Abstract
The “dilution effect” implies that where species vary in susceptibility to infection by a pathogen, higher diversity often leads to lower infection prevalence in hosts. For directly transmitted pathogens, non-host species may “dilute” infection directly (1) and indirectly (2). Competitors and predators may (1) alter host behavior to reduce pathogen transmission or (2) reduce host density. In a well-studied system, we tested the dilution of the zoonotic Puumala hantavirus (PUUV) in bank voles (Myodes glareolus) by two competitors and a predator. Our study was based on long-term PUUV infection data (2003–2013) in northern Sweden. The field vole (Microtus agrestis) and the common shrew (Sorex araneus) are bank vole competitors and Tengmalm’s owl (Aegolius funereus) is a main predator of bank voles. Infection probability in bank voles decreased when common shrew density increased, suggesting that common shrews reduced PUUV transmission. Field voles suppressed bank vole density in meadows and clear-cuts and indirectly diluted PUUV infection. Further, Tengmalm’s owl decline in 1980–2013 may have contributed to higher PUUV infection rates in bank voles in 2003–2013 compared to 1979–1986. Our study provides further evidence for dilution effect and suggests that owls may have an important role in reducing disease risk.
Land use change and habitat destruction contribute to loss of biodiversity and disruption of natural processes1. Disturbed ecosystems become “unhealthy”2 when hosts and vectors become dominant in depleted communities3,4. Ecosystem disturbance is thought to particularly affect zoonotic pathogens, i.e. those shared between humans and vertebrate animals, which comprise a majority of emerging infectious diseases of humans5. As human activities contributing to “unhealthy” ecosystems continue to accelerate6, interest in the role of diversity and community composition in modifying disease risk is growing7.
In disease systems where species vary in their susceptibility to infection by a pathogen, higher diversity often results in lower disease risk (reviewed in ref. 8). This is termed “the dilution effect”3 and acts on processes at different levels of the disease-cycle. The dilution effect framework in zoonotic systems was developed for the tick-borne Lyme disease system9. A key component of the dilution effect is that species-assemblages are nested, where reservoir hosts (those that maintain and transmit the pathogen) persist at low diversity10,11. Habitat specialist, predators, or species with a slow life history disappear from disturbed areas, while reservoir hosts tend to be habitat generalists, have fast life histories, and tolerate disturbance12,13,14. For example, in Central and South America, agricultural activities result in changes in the composition of rodent assemblages, which become restricted to few species. Those species that persist are often hosts for hantaviruses and their dominance of agricultural and peri-domestic areas increases human risk13.
The strength, scale, and generality of the dilution effect have been debated, but most caveats pertain to vector-borne pathogen systems15,16,17. For vector-borne pathogens with multiple hosts, complexities may arise if an increase in vector density associated with high species diversity counteracts the dilution effect18. However, community assembly is typically substitutive so that when diversity increases, individuals are replaced rather than added to maintain a constant total density in the community. Since total host density remains constant, vector density is unlikely to increase when diversity increases19. For directly-transmitted zoonotic viruses such as hantavirus, transmission rates and disease risk are not confounded by a vector, and the dilution effect depends on changes in host density or behavior20.
Puumala hantavirus (PUUV, family Bunyaviridae, genus Hantavirus) is a single-stranded RNA virus that causes hemorrhagic fever with renal syndrome in humans21. The natural and only competent host of PUUV, i.e. capable of furthering the infection cycle through shedding of viral particles upon infection22, is the bank vole (Myodes glareolus)23. It is a very common mammal in Europe24, and despite bank vole preference for forest habitats, it can reach high densities in other habitat types25 and often prevails at low species diversity. In Fennoscandina, its population and that of other small mammals undergo synchronous 3–4 year cycles26,27,28,29. PUUV is directly and horizontally transmitted within bank vole populations and viral particles are shed in the saliva, feces, and urine30. Human PUUV infections correlate with bank vole density and infection rates31,32,33,34,35 and have increased in the past decade both in Northern and Western-Central Europe35,36.
There are two mechanisms by which non-host species, including predators, may reduce PUUV infection in bank voles (reviewed in ref. 37). (1) The “encounter reduction” pathway occurs if non-host species change the behavior of bank voles, ultimately reducing encounter rate or duration between infected and susceptible individuals38. (2) The “susceptible host regulation”38 acts through suppression of bank vole density39. PUUV prevalence, i.e. proportion of infected bank voles in a population, has often been found to be density-dependent, e.g.31,40,41, so reduction in host density reduces PUUV transmission and prevalence among bank voles.
The potential of other species to dilute PUUV infection in bank voles is under-explored (but see refs 41 and 42). However, there is strong support for the dilution effect in hantavirus-host systems in North and Central America. Through experimental and observational studies, several studies reported lower Hantavirus infection rates in hosts at higher diversity of small mammals, e.g. refs 43, 44, 45, 46, 47. In a heterogeneous landscape where the bank vole and other small mammals fluctuate synchronously26,27,28,48, the relationship between different modes of inter-specific interactions and PUUV infection in bank voles is not trivial. To evaluate the validity of the dilution effect (see Fig. 1 in ref. 37), we use long-term data and account for habitat-specific, seasonal, and annual PUUV infection patterns.
Figure 1. Percentage of number of bank voles out of all trapped small mammals in spring in 1971–2013.
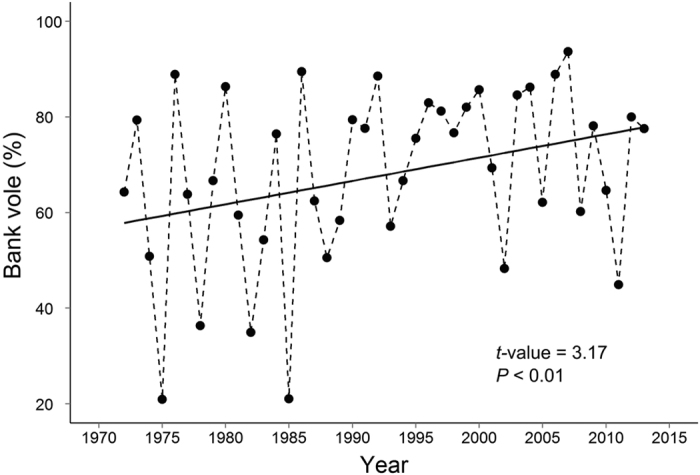
In our study area in northern Sweden, small mammals have been monitored since 197126. The bank vole is the most common species and can be found in most habitats25,49. The grey-sided vole Myodes rufocanus is the main competitor of the bank vole in coniferous forests50 and has declined and locally disappeared in the early 2000’s51,52. The field vole (Microtus agrestis) has also declined since the 1970’s (Figure S1a)25, yet persists in the landscape mainly in open areas dominated by grasses in the field layer, e.g. meadows and clear-cuts52. It is competitively superior to the bank vole and may exclude it from clear-cuts and young forests25. Hence, the field vole could cause a “dilution effect” due to its ability to affect both bank vole behavior and survival53. The common shrew (Sorex araneus) is a competitor and nest predator of bank voles54. This solitary small-sized insectivore can be found in most habitat types55. Recent studies have shown that the presence of common shrews influences the behavior and home range of lactating female bank voles54,56. Thus, the common shrew may dilute PUUV infection in bank voles through influencing bank vole behavior. While the grey-sided and field voles declined52, bank voles increased during the last decade (Figure S1), suggesting that drivers causing the decline in other vole species have not equally affected bank vole populations (Fig. 1).
