Abstract
Bile-resistant bacteria, particularly gram-positive Enterococcus faecalis and Enterococcus faecium, play an important role in biliary stent occlusion, because their sessile mode of growth protects them against host defenses and antimicrobial agents. Twelve E. faecalis and seven E. faecium strains isolated from occluded biliary stents have been investigated for slime production, presence of aggregation substance genes, and ability to adhere to Caco-2 cells. Ten isolates were strong producers of slime, and seven isolates produced clumps when exposed to pheromones of E. faecalis JH2-2 and/or OG1RF. The small E. faecium clumps differed from the large clumps of E. faecalis and were similar to those of E. faecium LS10(pBRG1) carrying a pheromone response plasmid. After induction with pheromones, the adhesion to Caco-2 cells of clumping-positive strains was found to increase from two- to fourfold. Amplicons of the expected size were detected in three clumping-positive and three clumping-negative E. faecalis isolates by using primers (agg) internal to a highly conserved region of the E. faecalis pheromone response plasmids pAD1, pPD1, and pCF10 and primers internal to prgB of the E. faecalis plasmid pCF10. The agg/prgB-positive E. faecalis strains were also positive in Southern hybridization experiments with a prgB-specific probe. No PCR products were obtained with the same primers from four clumping-positive isolates (one E. faecalis and three E. faecium strains), which were also Southern hybridization negative. Our results demonstrate that slime production and pheromone response are both present in isolated enterococci, suggesting that clinical strains with these features might have a selective advantage in colonizing biliary stents.
In patients with obstructive jaundice, decompression of an occluded common bile duct can be palliated by using endoscopic stenting, which represents a valid alternative to surgery in inoperable tumors, with a resultant regression of jaundice and perhaps an improvement in the quality of life. Stent insertion presents a low incidence of morbidity and mortality, with a markedly reduced hospital stay compared to that after surgery. However, a frequent complication is the stent occlusion caused by bacterial-biofilm formation and sludge accumulation, which requires removal of the device with increased risk for the patient and additional health care costs. It has been observed that Enterococcus faecalis and Enterococcus faecium are the prevalent bacterial species involved (2).
Enterococci are gram-positive bacteria that normally inhabit the gastrointestinal tracts of many animals, including humans. They are opportunistic bacteria that become pathogens when they colonize niches where they are not normally found. Enterococci are a major cause of nosocomial infections and are increasingly detected in bloodstream and urinary tract infections and in infected surgical sites. E. faecalis is responsible for ∼80 to 90% of all enterococcal infections, and E. faecium accounts for most of the others; the pathogenesis of enterococcal infections is poorly understood, but several virulence factors have been proposed (14).
Enterococci are also known to produce slime, an amorphous extracellular substance found to be polysaccharidic in nature, which is one of the major components of bacterial biofilm. In fact, a biofilm is presently defined as a microbial sessile community characterized by cells that are attached to a substrate and/or to each other and that produce a “glycocalyx” matrix in which they are embedded. Thus, slime production is closely related to biofilm establishment, and it can be used as a measurement of biofilm formation. Slime production and biofilm formation have been suggested as virulence determinants of clinical isolates (2, 7, 28). In previous studies, Baldassarri and coworkers have found no relation of slime production to the source of isolation for E. faecalis, while in E. faecium, slime production was more frequent in clinical isolates than in environmental strains or isolates from healthy individuals (1). Enterococci are known for their acquired multidrug resistance, and the recent emergence of vancomycin-resistant strains has generated major concern among clinicians (4, 18). Enterococci harbor transferable genetic elements, which have an unusually broad host range and can even be transferred to both gram-negative and gram-positive bacterial species (6). Conjugation systems involving plasmids and transposons are abundant in these organisms and contribute to the dissemination of both antibiotic resistance and virulence factors (6). In particular, the sex pheromone system of E. faecalis, first described by Dunny et al. (9), involves the production of pheromones by recipient strains, each being specific for a particular plasmid or a group of related plasmids (11). Donor strains exposed to a pheromone are induced to synthesize a specific plasmid-encoded surface protein, designated “aggregation substance” (AS), which facilitates the initiation of mating pair formation (5). In E. faecalis, four plasmids (pAD1, pCF10, pPD1, and pAM373) encoding AS have been described (6), AS-encoding genes have been sequenced, and highly conserved regions have been used to generate primers specific for AS (12). Sex pheromone plasmids have also been described in E. faecium, in which they have been found to be associated with vancomycin resistance (15, 16, 23).
