.1; other-gender: t(19) = 0.3, p = .77, Figure 1).
Based on evidence from an identical study on gender-related differences in neural responses to exclusion performed in adults (Bolling et al., 2012), we posited that in the current study activation in right vlPFC would be related to self-reported distress specifically during exclusion by the opposite gender. Testing this hypothesis in the current study using a structurally-defined region of right vlPFC did not reveal a significant correlation between activation to other-gender exclusion > fair play and self-reported distress to other-gender exclusion (p > .05). The correlation between activation to other gender exclusion > fair play and distress to other-gender exclusion in the current sample of children and adolescents was significantly different than the comparable correlation previously observed in typical adults performing the same task (z = 2.01, p = 0.04; Bolling et al., 2012). However, recalculating this correlation in the current study after removing moderate outliers (data > 2 SD from the mean on either vlPFC activation or other-gender exclusion distress; n = 2) revealed a trend negative correlation similar to our previous finding in adults (r(12) = −0.5, p = 0.1).
Correlations with age
Chronological age did not correlate with vACC activation to same-gender exclusion (r(18) = −0.02, p = 0.9), other-gender exclusion (r(18) = −0.25, p = 0.3) or the difference between same-versus other-gender exclusion (r(18) = 0.2, p = 0.4). Chronological age also did not correlate with dACC activation to same-gender exclusion (r(18) = 0.33, p = 0.16), other-gender exclusion (r(18) = −0.06, p = 0.8) or the difference between same-versus other-gender exclusion (r(18) = 0.3, p = 0.2). We did not strongly interpret these results because of the notable age difference between males and females in the current study (males: 13.6 years, females: 11.6 years; t(18) = 1.8, p = 0.08) that confounds age analyses.
DISCUSSION
The current study is the first to investigate the effects of group membership on brain responses to social exclusion in children and adolescents. Past research in adults has found that neural responses to exclusion by one’s in-group (race or gender) are greater than responses to exclusion by an out-group in ACC (Bolling et al., 2012; Krill & Platek, 2009). In addition, it is thought that exclusion from an out-group triggers a complex set of emotion regulation strategies that modulate the associated neural activation (Masten et al., 2011b). In the current study, children and adolescents failed to show the characteristic adult response to out-group exclusion, namely a decreased brain response in ACC. Instead, we found equal responses to social exclusion by both genders in vACC, with equivocal evidence for greater dACC activation to other-gender exclusion.
These data support the hypothesis that children and adolescents differ from adults in that they do not show decreased brain responses in ACC to out-group exclusion. This finding rests on a failure to detect differences between two experimental conditions, but we do not suspect that the results are due to a general failure to induce experimental effects. Responses to exclusion collapsed across group membership reliably identified regions typically responsive to social rejection (Bolling et al., 2011a, 2011b; Eisenberger, Lieberman & Williams, 2003; Karremans, Heslenfeld, van Dillen & Van Lange, 2011; Krill & Platek, 2009; Masten, et al., 2009, 2011a; Moor, van Leijenhorst, Rombouts, Crone & van der Molen, 2010; Onoda et al., 2009; Sebastian et al., 2011). Regions previously implicated in this network such as ventral ACC, right insula, bilateral hippocampus, posterior cingulate cortex, and left vlPFC all showed significant activation to social exclusion compared to fair play. Thus, the main effect of social exclusion in the brain is largely robust to variations in the gender(s) of the excluders. In relation to a study of brain responses to solely same-gender exclusion on participants within the same age range using a comparable paradigm (Bolling et al., 2011b), the current study demonstrated that combining responses to same- and other-gender exclusion yields activation in a very similar network of brain regions as identified by eliciting same-gender exclusion alone. In addition, the average item score on the self-reported distress measure (2.85 out of 5) was similar to those reported in previous studies of social exclusion in children and adolescents (Bolling et al., 2011b; Masten et al., 2009). Thus, the current study was successful in eliciting our predicted effects of social exclusion independent of the gender of the excluders.
