Abstract
Flight initiation distance (FID), a measure of an animal’s tolerance to human disturbance and a descriptor of its fear of humans, is increasingly employed for conservation purposes and to predict the response of species to urbanization. However, most work devoted to understanding variability in FID has been conducted at the population level and little is still known about inter-individual variability in this behaviour. We estimated the heritability of FID, a factor fundamental to understanding the strength and evolutionary consequences of selection of particular phenotypes associated with human disturbances. We used a population of burrowing owls (Athene cunicularia) monitored long-term and for which FID was previously shown to be highly consistent across an individual’s lifespan. Heritability estimates varied between 0.37 and 0.80, depending on the habitat considered (urban-rural) and method used (parent-offspring regressions or animal models). These values are unusually high compared with those previously reported for other behavioural traits. Although more research is needed to fully understand the underlying causes of this resemblance between relatives, selection pressures acting on this behaviour should be seriously considered as an important evolutionary force in animal populations increasingly exposed to human disturbance worldwide.
Human activities have transformed ecological patterns and processes around the world1, creating a wide range of unintentional experiments in which organisms either adapt to these changes or cease to exist when faced with severe and novel perturbations. Although traditionally the most adverse human activities for fauna have been related to natural landscape modification, a growing number of studies show that human presence per se can alter animal activities through behavioural changes2,3. Flight initiation distance (hereafter FID) is widely used as a quantitative measure of an animal’s tolerance to human disturbance4 and as a descriptor of its fear of humans5,6. FID has been largely employed as a species-specific trait useful for conservation purposes, such as in planning the recreational use of the countryside7,8,9 or predicting the response of species to urbanization5,10,11. A long-term monitoring study has recently shown that this behaviour is highly consistent across the individual’s lifespan of the burrowing owl Athene cunicularia (repeatability = 0.85–0.96), suggesting that the margin left for any existing inter-individual variance in plasticity would be very small6,12. Thus, the low fear of humans of some populations inhabiting anthropogenic habitats could not have resulted from individual habituation to human disturbance9,13,14,15,16 but to selective processes5,10,17. In this sense, it is compulsory to determine the additive genetic variation associated with the expression of this behavioural trait to understand the strength and evolutionary consequences of this selection. Although it has been shown that other risk-taking behaviours have a heritable component in vertebrates18 little is known about the fear of humans. The only study performed to date shows that the behavioural reactions of long-lived wandering albatross Diomedea exulans toward an approaching human are repeatable and heritable, with strong differences between breeding colonies19. However, most seabirds breed in remote places free of terrestrial predators, so they typically exhibit no instinctual fear of humans in their breeding colonies9. Moreover, the way that authors have measured fear is not the standard procedure used for FID, making extrapolations difficult.
Here, we estimated the heritability of FID in the burrowing owl in two adjacent habitats (urban and rural) differing in their degree of human disturbance. We used parent-offspring regressions and pedigree-based quantitative genetic models. Although there are numerous estimates of heritabilities for a wide range of traits, the great majority has been obtained for morphological and life-history traits and little is known about the heredability of behavioural traits20. To our knowledge, our results are the first to show that fear of humans, as measured by FID, is heritable in birds. Although more research is needed to fully understand the underlying causes of this resemblance between relatives, selection pressures acting on this behaviour should be seriously considered as an important evolutionary force for an increasing number of animal populations exposed to human disturbance.