Many enterococcal infections are endogenous, originating from the intestinal tract, and several experimental data suggest that AS may be involved in translocation across the intestinal barrier (14, 29). AS promotes enterococcal adhesion to and internalization into cultured human cells (21, 23, 26) either directly or simply by increasing the number of organisms taken up as a clump (C. M. Waters, C. L. Wells, and G. M. Dunny, Abstr. 6th Am. Soc. Microbiol. Conf. Streptococcal Genetics, abstr. 121, 2002). AS also promotes survival of E. faecalis in mouse peritoneal macrophages (13). The sequence of the structural gene for the pAD1-encoded AS revealed the presence of two Arg-Gly-Asp motifs (30), recognized by integrins, a family of eukaryotic receptors expressed on leukocytes, thrombocytes, and endothelial and various epithelial cells, including intestinal cells (19).
In this study, E. faecalis and E. faecium strains isolated from occluded biliary stents removed from patients with obstructive jaundice were investigated for the presence of AS genes and the ability to adhere to Caco-2 cells and to produce biofilms. The susceptibilities of isolates to various antibiotics were also evaluated.
MATERIALS AND METHODS
Bacterial strains.
A total of 19 clinical strains of enterococci (12 E. faecalis and seven E. faecium) were isolated from occluded biliary stents. Two strains of sex pheromone-producing enterococci (E. faecalis JH2-2 and E. faecalis OG1RF) and two strains containing pheromone-susceptible plasmids [E. faecalis OG1RF(pCF10) (10) and E. faecium LS10(pBRG1) (23)] were used as control strains.
Bacteria were grown in brain heart infusion (BHI) broth or agar (Oxoid Ltd., Basingstoke, United Kingdom). For the determination of slime production, strains were grown in tryptone soy broth (TSB; Oxoid) supplemented with 1% glucose (1).
Antibiotics and susceptibility testing.
Antibiotic resistance was detected by the Kirby-Bauer method according to standard procedures approved by the NCCLS in 2000. Antibiotic disks were obtained from Oxoid.
Slime production assay.
Biofilm formation was tested as previously described (1). Briefly, bacteria were grown overnight at 37°C with no shaking in 2 ml of TSB containing 1% glucose. The culture was diluted 1:10 in fresh TSB, and 200 μl of the suspension was used to inoculate sterile 96-well polystyrene microtiter plates (Corning Costar, Milan, Italy). After overnight incubation at 37°C in 5% CO2, the wells were washed three times in phosphate-buffered saline, dried in an inverted position for 1 h at 50 to 60°C, and stained with Hucker's crystal violet. After the staining, the optical densities (OD) of the biofilms were read at a wavelength of 570 nm by a spectrophotometer (Novapath Microplate Reader; Bio-Rad Laboratories Inc.). The slime index was defined as an estimate of the density of the biofilm generated by a culture with an OD at 600 nm of 0.5 [slime index = mean OD of the biofilm × (0.5/mean OD growth)].
Pheromones and clumping assay.
Pheromone-containing filtrates were prepared from strains of E. faecalis JH2-2 and OG1RF grown overnight in BHI broth at 37°C with shaking. Late-stationary-phase cultures were obtained by inoculating 1 ml of the overnight culture in 100 ml of fresh BHI broth and incubating it at 37°C with shaking to a final concentration of ∼5 × 108 bacteria per ml. The cultures were centrifuged at 7,000 × g for 10 min at 20°C, and the supernatants containing pheromones were then filtered through 0.45- and 0.22-μm-pore-size filters (Millipore, Milan, Italy). The filtrates were autoclaved at 121°C for 15 min and stored at 4°C prior to use. Clumping of clinical enterococci was evaluated by adding 20 μl of stationary-phase culture to 0.5 ml of pheromone-containing filtrate. Negative controls were prepared by replacing pheromone-containing filtrates with BHI broth. Samples were incubated at 37°C for 3 h with shaking, mounted on glass slides, and observed by phase-contrast microscopy.
Cell line.
Cells from the human colon carcinoma enterocyte-like cell line Caco-2 (ATCC HTB37) were routinely grown in 25-cm2 plastic tissue culture flasks (Corning Costar) and incubated at 37°C in a humidified atmosphere of 5% CO2 in air. The culture medium was Dulbecco's modified Eagle's medium (DMEM) containing 25 mM glucose, 4 mM l-glutamine, and 3.7 mg of sodium bicarbonate per ml (Euroclone, West York, United Kingdom) with 1% nonessential amino acids supplemented with 10% fetal bovine serum (Euroclone), 100 U of penicillin per ml, and 100 μg of streptomycin per ml.