Tengmalm’s owl (Aegolius funereus) is a predator specializing on small mammals, and field and bank voles constitute approximately 85% of its diet57. Nest box occupancy of breeding Tengmalm’s owls in the study area has declined since its monitoring began in 1980 and continues to fluctuate at low levels58. In theory, predators of hosts may reduce disease risk both by selectively taking infected host individuals59 and by regulating host density60. Empirical work on predation and dilution of infection is scarce, but Tengmalm’s owls probably suppress bank vole density61,62.
Here, we investigate the dilution effect in a well-studied system of a directly-transmitted zoonotic pathogen (PUUV) in boreal Sweden28,31,49,52,63. We hypothesize that both field voles and common shrews will dilute PUUV infection in bank voles through changing their movement patterns and reducing contact rates, i.e. cause a dilution effect via “encounter reduction”. Moreover, we expect field voles, but not common shrews, to indirectly reduce PUUV infection by suppressing bank vole densities, i.e. via “susceptible host regulation”. Effects of field voles on bank vole density and PUUV prevalence should be strongest in core field vole habitat. To test our hypotheses, we used long-term trapping data over a large area, while incorporating habitat at a local patch scale. Although areas of owl nest box monitoring and small mammal trapping only partially overlap, we discuss how long-term decline of Tengmalm’s owls may have affected PUUV infection and host density in 2003–2013.
Results
In 2003–2013, trapped small mammals in our analyses consisted of 4169 bank voles (84% of all trapped specimens), 545 field voles (11%) and 271 common shrews (5%). In total 942 bank voles were infected, and overall PUUV prevalence was 22.5%. Overall PUUV prevalence in spring (47%) was higher than in fall (17%). In 1971–2013, the percentage of bank voles relative to total number of small mammals increased in both spring (Fig. 1, t-value = 3.17, p < 0.01, dfresidual = 41) and fall (t-value = 2.03, p = 0.04, dfresidual = 41).
Encounter reduction
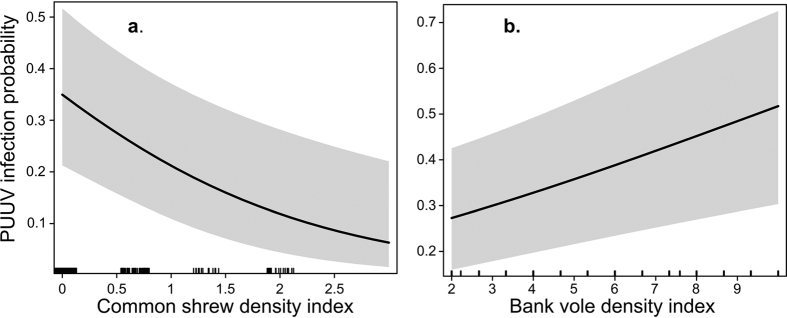
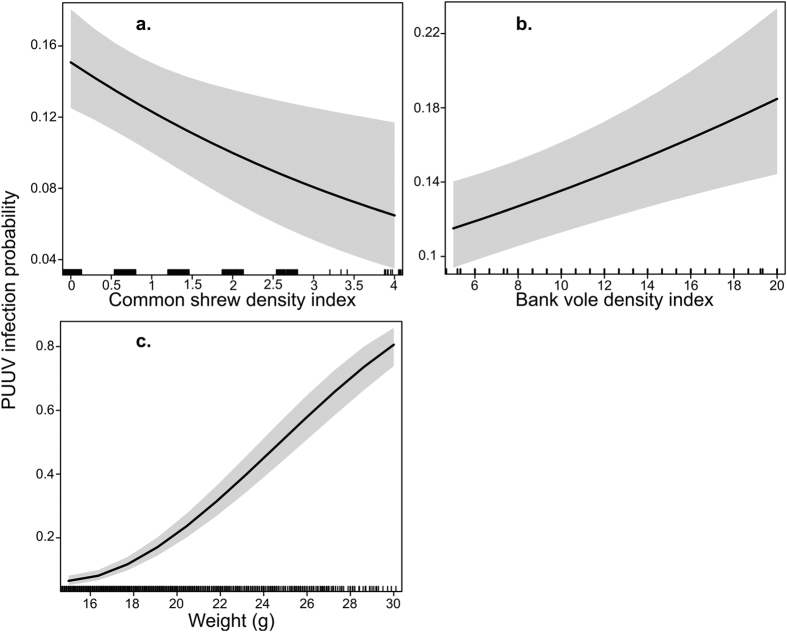
In both spring and fall, the best model (Table S1, models 1 and 2) predicting the probability of a bank vole being infected included common shrew density index and bank vole density index as predictors (Table 1, Figs 2 and 3). Infection probability increased with bank vole density index. However, infection probability decreased as common shrew density index increased. In fall, the best model suggested that infection probability also increased with bank vole weight (Table 1, Fig. 3). Neither habitat nor field vole density index influenced infection probability in either season, despite field voles showing a higher overall density index compared with common shrews (Figure S1).
Table 1. Models predicting Puumala virus-infection probability in bank voles and bank vole density index.
| Infection probability binomial | |||||||
|---|---|---|---|---|---|---|---|
| Fall |
Spring |
||||||
| OR | CI | P | OR | CI | P | ||
| Fixed Parts | |||||||
| Bank vole density | 1.04 | 1.02–1.06 | <0.001 | Bank vole density | 1.14 | 1.05–1.24 | 0.001 |
| Common shrew density | 0.79 | 0.67–0.94 | 0.007 | Common shrew density | 0.50 | 0.32–0.78 | 0.002 |
| Weight (g) | 1.31 | 1.27–1.35 | <0.001 | ||||
| Random Parts | |||||||
| Nplots | 54 | Nplots | 53 | ||||
| NYear | 11 | NYear | 10 | ||||
| ICCplots | 0.017 | ICCplots | 0.060 | ||||
| ICCYear | 0.015 | ICCYear | 0.203 | ||||
| Observations | 3330 | Observations | 839 | ||||
| Bank vole density poisson | |||||||
| Fixed Parts | |||||||
| Intercept | 3.79 | 2.35–6.14 | <0.001 | Intercept | 1.31 | 0.91–1.88 | <0.001 |
| Old forest | 1.34 | 0.97–1.87 | 0.08 | Old forest | 1.67 | 1.19–2.33 | 0.003 |
| Young forest | 0.94 | 0.70–1.26 | 0.69 | Young forest | 1.31 | 0.97–1.78 | 0.08 |
| Bank vole density (t-1) | 0.99 | 0.99–0.99 | <0.001 | Bank vole density (t-1) | 0.95 | 0.95–0.96 | <0.001 |
| Field vole density | 0.98 | 0.98–0.99 | <0.001 | Field vole density | 1.07 | 1.04–1.11 | <0.001 |
| Common shrew density | 1.07 | 1.06–1.07 | <0.001 | Common shrew density | 1.05 | 1.03–1.07 | <0.001 |
| Field vole density × Young forest | 1.03 | 1.02–1.04 | <0.001 | Field vole density × Young forest | 1.02 | 0.98–1.05 | 0.29 |
| Field vole density × Old forest | 1.01 | 1.01–1.02 | 0.001 | Field vole density × Old forest | 0.93 | 0.90–0.96 | <0.001 |
| Random Parts | |||||||
| Nplots | 50 | Nplots | 48 | ||||
| NYear | 11 | NYear | 11 | ||||
| ICCplots | 0.05 | ICCplots | 0.05 | ||||
| ICCYear | 0.13 | ICCYear | 0.06 | ||||
| Observations | 430 | Observations | 247 | ||||
The reference (intercept) is bank vole density index in meadows and clear-cuts. OR = odds ratio, CI = confidence interval, ICC = intra-class correlation coefficient.