Results
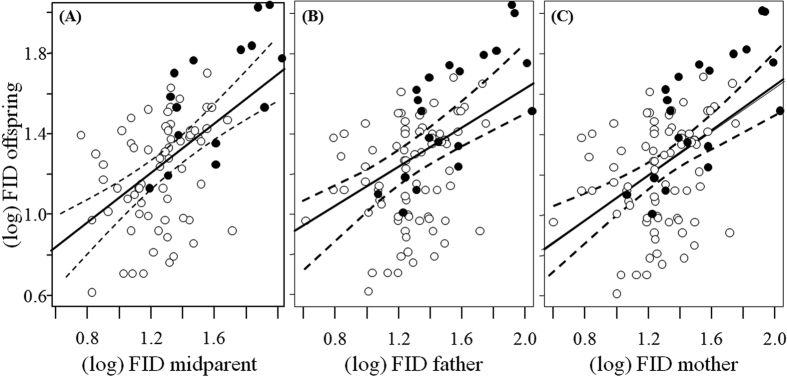
After measuring FID from 1748 individuals over eight years (2008–2015), we were able to establish 118 parent-offspring relationships (mother-offspring: FID range = 1–130 m, n = 96; father-offspring: FID range = 1–111 m, n = 103). Heritability (h2) of FID obtained through midparent-midoffspring regressions ranged from 0.15 to 0.8, without significant differences when calculated for the entire data set or separately for urban and rural birds (Table 1; Fig. 1A). Except for the urban father-offspring regression, all other mother- and father-offspring heritability estimates were significant and statistically similar, ranging from 0.37 to 0.80 (Table 1; Fig. 1B,C). Although heritabilities obtained from mother-offspring regressions were higher than those obtained from father-offspring regression, they were statistically equivalent (Table 1).
Table 1. Heritability (h 2 ) of FID and its standard error (SE) estimated from the midparent-midoffspring and mother/father-offspring regressions for urban and rural burrowing owls.
| Parent-offspring | Rural | Urban | Total |
|---|---|---|---|
| h2 | 0.69*** | 0.39** | 0.64**** |
| SE | 0.22 | 0.14 | 0.11 |
| n | 15 | 66 | 81 |
| rural-urban | rural-total | urban-total | |
| z | 1.15 | 0.20 | −1.40 |
| P | 0.2501 | 0.8415 | 0.1615 |
| father-offspring† | rural | urban | total |
| h2 | 0.75** | 0.15ns | 0.43*** |
| SE | 0.37 | 0.22 | 0.19 |
| n | 20 | 83 | 103 |
| rural-urban | rural-total | urban-total | |
| z | 1.40 | 0.78 | −0.97 |
| P | 0.1615 | 0.4354 | 0.3320 |
| mother-offspring† | rural | urban | total |
| h2 | 0.80** | 0.37* | 0.63**** |
| SE | 0.36 | 0.26 | 0.22 |
| n | 20 | 76 | 96 |
| rural-urban | rural-total | urban-total | |
| z | 0.97 | 0.40 | −0.76 |
| P | 0.3320 | 0.6892 | 0.4473 |
| father-mother rural | father-mother urban | father-mother | |
| z | −0.10 | −0.65 | −0.69 |
| P | 0.9203 | 0.5157 | 0.4902 |
Significance of the estimate is shown (****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05; ns: P > 0.05). Heritability values are compared through z-test (z). n: sample size.
†Values corrected for assortative mating: rurban birds = 0.61, rrural birds = 0.72, rall birds = 0.78.
Figure 1. Offspring FID regressed (dashed lines: 95% confidence interval) on parent FID for burrowing owls.
White and black dots represent urban and rural birds, respectively.
Animal models were performed using pedigree information from 1265 individuals, distributed through 3 generations. Heritability of FID using this method, which accounts for the significant lower FID of urban individuals (β = −0.42; 95% CI: −0.45–−0.39) and the larger FID of females (β = 0.06; 95% CI: 0.03–0.08), was 0.43 (95% CI: 0.19−0.60; heritabilities with models performed with different priors: 0.37−0.5; see Table S1 and Figure S1 for further details). The large 95% CI of this estimate suggests that it is not statistically different from heritability estimates obtained through parent-offspring regressions.