Adhesion to Caco-2 cells.
Confluent cell monolayers were trypsinized, counted, and adjusted at a concentration of ∼2.5 × 105 per ml in DMEM. One milliliter of the cell suspension was dispensed into each 22-mm-diameter well of a 12-well tissue culture plate (Corning Costar) and incubated to obtain semiconfluent monolayers at 37°C. The cells were washed with unsupplemented DMEM prior to bacterial infection. A late-exponential-phase enterococcal culture was diluted 1:10 in 10 ml of a 1:1 mixture of pheromone-containing filtrates and fresh BHI broth and incubated at 37°C with shaking to exponential-phase growth (∼5 × 108 bacteria per ml). Then, 1 ml of the bacterial suspension, vortexed and diluted in DMEM, was added to each well at a multiplicity of infection of 160 bacteria per cell. The plates were then incubated for 1 h at 37°C in 5% CO2. After incubation, the wells were rinsed three times with phosphate-buffered saline and the cells were lysed with Triton X-100. The number of CFU of bacteria was determined after plating of suitable dilutions of the lysates on BHI agar and incubation for 24 to 36 h at 37°C. Bacterial adherence was quantified by determining the ratio of cell-associated CFU to the total CFU of the initial inoculum.
PCR and restriction analysis of PCR products.
For total DNA extraction, the strains were grown overnight in BHI broth containing 0.5% glycine at 37°C. One milliliter of overnight culture was centrifuged at 2,739 × g for 10 min, and the pellet was resuspended in 1 ml of salt-Tris-EDTA buffer containing 20% saccharose and 2.5 mg of lysozyme/ml. Samples were incubated at 37°C for 60 min and centrifuged at 18,000 × g for 3 min. The pellet was resuspended in 1 ml of lysis buffer and incubated at 60°C for 1 h. The temperature was then raised to 95°C for 10 min for proteinase K inactivation and DNA denaturation.
PCR amplifications were performed using the Gene Amp PCR System 2400 (Perkin-Elmer Cetus, Norwalk, Conn.). Primers (Table 1) included those reported by Eaton and Gasson (12) internal to highly conserved regions in the AS genes of pAD1, pPD1, and pCF10 (agg primers) and the primers internal to the AS structural gene prgB and the regulatory prgX gene of pCF10 (16).
TABLE 1.
PCR primers used in this study
| Gene target | Primer pair sequence | Positions | PCR product length (bp) | Reference |
|---|---|---|---|---|
| agg | 5′-AAGAAAAAGAAGTAGACCAAC | 601-621 | 1,553 | 12 |
| 5′-AAACGGCAAGACAAGTAAATA | 2133-2153 | |||
| prgB | 5′-ATACAAAGCCAATGTCG | 1730-1746 | ||
| 5′-TACAAACGGCAAGACAAG | 2139-2156 | 427 | 16 | |
| prgX | 5′-ATCGTAATCTTTACCAAAGG | 393-412 | ||
| 5′-ATGTTTAAGATAGGTTCTGTCC | 932-953 | 560 | 16 |
EcoRI, DraI, and ScaI (Roche Molecular Biochemicals, Mannheim, Germany) restriction enzymes were used in accordance with the manufacturer's instructions. The DNA restriction fragments were separated by agarose (1%) gel electrophoresis and visualized by ethidium bromide staining. The gels were photographed under UV light with Polaroid black-and-white 667 film and an MP-4 Land camera. The molecular size marker (GeneRulerTM 100-bp DNA Ladder Plus) was from M-Medical Genenco (Cornaredo, Italy).
Plasmid analysis, PFGE, and Southern hybridization.
Plasmid DNA was extracted as previously described (3). The presence of plasmid DNA was monitored by 0.8% agarose gel electrophoresis. Genomic DNA for pulsed-field gel electrophoresis (PFGE) was prepared by following standard procedures. The chromosomal fragments obtained after restriction with SmaI (New England Biolabs, Beverly, Mass.) were separated using a CHEF Mapper system (Bio-Rad Laboratories). The SmaI-restricted DNA, obtained by PFGE, and the plasmid DNA were transferred onto nylon membranes and hybridized with a probe following standard procedures. Low Range PFG Marker (New England Biolabs) and Marker II (Roche Molecular Biochemicals) were used as molecular weight standards.
RESULTS
Antibiotic resistance and presence of plasmid DNA.