Figure 2. The model-predicted probability of a bank vole being Puumala virus-infected in spring.
Relative to (a) common shrew density index and (b) bank vole density index. The grey-shaded area represents the 95% confidence interval of coefficient estimates. Vertical black marks on the x-axis show how predictor values are distributed across predictor range, denser marks indicate a concentration of predictor values.
Figure 3. The model-predicted probability of a bank vole being infected in fall.
Relative to (a) common shrew density index, (b) bank vole density index and (c) weight (g). The grey-shaded area represents the 95% confidence interval of coefficient estimates. Vertical black marks on the x-axis show how predictor values are distributed across predictor range, denser marks indicate a concentration of predictor values.
Susceptible host regulation
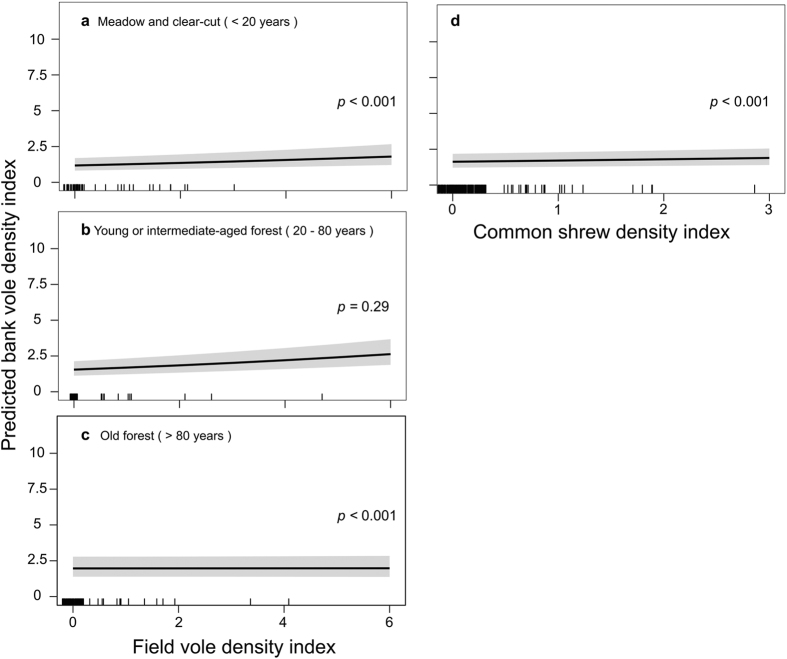
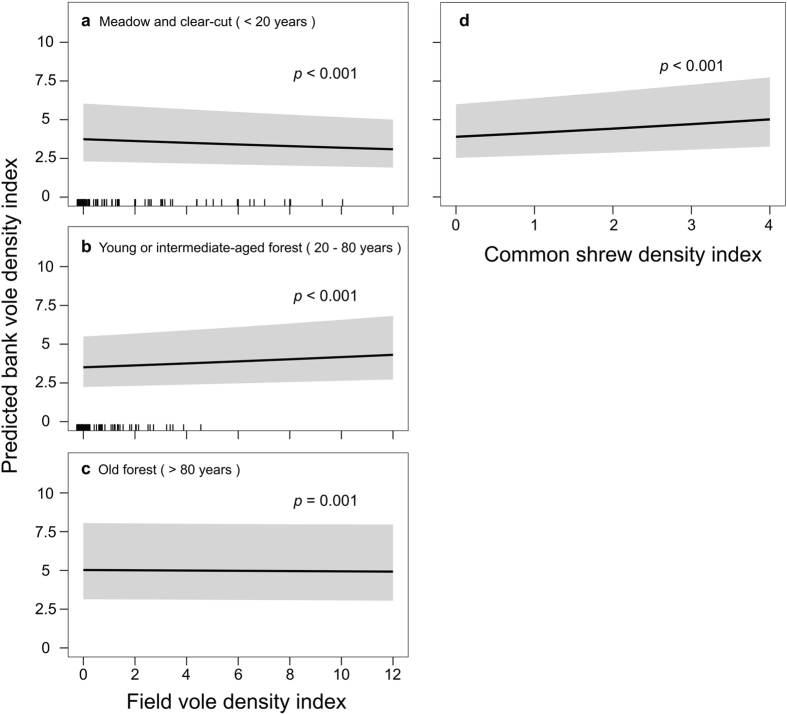
Factors predicting bank vole density index were similar in spring and fall models, but the direction of the relationships differed (Table S1, models 3 and 4). In both spring and fall, bank vole density index increased with common shrew density index, irrespective of habitat (Table 1). Current bank vole density index was negatively related to its previous density index (Yeart-1) (Table 1), and was higher in older forest compared to young and intermediate-aged forests and meadows and clear-cuts. There was an interaction between field vole density index and habitat in both seasons, but the direction of the relationship differed between spring and fall. In spring, bank vole density index increased as field vole density index increased in meadows and clear-cuts and intermediate-aged forests (Fig. 4a,b). In fall, we found the opposite scenario, and bank vole density index decreased when field vole density increased, but only in core field vole habitat, i.e. meadows and clear-cuts (Fig. 5a).
Figure 4. Model-predicted bank vole density index in spring.
Relative to (a–c) field vole density index in different habitat succession stages, and relative to (d) common shrew density index. The grey-shaded area represents the 95% confidence interval of coefficient estimates. Vertical black marks on the x-axis (rug plots) show how predictor values are distributed across predictor range, denser marks indicate a concentration of predictor values.
Figure 5. Model-predicted bank vole density index in fall.
Relative to (a–c) field vole density index in different habitat succession stages, and relative to (d) common shrew density index. The grey-shaded area represents the 95% confidence interval of coefficient estimates. Vertical black marks on the x-axis (rug plots) show how predictor values are distributed across predictor range, denser marks indicate a concentration of predictor values.