Discussion
To understand the evolutionary consequences of human pressures on animal behaviours, it is fundamental to know whether behavioural responses have a genetic basis, i.e. if they are heritable between generations21. Here, we assessed whether FID, a behaviour widely used to measure anti-predator responses and fear of humans in birds4,5,6,7,8,9,10,11 and shown to be highly repeatable along an individual’s adulthood in our study species6, has a heritable component. Our estimates of heritability varied from 0.37 to 0.80, depending on the habitat (urban – rural) and method used (parent-offspring regressions or animal models), and are high compared with those previously reported for other heritable antipredator behaviours (0.12–0.42)18,19,21. While parent-offspring regressions have largely been used to estimate heritabilities in a variety of behaviours, animal models usually perform better because they are able to explain total phenotypic variance by partitioning residual variance into additive and non-additive genetic variance22,23,24. However, animal models require high quality data that are often difficult to obtain in wild populations. In our case, after marking more than 2,000 individuals for 10 consecutive years and measuring FID in more than 1,700 individuals for 8 of those years, we were able to use animal models to estimate heritability (h2 = 0.43). However, we obtained a rather large 95% CI, perhaps due to the low pedigree depth25. As this value is not different from those obtained using parent-offspring regressions, our results support the validity of regressions to estimate heritability when pedigree information is not available26.
Non-genetic inheritance provides a faster means of adapting to rapid environmental change than genetic inheritance alone27,28, with cultural evolution theoretically providing an important source of biodiversity through speciation29,30. In our case, the high fidelity of burrowing owls to their natal habitat (urban or rural, author’s unpublished data) does not allow us to properly assess to what extent non-genetic inheritance (i.e., epigenetic, ecological and cultural inheritance, and parental effects) could be contributing to our high heritability estimates31,32. Nonetheless, heritability estimates were high and statistically similar in both rural and urban habitats, which largely differ in levels of human disturbance, thus suggesting that these estimates were not largely inflated by the similar exposure to humans experienced by parents and their offspring. In any case, independent of the mechanisms promoting the significant resemblance between relatives, our results indicate that selection acting on this behaviour can have long-term consequences for animal populations36. More research is thus needed using other study models to properly generalize and fully understand the consequences of population and individual variability in this behaviour.
Selection of particular phenotypes (in this case, behaviours) associated with human disturbances5,10 can have profound effects on populations. Several studies have shown that risk-taking behaviours, within which we can include fear of humans, are actually correlated with exploration and aggression33,34,35,36, and linked to life history traits37. Thus, selection of human-tolerant phenotypes can have important ecological and evolutionary implications due to the inherent trade-offs of a syndrome structure38. Human presence in the wild selecting positively for individuals with a low fear of humans would result in a population with more exploratory and aggressive individuals, thus changing intraspecific interactions among them35,36. Moreover, demographic parameters of those individuals can be different39, and potential consequences such as the non-random dispersal of individuals between humanized and non-humanized habitats, should be taken into account as an unappreciated ecological and evolutionary force5,17,40. A similar scenario of human-induced evolution has been shown for fisheries, as harvesting has imposed a directional and disruptive selection process to key life-history traits genetically or phenotypically correlated with a suite of interrelated physiological, behavioral, and morphological characters41,42. In this sense, even when human activities such as habitat transformation, persecution, pollution or species introductions have been claimed as the main drivers of species loss43, human presence per se should be considered as another, maybe less obvious but potentially important, cause of biodiversity change.
Methods
Study species and study area
The burrowing owl is found across American open landscapes, showing diurnal activity and nesting in burrows excavated by the owls or by mammals44. Breeding pairs are easily located within the surrounding of their nests (usually 30 m) due to their territorial and highly conspicuous behaviour during the daytime6,12,17.