The clinical isolates of E. faecalis and E. faecium were evaluated for antibiotic susceptibility to ampicillin, chloramphenicol, erythromycin, gentamicin, streptomycin, tetracycline, and vancomycin (Table 2). Most of the isolates exhibited different patterns of antibiotic resistance. The highest percentages of resistant isolates were observed in the presence of chloramphenicol, erythromycin, and tetracycline, while no vancomycin-resistant strains were observed (Table 2). Five strains of E. faecalis and one strain of E. faecium exhibited multidrug resistance (to four or five antibiotics).
TABLE 2.
Antibiotic resistance, presence of plasmid DNA, slime production, clumping, presence of AS genes, and Southern hybridization with a probe internal to the prgB gene of pCF10 in enterococci isolated from occluded biliary stents
| Strain | Antibiotic resistancea | Plasmid DNAb | Slime productionc | Clumpingd
|
PCRb
|
Hybridization with prgBb | |||
|---|---|---|---|---|---|---|---|---|---|
| JH2-2 | OGIRF | agg | prgB | prgX | |||||
| E. faecalis | |||||||||
| EFS-12 | Tc, Em, Sm, Gm, Cl | + | SP | − | − | + | + | − | + |
| EFS-13 | Tc, Em, Sm, Gm, Cl | + | NP | − | − | − | − | ND | ND |
| EFS-16 | Tc, Em, Gm, Cl | + | SP | − | − | − | − | ND | ND |
| EFS-20 | Em, Gm | + | SP | − | − | + | + | − | + |
| EFS-22 | Tc, Em | − | NP | − | − | − | − | ND | ND |
| EFS-27b | Tc, Gm, Cl | + | NP | +++ | ++ | + | + | − | + |
| EFS-28b | Em, Gm | + | SP | − | − | + | + | − | + |
| EFS-30d | Em | + | SP | +++ | − | + | + | − | + |
| EFS-32 | Em, Gm | − | SP | − | − | − | − | ND | ND |
| EFS-35 | Tc, Em | + | SP | − | − | − | − | ND | ND |
| EGL-19 | Tc, Em, Gm, Cl | + | SP | − | + | + | + | − | + |
| EFS-38 | Tc, Em, Gm, Cl | + | SP | − | + | − | − | ND | − |
| OGIRF(pCF10) | Tc, Rf, Fu | + | NDe | +++ | − | + | + | + | + |
| E. faecium | |||||||||
| EFM-13 | Tc, Gm | + | NP | − | − | − | − | ND | ND |
| EFM-14 | Em, Gm | − | NP | − | − | − | − | ND | ND |
| EFM-15 | Em, Gm | − | NP | − | − | − | − | ND | ND |
| EFM-26 | Tc, Em, Gm, Am | + | NP | + | + | − | − | ND | − |
| EFM-33 | Tc, Em | − | NP | − | − | − | − | ND | ND |
| CIUM-22 | Tc, Em, Cl, Am | − | NP | + | − | − | − | ND | − |
| CIUM-34 | Tc, Am | − | SP | + | + | − | − | ND | − |
| LS10(pBRG1) | Vm | + | ND | + | − | + | − | ND | − |
Am, ampicillin; Cl, chloramphenicol; Em, erythromycin; Gm, gentamicin; Sm, streptomycin; Tc, tetracycline; Vm, vancomycin; Rf, rifampin; Fu, fusidic acid.
+, present; −, absent.
SP, strong producers (OD > 0.240); WP, weak producers (0.120 < OD < 0.240); NP, nonproducers (OD < 0.120).
+, weak; ++, moderate; +++, strong.
ND, not determined.
Plasmid DNA was present in 10 of 12 E. faecalis strains and in 2 of 7 E. faecium strains.
Slime production.
All clinical enterococcal isolates were tested for slime production. Nine E. faecalis strains (EFS-12, EFS-16, EFS-20, EFS-28b, EFS-30d, EFS-32, EFS-35, EFS-30, and EGL-19) and one E. faecium strain (CIUM-34) were strong producers (Table 2).
Enterococcal clumping.
To assess responses to pheromones, the 19 enterococcal strains were microscopically examined for clumping after growth in the presence of pheromone-containing supernatants of E. faecalis JH2-2 and E. faecalis OG1RF. Among all isolates, seven strains (four E. faecalis [EFS-27b, EFS-30d, EFS-38, and EGL-19] and three E. faecium [EFM-26, CIUM-22, and CIUM-34]) were clumping positive. Growth of strains in the presence of pheromones gave rise to different levels of aggregation: some strains elicited a barely detectable effect, other strains generated small aggregates, while others did not aggregate at all (Table 2). Strains also exhibited different aggregation patterns when exposed to pheromone-containing supernatants of strain JH2-2 or OG1RF; moreover, E. faecium isolates generated aggregates smaller than those of E. faecalis (Fig. 1).