Owls nest box occupancy (%) decreased in 1980–2013 (Fig. 6, t-value = −5.4, p < 0.001, dfresidual = 32). Concurrently, the number of infected voles per cycle was higher in the 2003–2013 time-frame compared to that in 1979–1986. This difference was most evident in spring (Fig. 6a). In 1979–1986, there were 413 infected bank voles (206.5 per cycle), whereas there were 942 infected in 2003–2013 (314 per cycle). Also, mean prevalence in spring was 7% higher in 2003–2013 than in 1979–1986.
Figure 6.
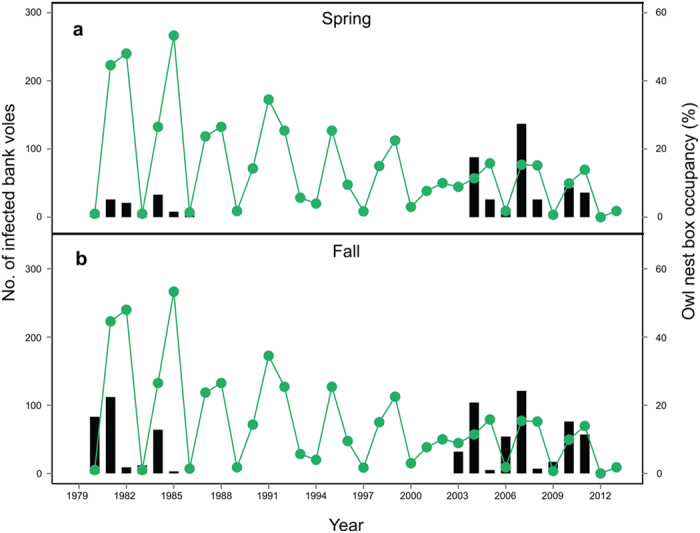
The number of infected bank voles (bars and left-hand y-axis) in (a) spring and (b) fall in two time periods: 1979–1986 and 2003–2013 and Tengmalm’s owl nest box occupancy (%) (line and right-hand y-axis) in spring in 1980–2013.
Discussion
As far as we know, this study is the first to investigate PUUV dilution by non-host small mammals through species-specific hypotheses. The probability of infection in a bank vole decreased with increasing common shrew density index (Figs 2 and 3). In addition, the field vole affected PUUV prevalence indirectly by suppressing bank vole density index in fall in meadows and clear-cuts. The decrease in nest box occupancy of Tengmalm’s owl during the past three decades was concurrent with an increase in overall density of infected voles and prevalence in spring in the 2003–2013 time-frame compared to that in 1979–1986. Our study thus found evidence for the dilution effect by two non-host species, and suggested that Tengmalm’s owls are important in reducing PUUV infection in bank voles.
Our results are part of a growing corpus of evidence for the dilution of hantavirus infection in a range of new and old world hantavirus-host systems. For example, in an experimental study in Panama, both infection prevalence and host density increased when small mammal diversity was reduced43. In the United States, Dizney and Dearing44 found that hosts of Sin Nombre hantavirus in more diverse sites spent less time engaged in behaviors related to pathogen transmission and were less likely to be infected. Similar results were found in Argentina in an observational study, as host individuals infected with Andes hantavirus were more likely to be found near human dwellings where small mammal diversity was low64. In Europe, Voutilainen et al.41 found evidence for the dilution of PUUV infection in bank voles through pooling densities of non-host small mammals. Here, by studying the potential of common shrews and field voles to influence PUUV infection in bank voles independently, we were able to infer mechanisms and conditions that promote dilution of PUUV.
The common shrew is found in a wide range of habitats55. It is smaller and competitively inferior than the bank vole48,65. They are unlikely to regulate bank vole densities and we found that the two species densities were positively related (Figs 4b and 5d). Correlated changes in density indices were expected due to the synchronous population fluctuations of small mammals regionally48. Nevertheless, dilution through “encounter reduction” reduces infection in host populations irrespective of host density47. In an experimental study, the presence of common shrews changed bank vole behavior, resulting in lactating females visiting fewer supplementary feeding stations56. Common shrews are opportunistic predators and may prey on vole nestlings, and the two species share above ground runways and tunnels54. As a response to risk, bank voles may avoid common shrews and increase time spent protecting nestlings. Ultimately, we expect that a reduction in movement of infected voles limited the spatial scale of PUUV shedding and number of encounters with susceptible voles. In North America, the short-tailed shrew (Blarina brevicauda) restricts spatial use of the meadow vole (Microtus pennsylvanicus)66 and may prey on it67. Dilution of PUUV through encounter reduction was also reported from Western Europe. In Belgium, PUUV prevalence in bank voles was lower when non-host wood mouse (Apodemus sylvaticus) density increased relative to bank voles42. Further, direct evidence for encounter reduction came from the Sin Nombre hantavirus system. Based on an experimental setup, Clay et al.47 reported that contact rates among hosts declined when non-host diversity increased.
Alternatively, competition can indirectly reduce infection prevalence by reducing host density. PUUV prevalence increased with bank vole density index in spring and fall (Figs 3 and 4), likely due to accelerated density-dependent transmission68. Nevertheless, infection prevalence was higher in spring than in fall despite fall density indices being higher. This is probably due to the influx of uninfected newborn voles into the population, which masks the increase in density-dependent transmission69. Field voles suppressed bank vole density in meadows and clear-cuts in fall (Fig. 5a), when bank vole density is often highest. In the reproductive season, field vole populations reach peak densities after bank voles48. Competition between the two species was most likely space-driven after reproduction70 and we detected interference competition by field voles only in our fall data (Table 1). Also, winter survival in field voles has declined, leading to lower spring densities28,52 and thus reduced spring competition between the two species. Bank voles may reach high densities in meadows71, but interference competition from field voles limits bank vole density53, and thereby PUUV transmission. Only in core field vole habitats - where field voles are more abundant than in other habitat types70 - bank vole density index declined as that of the field vole increased (Fig. 5a). Because field voles also alter bank vole behavior53, we expected field voles to also directly reduce PUUV infection in bank voles in meadows and clear-cuts. But we found no evidence for dilution through “encounter reduction” (Table S1, models 1 and 2). We speculate that space-driven interference competition occurred for a limited time-period after reproduction, outside of which bank vole behavior, encounter rates, and PUUV transmission were not sufficiently altered to be reflected in PUUV infection rates.
In meadows and clear-cuts and intermediate-aged forests in spring and in intermediate and old-aged forests in fall, bank voles and field vole density indices were positively related. Bank vole and common shrew density indices were correlated irrespective of habitat type or season. Fairly synchronous fluctuations in density are typical of cyclic small mammals in northern Fennoscandia27,48, suggesting common external drivers such as predators and food availability that synchronize fluctuations of small mammal species29, ultimately overwhelming competitive interactions. The negative relationship between field vole and bank vole density indices in meadows and clear-cuts in fall despite the synchronizing forces acting on the different species strengthens the evidence for “susceptible host regulation” hypothesis.