Our study site encompasses ca. 5,500 km2, including rural and urban areas around Bahía Blanca (Argentina). Rural habitats are mainly represented by large expanses of natural grasslands and pastures used for wide ranging livestock and low-intensive cereal crops. Human presence is extremely low and mostly restricted to a few paved or unpaved roads (with 0–0.1 pedestrians/h and 0.34–2.4 cars/h), so most owls breeding in rural habitats have little or even no close contact with humans (or only with the researchers). This strongly contrasts with urban owls, which excavate their nests in small (usually 0.01–0.1 ha) private and public gardens in urbanized residential areas, unbuilt spaces among houses, curbs of streets and even along large avenues. These owls are in constant contact with garden and house owners, children, pedestrians and intense car traffic. There is no clear habitat interface between urban and rural habitats, since urbanized areas are immediately surrounded by rural ones, and all birds sampled for FID unambiguously bred in urban or rural areas (for more details on the study area, human pressure and owl population, see refs 5,6,12,17,45).
Field procedures
We trapped 950 adults and 1,245 chicks from 2006 to 2015, using bow nets and ribbon carpets, to mark them with a plastic numbered colour-ring readable at distance. Pair members were first sexed based on plumage patterns and colouration, and then confirmed by molecular analyses18. FIDs were measured in a sample of 791 banded adult owls during the late breeding season stage, i.e. when they were rearing chicks (from late November to late January), in 2008–2015. We enlarged sample sizes by measuring FID in another 957 non-ringed birds. These birds were breeding adults for which we were able to ring their offspring and/or their mates, so we could confirm their parent-offspring and mate relatedness despite being unmarked. Due to the high within-individual repeatability of FID recorded both within breeding seasons (r = 0.84–0.92)12 and across breeding seasons covering the lifespan of individuals (r = 0.90–0.96)6, we used one FID per individual or the mean when more than one value was available (see ref. 15 for the same approach). The standard procedure used was to walk towards undisturbed focal individuals, which were perched on the ground or on small poles close to their nests, following a direct trajectory at a constant speed of 0.5 m/s, with no obstacles between the bird and the observer. Distances at which birds fled were measured using a laser telemeter incorporated into 10 × 42 binoculars (Leica Geovid, range: 10–1300 m) or counting paces for distances of less than 10 m5,6,12. FIDs were measured during the day, when owls were easily located at distance, given the bare ground and short vegetation surrounding their nests. As shown in previous studies of this burrowing owl population5,6,17, urban individuals included here for heritability analyses also showed shorted FIDs (median 17 m, range 1–55 m, n = 252) than their rural counterparts (median = 32 m, range = 10–130 m, n = 65) (GLM on log-transformed FID, F = 70.24, p < 0.0001).
Statistical analysis
Heritability (h2) of (log) FID was obtained from the slope of the regression of midoffspring on midparent and twice the slopes of midoffspring on each single-parent through GLMs with normal error distribution and identity link function, using the average value of full sibs to avoid pseudo-replication (see Figure S2 for actual values of parent-offspring FIDs). As heritability could differ between habitats, we obtained h2 for urban and rural birds separately as well as for all birds together. The different heritabilities obtained were compared by calculating z scores as (xi − xj)/√(SEi2 + SEj2), where xi and xj are the two different estimates, and SEi and SEj the respective standard errors. Assortative mating does not affect midparent–offspring regressions, but it increases the regression of offspring on single parents by a factor (1 + r), where r is the phenotypic correlation between mates46. Breeders did not mate at random regarding FID12 so heritability values obtained from father/mother-offspring regressions were corrected for (urban birds: r = 0.61, n = 293 breeding pairs; rural birds: r = 0.72, n = 264 breeding pairs; all birds: r = 0.78, n = 557 breeding pairs; all p < 0.0001).