A whole brain voxel-wise within-participant comparison of activation to social exclusion (versus fair play) by same-versus other-gender players revealed several regions showing differential responses based on the gender of the excluders. These regions included bilateral supramarginal gyrus, occipital cortex, left inferior frontal gyrus, posterior central sulcus, and right dorsal ACC, suggesting that participants were sensitive to the gender manipulation. However, all regions showing an effect of group membership were more sensitive to other-gender exclusion. An analysis of the structurally-defined ACC failed to reveal any significant modulation by the gender of the excluders. Similarly, sub-scores on the distress questionnaire calculated from items relating specifically to either exclusion by one’s own gender or the opposite gender did not significantly differ.
After failing to replicate the effects of group membership on ACC responses to social exclusion, we tested the relationship previously demonstrated in adults between vlPFC activation and self-reported distress exclusively to other-gender exclusion. Ventrolateral PFC is an emotion regulation region with hypothesized influence on psychological responses to out-group rejection (Masten et al., 2011b). In the current study, we did not find a significant correlation between right vlPFC activation to other-gender exclusion and self-reported distress in the 16 youth who completed this distress measure. In fact, the correlation between activation in right vlPFC and self-reported distress in children and adolescents significantly differed from the negative correlation found in adults. However, a careful investigation of this correlation removing moderate outliers (data > 2 SD from the mean on either vlPFC activation or other-gender exclusion distress; n = 2) revealed a trend negative correlation similar to our previous finding in adults (r(12) = −0.5, p = 0.1). Because this correlation includes only 70% of the participants in the current study, we interpret this result with caution. There may be evidence that a subset of the youth in the current study demonstrate evidence of some prefrontal regulation of distress. However, more sensitive measures of exclusion-related distress will be necessary to validate this hypothesis in a larger sample that is sufficiently powered to investigate such a correlation.
While we interpret the results of the vlPFC correlation analysis with caution due to the limited number of data points (n = 14), past research has demonstrated that neural functioning in vlPFC during rejection shows significant development from childhood to adulthood. Previous work has found that children and adolescents show decreased activation to social exclusion in right vlPFC compared to adults (Sebastian et al., 2011), that activation in this region increases with age from childhood to adolescence (Bolling et al., 2011b), and that the functional coupling between this region and ventral ACC during exclusion also increases with age (Bolling et al., 2011b). Thus, past research would suggest that during social exclusion, the ability to effectively use regulation techniques dependent on vlPFC would be decreased in youth. A direct comparison of vlPFC activation to out-group exclusion in children versus adults or an investigation of vlPFC activation to out-group exclusion across development would be well-suited to validate this interpretation. Unfortunately, the age disparity between males and females in the current study precludes the possibility of conducting the latter analysis within the current data set.
Interestingly, vlPFC (along with ACC) is part of a network of brain regions implicated in the pathology of childhood anxiety disorders (Blackford & Pine, 2012). Indeed, social anxiety has been shown to intensify the magnitude and duration of psychological responses to social exclusion (Oaten, Williams, Jones, & Zadro, 2008; Zadro, Boland, & Richardson, 2006). It is possible that brain responses to social exclusion in the current study are moderated by symptoms of social anxiety. While the current study was limited by the lack of anxiety metrics administered to participants, future studies involving more careful characterization of anxiety symptoms may elucidate the precise influences of such traits on brain responses to in-group and out-group exclusion.
While the results of the current study answer the previously unknown question of what brain regions are differentially responsive to social exclusion by the same versus opposite gender in youth, the current investigation has some limitations. First, while we found several brain regions sensitive to the gender relation of the excluders to the participant, self-report sub-scores indexing distress to same- or other-gender exclusion did not significantly differ. We acknowledge that this may be due to the potentially low sensitivity of a 5-point rating scale to discern subtle differences in distress levels. We also failed to find any brain regions showing a positive correlation with distress scores during social exclusion > fair play collapsed across gender conditions (p < .05, k = 35), supporting the idea that while the questionnaire is helpful for confirming a psychological effect of exclusion, it may lack sensitivity to measure subtle differences in distress levels between participants. In addition, we chose to administer the distress questionnaire after both Cyberball games were completed in order to avoid priming participants to notice exclusion more in the second game (which may have occurred if we administered the questionnaire after each game). We conclude that this precaution was successful, as a post-hoc assessment of regions differentially responsive to exclusion in the first versus second round of Cyberball (p < .05, k = 35) revealed that the only region modulated by the order of the games that we also reported as being responsive to social exclusion > fair play collapsed across gender conditions was right hippocampus. This region was more active to social exclusion in the first game compared to the second. The administration of the distress measure after both games were completed may have decreased the amount of distress participants reported, however we did find that participants reported significant distress, even after both games were played, suggesting that Cyberball indeed elicited veritable psychological responses to social exclusion. Future studies may estimate participants’ distress levels with more accuracy using more frequent or graded self-report measures, or physiological measures such as skin conductance which index arousal.