Tengmalm’s owls nest box occupancy declined in 1980–2013. Out of the three vole species that constituted >90% of Tengmalm’s owl diet, i.e. bank vole, field vole, and grey-sided vole57, only bank vole density index increased in the 2000’s (Figure S1). PUUV prevalence and infected bank vole density index in spring were higher in 2003–2013 compared to 1979–1986 (Fig. 6). We hypothesize that low field vole and grey-sided vole density indices contributed to Tengmalm’s owl persistent low numbers72. The negative relationship between PUUV prevalence (and number of infected bank voles) and owl decline suggests that Tengmalm’s owls may limit infection in bank vole populations. However, this relationship merits further investigation at spatially appropriate scales.
The study area is heavily managed by forestry52,73 with a species-poor small mammal community27. The drastic decline of the grey-sided vole51, driven by habitat loss52, probably released the bank vole from competition in forest habitats and allowed the latter to expand its niche (sensu50, Fig. 1). The decline in field voles, to which climate change was suggested to contribute28,74 may further increase utilization of meadows and clear-cuts by bank voles (Fig. 5). Competitive release of bank voles in new habitats may be associated with higher density and PUUV prevalence, especially in places where virus survival outside the host or transmission may be enhanced due to micro-habitat properties41. Identification of micro-habitat factors, e.g. resource distribution and structural and physical properties would facilitate predicting PUUV dynamics in habitats where the bank vole replaces its competitors.
Our results are based on long-term time series collected systematically, over a large area with plots 2.5 km apart. This enabled us to test the dilution effect at the mechanistically important local (plot) scale, while accounting for habitat differences. It is at the plot level where changes in bank vole density and behavior are expected to affect PUUV infection within populations. Also, the simple system with directly transmitted pathogen and few non-host small mammal species enabled us to include density indices of non-host species rather than species richness. Nevertheless, our inferences of dilution mechanisms were based on observational data. Experimental testing in large enclosures is needed to establish a direct link between behavioral and density changes in bank voles (e.g. refs 53, 75 and 76) to changes in transmission rates. For example, experimental work on the dilution effect is ongoing in the United States on Sin Nombre virus system (reviewed by ref. 77).
We highlighted the role of non-host species in directly and indirectly reducing PUUV infection prevalence in bank voles. We found evidence for the dilution effect by a competitor (field vole) that conditionally regulated bank vole density indices thereby indirectly reducing PUUV infection, and a nest predator (common shrew) that directly influenced bank vole infection probability. The long-term decline in Tengmalm’s owls coincided with a general increase in density indices and infection prevalence in bank voles in 2003–2013, and thus higher number of infected voles (Fig. 6). The increase in infected bank voles, including our study period 2003–2013, points to an increasing human risk in Northern Sweden. Our results provide evidence for the importance of functional diversity in a given community in reducing pathogen infection in hosts. Landscape and climate changes may increase risk of hantavirus infections in humans, especially if a generalist (here the bank vole) dominates when its competitors and predators decline.
Materials and Methods
Small mammal and habitat data
Small mammal data in 1971–2013 was available through the ongoing Swedish National Environmental Monitoring Program for small rodents, initiated in 1971 around Umeå in northern Sweden (64° N, 20° E)27. The area belongs to the middle boreal zone78. Within a 100 ×100 km area, trapping of small mammals takes place twice a year in 58 systematically placed 1-ha plots of at least 2.5 km inter-distance. Spring trapping is in late May whereas fall trapping is in late September. Each 1-ha plot is trapped for three nights along a 90 m line with 10 trapping stations. Each station has five snap traps placed within a 1 m radius circle. The total trapping effort was 150 trap nights per plot (see refs 27 and 28 for further details). For each species, a density index was calculated as number of individuals per 100 trap nights.
We characterized sampling plots in 2012–2013 according to habitat type and three forest succession stages: meadows and clear-cuts <20 years (n = 12), young and intermediate-aged forest 20–80 years (n = 24), and old forest >80 years (n = 14). Two sampling plots were on meadows dominated by grasses in the field layer; for small mammals a habitat type often functionally similar to clear-cuts79. The dominant forest age class along the trapping line was used in the analyses and forest age was estimated by increment coring at breast height combined with visual observations.
This study, including small mammal and owl monitoring, was approved by the Animal Ethics Committee in Umeå (Dnr A 11–14, A 12–14 and A 13–14), and all applicable institutional and national guidelines for the use of animals were followed.
Owl data
Data on Tengmalm’s owls breeding was collected since 1980 from nest boxes placed in trees at approximately 1 km interval in an area partially overlapping with the small mammal monitoring area57. We used nest box occupancy data in 1980–2013. The number of nest boxes checked per year varied and ranged between 275 and 50058,80. Breeding attempts were confirmed through systematic visits in spring. Tengmalm’s owl reproduction is largely dependent on vole density57 and is reflected in annual variation in box occupancy by breeding owls. Nest box occupancy was calculated as the percentage of boxes occupied.
Hantavirus infection data
In this study we focused on the 2003–2013 infection data, published for the first time, while we used available infection data in 1979–1986 (n = 2064 bank voles31,81) for comparison.
We analyzed lung samples from bank voles by enzyme-linked immunosorbent assay (ELISA) to detect anti-PUUV IgG antibodies and identify sero-positive individuals31,82. Sero-positivity points to an ongoing infection in bank voles since shedding of PUUV is life-long83. Thus, we use the term infected rather than sero-positive throughout this paper. Bank voles weighing <14.4 g may carry maternal antibodies41,84 and were excluded (n = 866) from further analyses since their sero-positivity may not reflect genuine infection. In subsequent analyses, PUUV infection data from 4169 bank voles in 2003–2013 was used.
Statistical analyses
Bank vole dominance
To confirm that bank voles have increased in proportion relative to other small mammals, we calculated the percentage of bank voles relative to total number of small mammals (% bank voles) in spring and fall. The time series of percentage of bank voles showed temporal autocorrelation in both seasons. We hence fitted a generalized least square model with a temporal autocorrelation structure (maximum lag = 3 in spring and 2 in fall) to % bank voles over time in 1971–2013.
Encounter reduction
We tested whether PUUV infection probability in bank voles in spring and fall (2003–2013) at local plot level was affected by common shrew and field vole density indices. Also, several studies found hantavirus prevalence to increase with host density (e.g. refs 31, 40, 85 and 86, which is common in horizontally-transmitted pathogens68. So we included bank vole density index as a predictor of infection probability at plot level in the analysis. We also included local habitat (meadows and clear-cuts, young and intermediate-aged forest, and old forest) since habitat influences PUUV dynamics (e.g. refs 41 and 81). Probability of PUUV infection often increases with weight, a surrogate of bank vole age (e.g. refs 40 and 87), so weight (g) was also used as a predictor.
We fitted a generalized linear mixed effects model with a binomial error distribution to predict the probability of a bank vole being infected. Models for spring and fall were fitted independently as there was little overlap between the two seasons in the ranges of predictors (density index and weight). The response was binary: infected versus uninfected. Candidate fixed effects were bank vole density index, common shrew density index, field vole density index, bank vole weight (g), and habitat. Plot identity and year were included as random effects. We did not have data on bank vole sex, hence we could not test for sex differences in infection probability. Often, males are more likely to be infected with hantaviruses than females40,88. However, we do not expect sex differences in infection probability to influence our results in relation to the dilution effect.