We ran animal models to estimate the heritability of FID (log-transformed) by using a Bayesian Markov chain Monte Carlo (MCMC) technique implemented in the MCMCglmm package in R47. Animal models are a type of mixed effect model, which estimates quantitative genetic parameters of a trait by assessing the phenotypic covariance between all pairs of relatives in the pedigree48. MCMC pedigree-based models are particularly useful to partition phenotypic variance into additive and non-additive genetic variance of phenotypes with Gaussian and non-Gaussian distributions, while simultaneously accounting for any potentially confounding effects. We assessed heritability as VA/VP, where VA is the additive genetic variance and VP the total phenotypic variance of FID, calculated as VA + VR. In the ‘animal model’, VA and VP were inferred from the pedigree information, including both father and mother identities, and considering the effect of habitat and individual sex as fixed effects. Since FID is highly repeatable across the life span of burrowing owls6, we did not include year as a controlling variable in models. We used an uninformative prior for the fixed and random variances (V = 1, nu = 0.0224; but see Appendix 1 for results with other priors) for 1,000,000 iterations, preceded by a burn-in of 10,000 iterations and storing estimates of every 200th iteration to reduce autocorrelation. This provides us with the posterior probability density functions for the fixed term and all variance components, and thereby with the most probable size of each variance component, as well as its 95% credible interval (CI). We tested the statistical support of the fixed effect (habitat) by evaluating the extent to which their posterior distributions (95% CI) overlapped zero.
Parent-offspring regressions as well as animal models may underestimate heritability if social parents are not the genetic parents of all or some of the offspring49. However, burrowing owls are territorial birds that can be mostly considered as genetically monogamic, since extra-pair fertilizations and brood parasitism are infrequent even when individuals breed at very high densities in urban sites45.
Ethics statements
Capture, banding and FID measures of burrowing owls were conducted under permits from the Argentinean wildlife agency (22500-4102/09), and the owners of private properties, in accordance with the approved guidelines of the Ethics Committee of CSIC (CEBA-EBD-11-28).
Additional Information
How to cite this article: Carrete, M. et al. Heritability of fear of humans in urban and rural populations of a bird species. Sci. Rep. 6, 31060; doi: 10.1038/srep31060 (2016).
Supplementary Material
Acknowledgments
We thank N. Lois, S. Briones, M. Santillán, P. Laiolo, M. de la Riva, N. Tella-Carrete, and M. Vázquez for their help in capturing and monitoring owls over the years. Field work was conducted under permits from Argentinean wildlife agencies and the owners of private properties. This work was supported by Canal Sur TV, Fundación Repsol, Projects CGL2012-31888 and CGL2015-71378 from MEC, and COOPA20049 from CSIC (Spain). N.R.I. and S.R.M. were supported by CONICET (Argentina), A.P. by a La Caixa - Severo Ochoa foundation, J.M.P. by CGL2015-70639-P (Ministerio Economía y Competitividad, Spain) and M.C. by a Ramón y Cajal contract (RYC-2009-04860) and a contract of the UPO. S. Young revised the English.
Footnotes
Author Contributions M.C., J.M.P. and J.L.T. conceived the idea. M.C., S.R.M., N.R.I., A.P. and J.L.T. conducted field work. M.C., S.R.M. and J.M.P. analyzed the data. M.C., J.M.P. and J.L.T. wrote the paper. M.C., J.M.P., S.R.M., N.R.I., A.P. and J.L.T. discussed the results and commented on the manuscript.