FIG. 1.
Clumping phenomenon in enterococci induced by the presence of pheromone-responsive plasmids produced by E. faecalis strain JH2-2 or OG1RF. (a) E. faecalis EFS-27b (JH2-2); (b) E. faecalis EFS-30d (JH2-2); (c) E. faecalis EGL-19 (OG1RF); (d) E. faecium CIUM-22 (JH2-2); (e) E. faecium CIUM-34 (JH2-2); (f) E. faecium LS10(pBRG1) (JH2-2).
Adhesion to Caco-2 cells.
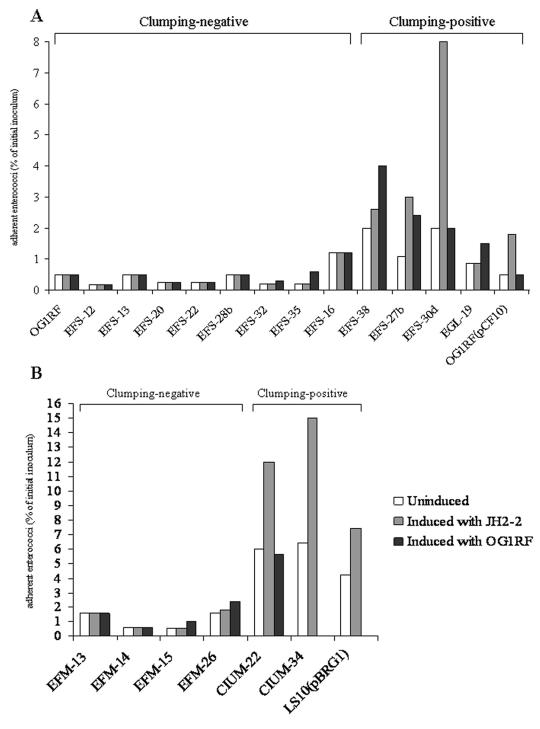
All enterococcal isolates were tested in vitro for the ability to adhere to intestinal epithelial cells. In adhesion experiments, E. faecalis OG1RF(pCF10) and E. faecium LS10(pBRG1) were used as positive controls. The abilities of enterococcal isolates to adhere to Caco-2 cells, expressed as the percentage of adhering bacteria with respect to the initial inoculum, were between 0.18 and 6.4% (Fig. 2). Among E. faecium isolates, clumping-positive strains were found to be more adhesive than clumping-negative strains. Moreover, after induction with pheromones, the adhesion of clumping-positive strains was found to increase from two- to fourfold. Particularly after induction with sex pheromones produced by E. faecalis JH2-2, adhesiveness increased significantly in E. faecalis EFS-27b (from 1.1 to 3%) and EFS-30d (from 2 to 8%) and E. faecium CIUM-34 (from 6.4 to 15%). In E. faecalis EFS-38 (a weakly clumping-positive strain), the percentage of adhesion was doubled after induction with sex pheromones produced by E. faecalis OG1RF (Fig. 2).
FIG. 2.
Adherence of clumping-negative and clumping-positive E. faecalis (A) and E. faecium (B) strains to Caco-2 cells before and after induction with sex pheromones produced by E. faecalis strain JH2-2 or OG1RF. The efficiency of adherence is expressed as the percentage of the inoculum remaining attached to eukaryotic cells and was calculated as follows: percentage of adhering bacteria = (number of adherent bacteria × 100)/number of bacteria in inoculum.
Presence of AS genes.
All enterococcal isolates were screened for the presence of AS genes by using the primers internal to a highly conserved region of the E. faecalis pheromone response plasmids pAD1, pPD1, and pCF10 and those internal to prgB and prgX of the E. faecalis plasmid pCF10 (Table 1). E. faecalis OG1RF(pCF10) and E. faecium LS10(pBRG1) were used as controls. Strain OG1RF containing pCF10 was also used to generate a prgB-specific probe. agg primers produced an amplicon of the expected size in six E. faecalis strains (three clumping-positive [EFS-27b, EFS-30d, and EGL-19] and three clumping-negative [EFS-12, EFS-20, and EFS-28b] strains) but not in clumping-positive E. faecium strains (Table 2). In all agg-positive E. faecalis strains, an amplicon of the expected size was also obtained with primers internal to prgB, whereas no PCR products were obtained with primers internal to prgX. The agg/prgB-positive E. faecalis strains were also positive in Southern hybridization experiments with a prgB-specific probe (Fig. 3). The prgB probe strongly hybridized with SmaI-restricted genomic DNA obtained by PFGE from strains EFS12, EGL19, and EFS20 (Fig. 3B, lanes 4, 5, and 6, respectively) and with plasmid DNAs from strains EFS27B, EFS28B, EFS30D, and OG1RF(pCF10) (Fig. 3D, lanes 7, 8, 9, and 11, respectively). In Fig. 3B, hybridizing bands corresponding to the plasmid DNA—in some cases not entering the gel—are also visible (lanes 7, 8, 9, and 11).