A second concern in the current study is that participants may not have believed that they were playing with real people. When verbally questioned by experimenters after the scanning session, 12 of the 20 participants voiced suspicions that the players were not real. Previous literature has suggested that the psychological responses to social exclusion are not affected by the belief that the excluding players are real (Zadro, Williams & Richardson, 2004). Indeed, participants in the current study reported significant distress to exclusion, suggesting that the experience was upsetting even if they did suspect that the players were fictional. While it is desirable for all participants to believe that they are being excluded by real people, the extent to which results of the current study are consistent with past investigations of brain responses to social exclusion with varying levels of participant belief in the veracity of the players supports the idea that differences in belief levels do not significantly alter the brain regions active to exclusion.
Third, while we manipulated the gender relation of the excluders to the excluded participant, we did not investigate gender differences with respect to effects of the group-membership manipulation. Whereas previous studies have reported gender differences in brain responses to peer feedback (Guyer, McClure-Tone, Shiffrin, Pine & Nelson, 2009; Guyer, Choate, Pine & Nelson, 2012), the current investigation was focused on the effects of group membership on brain responses to social exclusion. It is entirely possible that gender differences exist in respect to this investigation; however, the current study was not designed to address these differences and thus was not sufficiently powered to do so. Future studies could add to the field by elucidating the influence of gender differences on the effects reported in the current study.
Finally, while we did not identify any regions of ACC showing greater activation to social exclusion by one’s same (versus other) gender excluders, we did identify a region of dorsal ACC that showed greater activation to exclusion by the opposite gender. There was also a trend to this effect in our structurally-defined dACC ROI analysis. This effect was driven by a difference in activation during same-gender exclusion, with activation to fair play being significantly greater than activation to social exclusion. Because this region was not significantly active to social exclusion in either game, and because this region did not overlap with the region of ventral ACC that showed a main effect of exclusion in the current study, we did not interpret this difference in ACC activation as supporting or opposing the study hypotheses.
Conclusions
The current investigation is the first to explore brain responses to social exclusion in children and adolescents that are modulated by the gender relation of the excluded participant to his or her excluders. While overall neural responses to exclusion in this age group were similar to those seen in adults, the data did not reveal greater activation in ACC to same- versus other-gender exclusion found in older participants (Bolling et al., 2012). Thus, it appears that modulation of ACC activation, which may reflect the ameliorating effects of attributing out-group exclusion to discrimination, may not develop until late adolescence or adulthood. Previously cited developmental effects on emotion regulation in adolescence may underlie the delayed development of this effect.
Acknowledgments
This work was supported by the NIMH under grant R01 MH084080, and DZB was supported by the NINDS under the T32 training grant T32 NS07224.
The research presented herein was supported by National Institute of Mental Health grant R01MH084080. DZB was supported by NINDS T32 training grant (T32 NS07224).