References
- Ellis E. C. & Ramankutty N. Putting people in the map: anthropogenic biomes of the World. Front. Ecol. Environ. 6, 439–447 (2008). [Google Scholar]
- Sih A., Ferrari M. C. O. & Harris D. J. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367–387 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuti S. et al. Effects of humans on behaviour of wildlife exceed those of natural predators in a landscape of fear. Plos One 7, e50611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. et al. Does avian flight initiation distance indicate tolerance towards urban disturbance? Ecol. Indic. 15, 30–35 (2012). [Google Scholar]
- Carrete M. & Tella J. L. Inter-Individual Variability in Fear of Humans and Relative Brain Size of the Species Are Related to Contemporary Urban Invasion in Birds. Plos One 6, e18859 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrete M. & Tella J. L. High individual consistency in fear of humans throughout the adult lifespan of rural and urban burrowing owls. Sci. Rep. 3, 3524 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein D. T., Anthony L. L., Harcourt R. & Ross G. Testing a key assumption of wildlife buffer zones: is flight initiation distance a species-specific trait? Biol. Cons. 110, 97–100 (2003). [Google Scholar]
- Tarlow E. & Blumstein D. T. Evaluating methods to quantify anthropogenic stressors on animals. Appl. Anim. Behav. Sci. 102, 429–451 (2007). [Google Scholar]
- Martínez-Abraín A., Oro D., Conesa D. & Jiménez J. Compromise between seabird enjoyment and disturbance: the role of observed and observers. Environ. Conserv. 35, 104–110 (2008). [Google Scholar]
- Møller A. P. Interspecific variation in fear responses predicts urbanization in birds. Behav. Ecol. 21, 365–371 (2010). [Google Scholar]
- Díaz M. et al. The Geography of Fear: A Latitudinal Gradient in Anti-Predator Escape Distances of Birds across Europe. Plos One 8, e64634 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrete M. & Tella J. L. Individual consistency in flight initiation distances in burrowing owls: a new hypothesis on disturbance-induced habitat selection. Biol. Lett. 6, 167–170 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke A. S. Observations on how close certain passerine species will tolerate an approaching human in rural and suburban areas. Biol. Cons. 18, 85–88 (1980). [Google Scholar]
- Stankowich T. & Blumstein D. Fear in animals: a meta-analysis and review of risk assessment. Proc. R. Soc. B. 272, 2627–2634 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Prieto I., Fernández-Juricic E., Martín J. & Regis Y. Antipredator behavior in blackbirds: habituation complements risk allocation. Behav. Ecol. 20, 371–377 (2009). [Google Scholar]
- Li C., Monclús R., Maul T. L., Jiang Z. & Blumstein D. T. Quantifying human disturbance on antipredator behavior and flush initiation distance in yellowbellied marmots. App An Behav Sci 129, 146–152 (2011). [Google Scholar]
- Rebolo-Ifrán N. et al. Links between fear of humans, stress and survival support a non-random distribution of birds among urban and rural habitats. Sci. Rep. 5, e13723 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D. G., Réale D. & Roff D. A. Selection, structure and the heritability of behaviour. J. Evol. Biol. 15, 277–289 (2002). [Google Scholar]
- Patrick S. C., Charmantier A. & Weimerskirch H. Differences in boldness are repeatable and heritable in a long-lived marine predator. Ecol. Evol. 3, 4291–4299 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma E. In Quantitative Genetics in the Wild (eds Charmantier A., Garant D. & Kruuk L. E. B.) Ch. 2, 16–33 (Oxford, 2014). [Google Scholar]
- Réale D., Reader S. M., Sol D., Mcdougall P. T. & Dingemanse N. J. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 1–28 (2007). [DOI] [PubMed] [Google Scholar]
- Wilson A. J. et al. An ecologist’s guide to the animal model. J. Anim. Ecol. 79, 13–26 (2009). [DOI] [PubMed] [Google Scholar]
- de Villemereuil P., Gimenez O. & Doligez B. Comparing parent–offspring regression with frequentist and Bayesian animal models to estimate heritability in wild populations: a simulation study for Gaussian and binary traits. Meth. Ecol. Evol. 4, 260–275 (2013). [Google Scholar]
- de Villemeuril P. Estimation of a biological trait heritability using the animal model. Available at: http://devillemereuil.legtux.org/wp-content/uploads/2012/12/tuto_en.pdf (date of access: 27/06/2016) (2012).