FIG. 3.
PFGE and Southern hybridization of SmaI-digested genomic DNA and plasmid DNA from enterococcal isolates with a psoralen-biotin-labeled probe specific for prgB. (A and B) PFGE patterns of genomic DNA (A) and corresponding hybridizing bands (B). (C and D) PFGE profiles of plasmid DNA (C) and corresponding hybridizing bands (D). Lanes: 1, CIUM22; 2, CIUM34; 3, EFM26; 4, EFS12; 5, EGL19; 6, EFS20; 7, EFS27B; 8, EFS28B; 9, EFS30D; 10, EFS38; 11, OG1RF(pCF10); 12, OG1RF; M1, Low Range PFG Marker; M2, Marker II. The arrows indicate chromosomal bands (B, lanes 4, 5, and 6) or plasmid bands (B and D, lanes 7, 8, 9, and 11) hybridizing with the prgB probe.
PCR amplicons, obtained with agg and prgB primers, from the three clumping-positive (EFS-27b, EFS-30d, and EGL-19) and the three clumping-negative (EFS-12, EFS-20, and EFS-28b) E. faecalis isolates were digested with DraI, EcoRI, and/or ScaI restriction enzyme, and the restriction profiles were compared with that derived from E. faecalis OG1RF(pCF10) after digestion with the same enzymes. Restriction profiles obtained by DraI digestion of 427-bp amplicons obtained with prgB primers (Fig. 4) and by ScaI digestion of 1,553-bp amplicons obtained with agg primers (data not shown) were identical in E. faecalis clinical isolates and OG1RF(pCF10). The EcoRI restriction profiles of 1,553-bp amplicons obtained with agg primers (Fig. 4) were identical in all clinical isolates but differed from that of OG1RF(pCF10), showing a three-band difference resulting from the disappearance of a fragment of ∼1,170 bp and the appearance of two new fragments (∼730 and 440 bp).
FIG. 4.
Restriction analysis of PCR products obtained using primers internal to prgB and agg genes of clumping-positive (EFS-27b, EFS-30d, and EGL-19) and clumping-negative (EFS-12, EFS-20, and EFS-28b) clinical enterococcal isolates and of E. faecalis OG1RF(pCF10). (A) DraI digests from 427-bp amplicons (prgB). (B) EcoRI digests from 1,553-bp amplicons (agg). Lanes: 1, EFS-28b undigested; 2, EFS-28b digested; 3, EFS-30d undigested; 4, EFS-30d digested; 5, OG1RF(pCF10) undigested; 6, OG1RF(pCF10) digested; 7, EFS-20 undigested; 8, EFS-20 digested; 9, EFS-27b undigested; 10, EFS-27b digested; 11, EFS-12 undigested; 12, EFS-12 digested; 13, EGL-19 undigested; 14, EGL-19 digested; 15, Marker Gene Ruler 100-bp DNA ladder. The arrows indicate the sizes of fragments in base pairs.
DISCUSSION
Previous studies have indicated that bacterial contamination is a major factor in the pathogenesis of stent occlusion (2, 25, 28). Other authors have reported that stent clogging is significantly associated with polymeric surface irregularities promoting bacterial adherence, biofilm formation, and the accumulation of biliary sludge (24, 32). Furthermore, in vitro and in vivo studies have demonstrated the inhibitory effect of long-term treatment with antibiotics or aspirin on sludge formation, suggesting that both bacteria and mucus glycoproteins play significant roles in the clogging of biliary endoprostheses (22, 27). In this process, bacteria of the genus Enterococcus, particularly E. faecalis and E. faecium, are among the most frequently isolated gram-positive bacteria that translocate from the duodenum to colonize the biliary stent (7). It is well known that enterococcal pheromone-susceptible plasmids carry genes coding for AS, which is expressed as a response to a sex pheromone stimulus produced from strains that lack the specific plasmid (5). In addition to promoting conjugation processes, the presence of AS also increases the ability of enterococci to adhere to host surfaces. The reported increase in uptake of AS-expressing cells into intestinal epithelial cells (20, 26, 31) suggests the possible involvement of AS in the translocation of E. faecalis from the intestine to the bloodstream. On the other hand, AS of E. faecalis has been implicated as a virulence factor in several model systems (21), and its in vivo induction has recently been demonstrated (17).