Susceptible host regulation
We found that PUUV infection probability increased with bank vole density index (results). Hence, we investigated whether the common shrew and the field vole indirectly reduced PUUV infection by regulating bank vole density index at plot level. Also, we included bank vole density index in the previous year as a predictor to account for delayed-density dependence27,28. We included the interaction between habitat on one hand and field vole density indicex on the other to account for differences in interaction outcomes at different forest succession stages (meadows and clear-cuts, young and intermediate-aged forests, and old forests). Hence, we fitted a generalized linear mixed effects model with a poisson distribution error with bank vole density index as response variable. Candidate predictors were field vole density index, common shrew density index, previous bank vole density index (Yeart-1), habitat, and the interaction between habitat and field density indices. Plot identity and year were included as random effects.
Tengmalm’s owl nest boxes did not entirely overlap with bank vole trapping areas. We thus did not formally test the relationship between nest box occupancy (%) and PUUV infection in bank voles. However, we discuss how the temporal patterns in owl occupancy (%) in 1980–2013 were related to changes in bank vole PUUV infection between 1979–1986 and 2003–2013. The infection data from 1979–1986 covered two vole cycles whereas 2003–2013 infection data covered three cycles. We fitted a generalized least square model with temporal autocorrelation (maximum lag = 3) to the time series of owl nest box occupancy to determine if it declined. We related the temporal change in nest box occupancy to PUUV prevalence and number of infected voles per cycle between the two different time periods (1979–1986 versus 2003–2013).
All analyses were performed in R using the “nlme”89 and “lme4”90 packages in R91. All models were checked for violations of assumptions and correlation among explanatory variables. Model residuals were checked for patterns to investigate model fit. Selection of best models was based on AICc criteria. If two or more models had a ∆AICc < 2, only significant predictors were included. Significance was assumed below a probability p < 0.05.
Additional Information
How to cite this article: Khalil, H. et al. Declining ecosystem health and the dilution effect. Sci. Rep. 6, 31314; doi: 10.1038/srep31314 (2016).
Supplementary Material
Acknowledgments
We thank Åke Nordström for his help surveying the nest boxes and trapping small mammals. This study was funded by the Swedish Research Council Formas (grant no. 221-2012-1568) http://www.formas.se/. The study was also supported by The Swedish Natural Science Research Council, Stiftelsen Seth M. Kempes Minne, Olle och Signhild Engkvists Stiftelser, the Swedish Environmental Protection Agency, Helge Ax.son Johnsons Stiftelse and Alvins fond.
Footnotes
Author Contributions H.K., B.H. and F.E. conceptualized the study. B.H., F.E., M.M. and H.K. collected the data. H.K. and M.E. analyzed the samples. H.K. analyzed the data and wrote the manuscript. B.H., F.E., M.M. and M.E. provided comments on the manuscript.
References
- Baillie J. E. M., Hilton Taylor C., Stuart S. N. (eds). 2004 IUCN red list of threatened species: a global species assessment. (IUCN–The World Conservation Union, 2004). [Google Scholar]
- Patz J. A. et al. Unhealthy Landscapes: Policy Recommendations on Land Use Change and Infectious Disease Emergence. Environ. Health Perspect. 112, 1092–1098 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld R. S. & Keesing F. Biodiversity series: The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. Zool. 78, 2061–2078 (2000). [Google Scholar]
- LoGiudice K., Ostfeld R. S., Schmidt K. A. & Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. 100, 567–571 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L. H., Latham S. M. & Woolhouse M. E. J. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356, 983–989 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse M. & Gaunt E. Ecological Origins of Novel Human Pathogens. Crit. Rev. Microbiol. 33, 231–242 (2007). [DOI] [PubMed] [Google Scholar]
- Johnson P. T. J., Ostfeld R. S. & Keesing F. Frontiers in research on biodiversity and disease. Ecol. Lett. 18, 1119–1133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitello D. J. et al. Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc. Natl. Acad. Sci. 112, 8667–8671 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K. A. & Ostfeld R. S. Biodiversity and the Dilution Effect in Disease Ecology. Ecology 82, 609–619 (2001). [Google Scholar]
- Johnson P. T. J., Preston D. L., Hoverman J. T. & Richgels K. L. D. Biodiversity decreases disease through predictable changes in host community competence. Nature 494, 230–233 (2013). [DOI] [PubMed] [Google Scholar]
- Huang Z. Y. X. et al. Dilution effect in bovine tuberculosis: risk factors for regional disease occurrence in Africa. Proc. R. Soc. Lond. B Biol. Sci. 280, 20130624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. E., Tilman D. & Groth J. V. Effects of Grassland Plant Species Diversity, Abundance, and Composition on Foliar Fungal Disease. Ecology 83, 1713–1726 (2002). [Google Scholar]
- Mills J. N. Biodiversity loss and emerging infectious disease: An example from the rodent-borne hemorrhagic fevers. Biodiversity 7, 9–17 (2006). [Google Scholar]
- Han B. A., Schmidt J. P., Bowden S. E. & Drake J. M. Rodent reservoirs of future zoonotic diseases. Proc. Natl. Acad. Sci. 112, 7039–7044 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph S. E. & Dobson A. D. M. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology 139, 847–863 (2012). [DOI] [PubMed] [Google Scholar]
- Salkeld D. J., Padgett K. A. & Jones J. H. A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol. Lett. 16, 679–686 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld R. S. A Candide response to Panglossian accusations by Randolph and Dobson: biodiversity buffers disease. Parasitology 140, 1196–1198 (2013). [DOI] [PubMed] [Google Scholar]
- Lafferty K. D. & Wood C. L. It’s a myth that protection against disease is a strong and general service of biodiversity conservation: Response to Ostfeld and Keesing. Trends Ecol. Evol. 28, 503–504 (2013). [DOI] [PubMed] [Google Scholar]
- Levi T. et al. Does biodiversity protect humans against infectious disease? Comment. Ecology 97, 536–542 (2016). [DOI] [PubMed] [Google Scholar]
- Peixoto I. D. & Abramson G. The Effect of Biodiversity on the Hantavirus Epizootic. Ecology 87, 873–879 (2006). [DOI] [PubMed] [Google Scholar]