References
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Riess AL. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Pine DS. Neural substrates of childhood anxiety disorders: A review of neuroimaging findings. Child and Adolescent Psychiatris Clinics of North America. 2012;21:501–525. doi: 10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackhart GC, Nelson BC, Knowles ML, Baumeister RF. Rejection elicits emotional reactions but neither causes immediate distress nor lowers self-esteem: A meta-analysis review of 192 studies on social exclusion. Personality and Social Psychology Review. 2009;13:269–309. doi: 10.1177/1088868309346065. [DOI] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Mayes LC, Pelphrey KA. Dissociable brain mechanisms for processing social exclusion and rule violation. NeuroImage. 2011a;54:2462–2471. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, Mayes LC, Pelphrey KA. Development of neural systems for processing social exclusion from childhood to adolescence. Developmental Science. 2011b;14:1431–1444. doi: 10.1111/j.1467-7687.2011.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pelphrey KA, Vander Wyk BC. Differential brain responses to social exclusion by one’s own versus opposite-gender peers. Social Neuroscience. 2012;7:331–346. doi: 10.1080/17470919.2011.623181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Archives of general psychiatry. 2003;60(5):524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Crandall CS, Tsang J, Harvey RD, Britt TW. Group identity-based self-protective strategies: the stigma of race, gender, and garlic. European Journal of Social Psychology. 2000;30:355–381. doi: 10.1002/(SICI)1099-0992(200005/06)30:3<355::AID-EJSP995>3.0.CO;2-M. [DOI] [Google Scholar]
- Crocker J, Voelkl K, Testa M, Major B. Social Stigma: The affective consequences of attributional ambiguity. Journal of Personality and Social Psychology. 1991;60:218–228. doi: 10.1037/0022-3514.60.2.218. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential Ability Scales, 2nd edition: Introductory and technical handbook. San Antonio, TX: The Psychological Corporation; 2007. [Google Scholar]
- Feiring C. Other-sex friendship networks and the development of romantic relationships in adolescence. Journal of Youth and Adolescence. 1999;28:495–512. doi: 10.1023/A:1021621108890. [DOI] [Google Scholar]
- Gerber J, Wheeler L. On being rejected: A meta-analysis of experimental research on rejection. Perspectives on Psychological Science. 2009;4:468–488. doi: 10.1111/j.1745-6924.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- Gilboa-Schechtman E, Shachar-Lavie I. More than a face: a unified theoretical perspective on nonverbal social cue processing in social anxiety. Frontiers in human neuroscience. 2013;7:904. doi: 10.3389/fnhum.2013.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and using the implicit association test: I. an improved scoring algorithm. Journal of Personality and Social Psychology. 2003;85:197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80:1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience. 2012;7:81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren A, Peschard V, Philippot P. The causal role of attentional bias for threat cues in social anxiety: a test on a cyber-ostracism task. Cognitive Therapy and Research. 2012;36:512–521. doi: 10.1007/s10608-011-9394-7. [DOI] [Google Scholar]
- Karremans JC, Heslenfeld DJ, van Dillen LF, Van Lange PAM. Secure attachment partners attenuate neural responses to social exclusion: and fMRI investigation. International Journal of Psychophysiology. 2011;81:44–50. doi: 10.1016/j.ijpsycho.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Killen M, Stangor C. Children’s social reasoning about inclusion and exclusion in gender and race peer group contexts. Child Development. 2001;72:174–186. doi: 10.1111/1467-8624.00272. [DOI] [PubMed] [Google Scholar]
- Krill A, Platek SM. In-group and out-group membership mediates anterior cingulate activation to social exclusion. Frontiers in Evolutionary Neuroscience. 2009;1:1–7. doi: 10.3389/neuro.18.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, et al. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapping. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccoby EE. Gender as a social category. Developmental Psychology. 1988;24:755–765. doi: 10.1037/0012-1649.24.6.755. [DOI] [Google Scholar]