In this study, enterococcal strains isolated from occluded biliary stents, all resistant to antibiotics, were studied for slime production and response to sex pheromones produced by E. faecalis JH2-2 and E. faecalis OG1RF.
As far as slime production is concerned, 9 of 12 E. faecalis strains were strong producers compared to 1 of 7 E. faecium strains. This finding is particularly interesting because slime production in enterococci has not been extensively described (1). In fact, the production of slime exhibited by most of our E. faecalis isolates could play a significant role in the colonization and occlusion of biliary stents. This role in the impairment of indwelling medical devices is well established for staphylococci, for which slime is considered a significant biofilm component and a determining factor involved in the occlusion of intravascular catheters (8).
Our data also demonstrate a pheromone response in enterococcal strains isolated from occluded biliary stents, suggesting that sex pheromone response may play a role in biliary stent occlusion. Actually, clumping assays demonstrated a response to pheromones produced by E. faecalis JH2-2 and/or E. faecalis OG1RF in 7 (4 E. faecalis and 3 E. faecium) of 19 strains. Interestingly, clumping-positive E. faecium strains generated aggregates that were different (i.e., smaller) from the large aggregates generated by clumping-positive E. faecalis strains and similar to those of E. faecium LS10 carrying the pheromone-susceptible plasmid pBRG1 (23) and that showed a greater ability to adhere in vitro to cultured intestinal cells (Caco-2) than clumping-negative strains. Adherence to Caco-2 cells by clumping-positive E. faecalis and E. faecium strains was enhanced after induction with pheromones. These findings are relevant, especially for E. faecium species, in which a response to sex pheromones has rarely been described. Moreover, among tested enterococci, PCR products of the expected size were obtained in six E. faecalis strains (including three clumping-positive and three clumping-negative strains, all carrying plasmid DNA) using primers internal to AS genes of E. faecalis. No PCR products were obtained with the same primers among E. faecium isolates, either in clumping-positive or clumping-negative strains, only two of which carried plasmid DNA.
Among agg/prgB-positive E. faecalis clinical isolates, restriction profiles obtained by digesting PCR products with DraI and ScaI were identical to those obtained from E. faecalis OG1RF(pCF10), whereas EcoRI profiles showed a three-band difference, suggesting the presence of one more EcoRI site. All agg/prgB-positive E. faecalis strains were also positive in Southern hybridization experiments with SmaI-restricted genomic DNA, using as a probe a DNA fragment, amplified by PCR, internal to prgB of E. faecalis OG1RF(pCF10). The prgB probe strongly hybridized with plasmid DNAs from three E. faecalis strains, suggesting a plasmid location. These results strongly suggest that these clinical isolates contain AS genes. Considering that agg primers amplify a highly conserved 1,553-bp region (12), the slight difference revealed only by EcoRI restriction analysis might be attributed to a minor mutational event.
The agg/prgB-positive E. faecalis strains were negative for prgX, the gene involved in negative regulation of the pheromone response in pCF10; among these, three strains were clumping positive, and their adhesion to Caco-2 cells was enhanced by sex pheromones, suggesting that AS was expressed. Apart from the possible presence of a different gene, an alternative explanation might be found in the occurrence of point mutations in the prgX genes of these strains, affecting the affinity of the prgX primers for the target gene. The remaining strains were clumping negative, and their adhesion to Caco-2 cells was not enhanced by sex pheromones, suggesting that AS is not expressed. Another significant issue is represented by the four clumping-positive but PCR- and Southern hybridization-negative strains (one E. faecalis and three E. faecium strains), whose adhesion to Caco-2 cells increased after induction with sex pheromones. As for E. faecalis, it can be hypothesized that the AS gene was not recognized by primers, while for E. faecium, it is possible that clumping is AS independent, even if E. faecium LS10(pBRG1) was clumping and agg positive.
Overall, our results emphasize that slime production and/or response to pheromones is present in E. faecalis and E. faecium strains isolated from occluded biliary stents. Furthermore, these data support a pheromone response by enterococcal strains involved in clogging of biliary stents, suggesting the possible implication of the aggregation substance as one of the most important causative factors in the occlusion process. It may also be hypothesized that enterococci carrying AS genes and/or producing slime have a selective advantage in colonizing biliary stents.