- Vaheri A. et al. Hantavirus infections in Europe and their impact on public health: Hantavirus infections in Europe. Rev. Med. Virol. 23, 35–49 (2013). [DOI] [PubMed] [Google Scholar]
- Klingström J. et al. Rodent host specificity of European hantaviruses: Evidence of Puumala virus interspecific spillover. J. Med. Virol. 68, 581–588 (2002). [DOI] [PubMed] [Google Scholar]
- Brummer-Korvenkontio M. et al. Nephropathia Epidemica: Detection of Antigen in Bank Voles and Serologic Diagnosis of Human Infection. J. Infect. Dis. 141, 131–134 (1980). [DOI] [PubMed] [Google Scholar]
- Mitchell-Jones T., etc, Amori G., Bogdanowicz W. et al.The Atlas of European Mammals. (Poyser, 1999). [Google Scholar]
- Henttonen H., Kaikusalo A., Tast J. & Viitala J. Interspecific Competition between Small Rodents in Subarctic and Boreal Ecosystems. Oikos 29, 581–590 (1977). [Google Scholar]
- Hörnfeldt B. Synchronous population fluctuations in voles, small game, owls, and tularemia in northern Sweden. Oecologia 32, 141–152 (1978). [DOI] [PubMed] [Google Scholar]
- Hörnfeldt B. Delayed Density Dependence as a Determinant of Vole Cycles. Ecology 75, 791–806 (1994). [Google Scholar]
- Hörnfeldt B. Long-term decline in numbers of cyclic voles in boreal Sweden: analysis and presentation of hypotheses. Oikos 107, 376–392 (2004). [Google Scholar]
- Hansson L. & Henttonen H. Gradients in density variations of small rodents: the importance of latitude and snow cover. Oecologia 67, 394–402 (1985). [DOI] [PubMed] [Google Scholar]
- Hardestam J. et al. Puumala Hantavirus Excretion Kinetics in Bank Voles (Myodes glareolus). Emerg. Infect. Dis. 14, 1209–1215 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklasson B., Hörnfeldt B., Lunkvist Å., Björsten S. & LeDuc J. Temporal dynamics of Puumala virus antibody prevalence in voles and of nephropathia epidemica incidence in humans. Am. J. Trop. Med. Hyg. 53, 134–140 (1995). [DOI] [PubMed] [Google Scholar]
- Kallio E. R. et al. Cyclic hantavirus epidemics in humans — Predicted by rodent host dynamics. Epidemics 1, 101–107 (2009). [DOI] [PubMed] [Google Scholar]
- Tersago K. et al. Hantavirus outbreak in Western Europe: reservoir host infection dynamics related to human disease patterns. Epidemiol. Infect. 139, 381–390 (2010). [DOI] [PubMed] [Google Scholar]
- Khalil H. et al. The Importance of Bank Vole Density and Rainy Winters in Predicting Nephropathia Epidemica Incidence in Northern Sweden. PLoS ONE 9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reil D., Imholt C., Eccard J. A. & Jacob J. Beech Fructification and Bank Vole Population Dynamics - Combined Analyses of Promoters of Human Puumala Virus Infections in Germany. PLoS ONE 10, e0134124 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson G. E., Hjertqvist M., Lundkvist Å. & Hörnfeldt B. Predicting High Risk for Human Hantavirus Infections, Sweden. Emerg. Infect. Dis. 15, 104–106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil H. et al. Dynamics and Drivers of Hantavirus Prevalence in Rodent Populations. Vector-Borne Zoonotic Dis. 14, 537–551 (2014). [DOI] [PubMed] [Google Scholar]
- Keesing F., Holt R. D. & Ostfeld R. S. Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498 (2006). [DOI] [PubMed] [Google Scholar]
- Ostfeld R. S., Keesing F. & Eviner V. T. Infectious Disease Ecology: Effects of Ecosystems on Disease and of Disease on Ecosystems. (Princeton University Press, 2010). [Google Scholar]
- Olsson G. E. et al. Hantavirus antibody occurrence in bank voles (Clethrionomys glareolus) during a vole population cycle. J. Wildl. Dis. 39, 299–305 (2003). [DOI] [PubMed] [Google Scholar]
- Voutilainen L. et al. Environmental Change and Disease Dynamics: Effects of Intensive Forest Management on Puumala Hantavirus Infection in Boreal Bank Vole Populations. PLoS ONE 7, e39452 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersago K. et al. Population, Environmental and Community Effects on Local Bank Vole (Myodes glareolus) Puumala Virus Infection in an Area with Low Human Incidence. Vector-Borne Zoonotic Dis. 8, 235–244 (2008). [DOI] [PubMed] [Google Scholar]
- Suzán G. et al. Experimental Evidence for Reduced Rodent Diversity Causing Increased Hantavirus Prevalence. PLoS ONE 4, e5461 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizney L. & Dearing M. D. Behavioural differences: a link between biodiversity and pathogen transmission. Anim. Behav. 111, 341–347 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver S. et al. A temporal dilution effect: hantavirus infection in deer mice and the intermittent presence of voles in Montana. Oecologia 166, 713–721 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruedas L. A. et al. Community ecology of small mammal populations in Panama following an outbreak of Hantavirus pulmonary syndrome. J. Vector Ecol. 29, 177–191 (2004). [PubMed] [Google Scholar]
- Clay C. A., Lehmer E. M. St. Jeor S. & Dearing M. D. Testing Mechanisms of the Dilution Effect: Deer Mice Encounter Rates, Sin Nombre Virus Prevalence and Species Diversity. EcoHealth 6, 250–259 (2009). [DOI] [PubMed] [Google Scholar]
- Huitu O., Norrdahl K. & Korpimäki E. Competition, predation and interspecific synchrony in cyclic small mammal communities. Ecography 27, 197–206 (2004). [Google Scholar]
- Ecke F., Löfgren O. & Sörlin D. Population dynamics of small mammals in relation to forest age and structural habitat factors in northern Sweden. J. Appl. Ecol. 39, 781–792 (2002). [Google Scholar]
- Löfgren O. Niche Expansion and Increased Maturation Rate of Clethrionomys glareolus in the Absence of Competitors. J. Mammal. 76, 1100–1112 (1995). [Google Scholar]
- Hörnfeldt B., Christensen P., Sandström P. & Ecke F. Long-term decline and local extinction of Clethrionomys rufocanus in boreal Sweden. Landsc. Ecol. 21, 1135–1150 (2006). [Google Scholar]
- Magnusson M., Hörnfeldt B. & Ecke F. Evidence for different drivers behind long-term decline and depression of density in cyclic voles. Popul. Ecol. 57, 569–580 (2015). [Google Scholar]
- Eccard J. A. & YlöNen H. Costs of coexistence along a gradient of competitor densities: an experiment with arvicoline rodents. J. Anim. Ecol. 76, 65–71 (2007). [DOI] [PubMed] [Google Scholar]
- Liesenjohann M. et al. From interference to predation: type and effects of direct interspecific interactions of small mammals. Behav. Ecol. Sociobiol. 65, 2079–2089 (2011). [Google Scholar]
- Hanski I. & Kaikusalo A. Distribution and habitat selection of shrews in Finland. Ann. Zool. Fenn. 26, 339–348 (1989). [Google Scholar]