- Pemberton J. M. Wild pedigrees: the way forward. Proc. R. Soc. B. 275, 613–621 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åkesson M., Bensch S., Hasselquist D., Tarka M. & Hansson B. Estimating heritabilities and genetic correlations: comparing the ‘Animal model’ with parent-offspring regression using data from a natural population. Plos One 3, e1739 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M. W. & Cavalli-Sforza L. L. Cultural and biological evolutionary processes: gene-culture disequilibrium. Proc. Natl Acad. Sci. USA 81, 1604–1607 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson L. S., Davies W. & Isles A. R. Genomic imprinting effects on brain development and function. Nature Rev. Neurosci. 8, 832–843 (2007). [DOI] [PubMed] [Google Scholar]
- Hochberg M. E., Sinervo B. & Brown S. P. Socially mediated speciation. Evolution 57, 154–158 (2003). [DOI] [PubMed] [Google Scholar]
- Grant P. R. & Grant B. R. The secondary contact phase of allopatric speciation in Darwin’s finches. Proc. Natl Acad. Sci. USA 106, 20141–20148 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin E. et al. Beyond DNA: integrating inclusive inheritance into an extended theory of evolution. Nature Rev. Genetics 12, 475–485 (2011). [DOI] [PubMed] [Google Scholar]
- Danchin E. Avatars of information: towards an inclusive evolutionary synthesis. Trends Ecol. Evol. 28, 351–358 (2013). [DOI] [PubMed] [Google Scholar]
- van Oers K., de Jong G., Drent P. J. & van Noordwijk A. J. A genetic analysis of avian personality traits: correlated response to artificial selection. Behav. Genet. 34, 611–619 (2004). [DOI] [PubMed] [Google Scholar]
- van Oers K., Drent P. J., de Goede P. & van Noordwijk A. J. Repeatability and heritability of risk-taking behaviour in relation to avian personalies. Proc. R. Soc. B. 271, 65–71 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garamszegi L. Z., Eens M. & Török J. Behavioural syndromes and trappability in free-living collared flycatchers, Ficedula hypoleuca. Anim. Behav. 77, 803–812 (2009). [Google Scholar]
- Evans J., Boudreau K. & Jeremy Hyman J. Behavioural syndromes in urban and rural populations of song sparrows. Ethology 116, 588–595 (2010). [Google Scholar]
- Wolf M., van Doorn G. S., Leimar O. & Weissing F. J. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584 (2007). [DOI] [PubMed] [Google Scholar]
- Sih A., Bell A. & Johnson J. C. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 (2004). [DOI] [PubMed] [Google Scholar]
- Dingemanse N. J. & Réale D. Natural selection and animal personality. Behaviour 142, 1159–1184 (2005). [Google Scholar]
- Edelaar P. & Bolnick D. I. Non-random gene flow: an underappreciated force in evolution and ecology. Trends Ecol. Evol. 27, 659–665 (2012). [DOI] [PubMed] [Google Scholar]
- Hard J. J. et al. Evolutionary consequences of fishing and their implications for salmon. Evol. Appl. 1, 388–408 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas S. et al. The response of correlated traits following cessation of fishery-induced selection. Evol. Appl. 5, 657–663 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens I. P. F. & Bennett P. M. Ecological basis of extinction risk in birds: Habitat loss versus human persecution and introduced predators. P Natl Acad Sci USA 97, 12144–12148 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Hoyo J., Elliot A. & Sargatal J. Handbook of the birds of the world. Lynx, Barcelona (1999). [Google Scholar]
- Rodriguez-Martínez S., Carrete M., Roques S., Rebolo-Ifrán N. & Tella J. L. High urban breeding densities do not disrupt genetic monogamy in a bird species. Plos One 9, e91314 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer D. S. & Mackay T. F. Introduction to Quantitative Genetics. 4th edn, Benjamin Cummings,Harlow, Essex, UK (1996). [Google Scholar]
- Hadfield J. D. MCMC methods for multi-response generalised linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22 (2010).20808728 [Google Scholar]
- Kruuk L. E. B. Estimating genetic parameters in natural populations using the ‘animal model’. Phil. Trans. R. Soc. Lond. B 359, 873–890 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmantier A. & Réale D. How do misassigned paternities affect the estimation of heritability in the wild? Mol Ecol 14, 2839–2850 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.