- Major B, Kaiser CR, McCoy SK. It’s not my fault: When and why attributions to prejudice protect self-esteem. Personality and Social Psychology Bulletin. 2003;29:772–781. doi: 10.1177/0146167203029006009. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: A marker of adolescents’ risk for depression. Development and Psychopathology. 2011a;23:283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, et al. Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Eisenberger NI. An fMRI investigation of attributing negative social treatment to racial discrimination. Journal of Cognitive Neuroscience. 2011b;23:1042–1051. doi: 10.1162/jocn.2010.21520. [DOI] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Gabrieli JDE, Ochsner KN. The development of emotion regulation: an fNRI study of cognitive reappraisal in children, adolescents, and young adults. Social Cognitive and Affective Neuroscience. 2012;7:11–22. doi: 10.1093/scan/nsr093. doi:0.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta CM, Strough J. Sex segregation in friendships and normative context across the lifespan. Developmental Review. 2009;9:201–220. doi: 10.1016/j.dr.2009.06.001. [DOI] [Google Scholar]
- Miller CL. Developmental changes in male/female voice classification by infants. Infant Behavior and Development. 1983;6:313–330. doi: 10.1016/S0163-6383(83)80040-X. [DOI] [Google Scholar]
- Moor BG, van Leijenhorst L, Rombouts SARB, Crone EA, van der Molen MW. Do you like me? Neural correlates of social evaluation and developmental trajectories. Social Neuroscience. 2010;5:461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Moor BG, Güroğlu B, Op de Macks ZA, Rombouts SARB, Van der Molen MW, Crone EA. Social exclusion and punishment of excluders: Neural correlates and developmental trajectories. NeuroImage. 2012;59:708–717. doi: 10.1016/j.neuroimage.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Oaten M, Williams KD, Jones A, Zadro L. The effects of ostracism on self-regulation in the socially anxious. Journal of Social and Clinical Psychology. 2008;27:471–504. doi: 10.1521/jscp.2008.27.5.471. [DOI] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Social Neuroscience. 2009;4:443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Pharo H, Gross J, Richardson R, Hayne H. Age-related changes in the effect of ostracism. Social Influence. 2011;6:22–38. doi: 10.1080/15534510.2010.525852. [DOI] [Google Scholar]
- Powlishta KK, Serbin LA, Doyle AB, White DR. Gender, ethnic, and body type biases: The generality of prejudice in childhood. Developmental Psychology. 1994;30:526–536. doi: 10.1037/0012-1649.30.4.526. [DOI] [Google Scholar]
- Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Development. 1999;70:660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Johnson J, Hornig CD, Liebowitz MR, Weissman MM. Social phobia: comorbidity and morbidity in an epidemiologic sample. Archives of General Psychiatry. 1992;49:282–288. doi: 10.1001/archpsyc.1992.01820040034004. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Tan GCY, Roiser JP, Viding E, Dumontheil I, Blakemore SJ. Developmental influences on the neural bases of responses to social rejection: Implications of social neuroscience for education. NeuroImage. 2011;57:686–694. doi: 10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine & Child Neurology. 2002;44:4–16. doi: 10.1111/j.1469-8749.2002.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- van Beest I, Williams KD. When inclusion costs and ostracism pays, ostracism still hurts. Journal of Personality and Social Psychology. 2006;91:918–928. doi: 10.1037/0022-3514.91.5.918. [DOI] [PubMed] [Google Scholar]
- Weinraub M, Clemens LP, Sockloff A, Ethridge T, Gracely E, Myers B. The development of sex role stereotypes in the third year: Relationships to gender labeling, gender identity, sex-types toy preference, and family characteristics. Child Development. 1984;55:1493–1503. doi: 10.2307/1130019. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Williams KD. Ostracism. Annual Review of Psychology. 2007;58:425–452. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]
- Williams KD, Cheung CKT, Choi W. Cyberostracism: effects of being ignored over the internet. Journal of Personality and Social Psychology. 2000;79:748–762. doi: 10.1037/0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Wittenbaum GM, Shulman HC, Braz ME. Social ostracism in task groups: The effects of group composition. Small Groups Research. 2010;41:330–353. doi: 10.1177/1046496410363914. [DOI] [Google Scholar]
- Xiong J, Gao JH, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Human Brain Mapping. 1995;3:287–301. doi: 10.1002/hbm.460030404. [DOI] [Google Scholar]
- Zadro L, Boland C, Richardson R. How long does it last? The persistence of the effects of ostracism in the socially anxious. Journal of Experimental Social Psychology. 2006;42:692–697. doi: 10.1016/j.jesp.2005.10.007. [DOI] [Google Scholar]
- Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. Journal of Experimental and Social Psychology. 2004;40:560–567. doi: 10.1016/j.jesp.2003.11.006. [DOI] [Google Scholar]