- Liesenjohann T. et al. State-dependent foraging: lactating voles adjust their foraging behavior according to the presence of a potential nest predator and season. Behav. Ecol. Sociobiol. 69, 747–754 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörnfeldt B., Carlsson B.-G., Löfgren O. & Eklund U. Effects of cyclic food supply on breeding performance in Tengmalm’s owl (Aegolius funereus). Can. J. Zool. 68, 522–530 (1990). [Google Scholar]
- Hipkiss T., Gustafsson J., Eklund U. & Hörnfeldt B. Is the Long-term Decline of Boreal Owls in Sweden Caused by Avoidance of Old Boxes? J. Raptor Res. 47, 15–20 (2013). [Google Scholar]
- Ostfeld R. S. & Holt R. D. Are predators good for your health? Evaluating evidence for top-down regulation of zoonotic disease reservoirs. Front. Ecol. Environ. 2, 13–20 (2004). [Google Scholar]
- Levi T., Kilpatrick A. M., Mangel M. & Wilmers C. C. Deer, predators, and the emergence of Lyme disease. Proc. Natl. Acad. Sci. 109, 10942–10947 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpimäki E. Rapid Tracking of Microtine Populations by Their Avian Predators: Possible Evidence for Stabilizing Predation. Oikos 45, 281–284 (1985). [Google Scholar]
- Korpimäki E. & Norrdahl K. Predation of Tengmalm’s Owls: Numerical Responses, Functional Responses and Dampening Impact on Population Fluctuations of Microtines. Oikos 54, 154–164 (1989). [Google Scholar]
- Ecke F., Christensen P., Sandström P. & Hörnfeldt B. Identification of Landscape Elements Related to Local Declines of a Boreal Grey-sided Vole Population. Landsc. Ecol. 21, 485–497 (2006). [Google Scholar]
- Piudo L., Monteverde M. J., Walker R. S. & Douglass R. J. Rodent Community Structure and Andes Virus Infection in Sylvan and Peridomestic Habitats in Northwestern Patagonia, Argentina. Vector-Borne Zoonotic Dis. 11, 315–324 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henttonen H. et al. Long-term population dynamics of the common shrew Sorex araneus in Finland. Ann. Zool. Fenn. 26, 349–355 (1989). [Google Scholar]
- Fulk G. W. The Effect of Shrews on the Space Utilization of Voles. J. Mammal. 53, 461–478 (1972). [Google Scholar]
- Martinsen D. L. Energetics and Activity Patterns of Short-Tailed Shrews (Blarina) on Restricted Diets. Ecology 50, 505–510 (1969). [Google Scholar]
- Anderson R. M. & May R. M. Population biology of infectious diseases: Part I. Nature 280, 361–367 (1979). [DOI] [PubMed] [Google Scholar]
- Roche B., Dobson A. P., Guégan J.-F. & Rohani P. Linking community and disease ecology: the impact of biodiversity on pathogen transmission. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 2807–2813 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllymäki A. Interactions between the Field Vole Microtus agrestis and Its Microtine Competitors in Central-Scandinavian Populations. Oikos 29, 570–580 (1977). [Google Scholar]
- Ylönen H., Kojola T. & Viitala J. Changing female spacing behaviour and demography in an enclosed breeding population of Clethrionomys glareolus. Ecography 11, 286–292 (1988). [Google Scholar]
- Hörnfeldt B., Hipkiss T. & Eklund U. Fading out of vole and predator cycles? Proc. R. Soc. Lond. B Biol. Sci. 272, 2045–2049 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecke F., Magnusson M. & Hörnfeldt B. Spatiotemporal changes in the landscape structure of forests in northern Sweden. Scand. J. For. Res. 28, 651–667 (2013). [Google Scholar]
- Cornulier T. et al. Europe-Wide Dampening of Population Cycles in Keystone Herbivores. Science 340, 63–66 (2013). [DOI] [PubMed] [Google Scholar]
- Ylönen H., Mappes T. & Viitala J. Different demography of friends and strangers: an experiment on the impact of kinship and familiarity in Clethrionomys glareolus. Oecologia 83, 333–337 (1990). [DOI] [PubMed] [Google Scholar]
- Sundell J., Eccard J. A., Tiilikainen R. & Ylönen H. Predation rate, prey preference and predator switching: experiments on voles and weasels. Oikos 101, 615–623 (2003). [Google Scholar]
- Dearing M. D., Clay C., Lehmer E. & Dizney L. The roles of community diversity and contact rates on pathogen prevalence. J. Mammal. 96, 29–36 (2015). [Google Scholar]
- Ahti T., Hämet-Ahti L. & Jalas J. Vegetation zones and their sections in northwestern Europe. Ann. Bot. Fenn. 5, 169–211 (1968). [Google Scholar]
- Hansson L. Spatial Dynamics of Field Voles Microtus agrestis in Heterogeneous Landscapes. Oikos 29, 539–544 (1977). [Google Scholar]
- Löfgren O., Hörnfeldt B. & Carlsson B.-G. Site tenacity and nomadism in Tengmalm’s owl (Aegolius funereus (L.)) in relation to cyclic food production. Oecologia 69, 321–326 (1986). [DOI] [PubMed] [Google Scholar]
- Magnusson M. et al. Spatial and temporal variation of hantavirus bank vole infection in managed forest landscapes. Ecosphere 6, 1–18 (2015). [Google Scholar]
- Lindkvist M., Näslund J., Ahlm C. & Bucht G. Cross-reactive and serospecific epitopes of nucleocapsid proteins of three hantaviruses: Prospects for new diagnostic tools. Virus Res. 137, 97–105 (2008). [DOI] [PubMed] [Google Scholar]
- Voutilainen L. et al. Life-long shedding of Puumala hantavirus in wild bank voles (Myodes glareolus). J. Gen. Virol. 96, 1238–1247 (2015). [DOI] [PubMed] [Google Scholar]
- Kallio E. R. Prolonged survival of Puumala hantavirus outside the host: evidence for indirect transmission via the environment. J. Gen. Virol. 87, 2127–2134 (2006). [DOI] [PubMed] [Google Scholar]
- Adler F. R., Pearce-Duvet J. M. C. & Dearing M. D. How Host Population Dynamics Translate into Time-Lagged Prevalence: An Investigation of Sin Nombre Virus in Deer Mice. Bull. Math. Biol. 70, 236–252 (2007). [DOI] [PubMed] [Google Scholar]
- Carver S., Trueax J. T., Douglass R. & Kuenzi A. Delayed density-dependent prevalence of sin nombre virus infection in deer mice (Peromyscus maniculatus) in central and western montana. J. Wildl. Dis. 47, 56–63 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersago K., Crespin L., Verhagen R. & Leirs H. Impact of Puumala virus infection on maturation and survival in bank voles: a capture-mark-recapture analysis. J. Wildl. Dis. 48, 148–156 (2012). [DOI] [PubMed] [Google Scholar]
- Kallio E. R. et al. Hantavirus infections in fluctuating host populations: the role of maternal antibodies. Proc. R. Soc. B Biol. Sci. 277, 3783–3791 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., DebRoy S., Sarkar D. & Core Team R. Nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-122. at http://CRAN.R-project.org/package=nlme (2015).
- Bates D. et al. lme4: Linear Mixed-Effects Models using ‘Eigen’ and S4. at https://cran.r-project.org/web/packages/lme4/index.html (2015).
- R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/ (2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.