Acknowledgments
We thank G. M. Dunny for kindly supplying E. faecalis strains OG1RF and OG1RF(pCF10).
This study was supported in part by the European research project Antimicrobial Resistance Transfer from and between Gram-Positive Bacteria of the Digestive Tract and Consequences for Virulence, contract QLK2-CT-2002-00843 “ARTRADI.”
REFERENCES
- 1.Baldassarri, L., R. Cecchini, L. Bertuccini, M. G. Ammendolia, F. Iosi, C. R. Arciola, L. Montanaro, R. Di Rosa, G. Gherardi, G. Dicuonzo, G. Orefici, and R. Creti. 2001. Enterococcus sp. produces slime and survives in rat peritoneal macrophages. Med. Microbiol. Immunol. 190:113-120. [DOI] [PubMed] [Google Scholar]
- 2.Basoli, A., F. Fiocca, R. Di Rosa, L. Baldassarri, and G. Donelli. 1999. Biliary stent occlusion: a microbiological and scanning electron microscopy (SEM) investigation, p. 69-80. In E. Zanella (ed.) Advances in abdominal surgery. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 3.Biavasco, F., E. Giovanetti, A. Miele, C. Vignaroli, B. Facinelli, and P. E. Varaldo. 1996. In vitro conjugative transfer of VanA vancomycin resistance between enterococci and listeriae of different species. Eur. J. Clin. Microbiol. Infect. Dis. 15:50-59. [DOI] [PubMed] [Google Scholar]
- 4.Cetinkaya, Y., P. Falk, and C. G. Mayhall. 2000. Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 13:686-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clewell, D. B. 1993. Bacterial sex pheromone-induced plasmid transfer. Cell 73:9-12. [DOI] [PubMed] [Google Scholar]
- 6.Clewell, D. B., and G. M. Dunny. 2002. Conjugation and genetic exchange in enterococci, p. 265-300. In M. S. Gilmore (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, D.C.
- 7.Di Rosa, R., A. Basoli, G. Donelli, A. Penni, F. M. Salvatori, F. Fiocca, and L. Baldassarri. 1999. A microbiological and morphological study of blocked biliary stents. Microb. Ecol. Health Dis. 11:84-88. [Google Scholar]
- 8.Donelli, G., P. De Paoli, G. Fadda, P. Marone, G. Nicoletti, P. E. Varaldo, and the CVC Study Group. 2001. A multicenter study on central venous catheter-associated infections in Italy. J. Chemother. 13:251-262. [DOI] [PubMed] [Google Scholar]
- 9.Dunny, G. M., B. L. Brown, and D. B. Clewell. 1978. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc. Natl. Acad. Sci. USA 75:3479-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunny, G. M., C. Funk, and J. Adsit. 1981. Direct stimulation of the transfer of antibiotic resistance by sex pheromones in Streptococcus faecalis. Plasmid 6:270-278. [DOI] [PubMed] [Google Scholar]
- 11.Dunny, G. M., M. H. Antiporta, and H. Hirt. 2001. Peptide pheromone-induced transfer of plasmid pCF10 in Enterococcus faecalis: probing the genetic and molecular basis for specificity of the pheromone response. Peptides 22:1529-1539. [DOI] [PubMed] [Google Scholar]
- 12.Eaton, T. J., and M. J. Gasson. 2001. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl. Environ. Microbiol. 67:1628-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentry-Weeks, C. R., R. Karkhoff-Schweizer, A. Pikis, M. Estay, and J. M. Keith. 1999. Survival of Enterococcus faecalis in mouse peritoneal macrophages. Infect. Immun. 67:2160-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock, L. E., and M. S. Gilmore. 2000. Pathogenicity of enterococci, p. 251-258. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 15.Handwerger, S., M. J. Pucci, and A. Kolokathis. 1990. Vancomycin resistance is encoded on a pheromone response plasmid in Enterococcus faecium 228. Antimicrob. Agents Chemother. 34:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaton, M. P., and S. Handwerger. 1995. Conjugative mobilization of a vancomycin resistance plasmid by a putative Enterococcus faecium sex pheromone response plasmid. Microb. Drug Resist. 1:177-183. [DOI] [PubMed] [Google Scholar]
- 17.Hirt, H., P. M. Schlievert, and G. M. Dunny. 2002. In vivo induction of virulence and antibiotic resistance transfer in Enterococcus faecalis mediated by the sex pheromone-sensing system of pCF10. Infect. Immun. 70:716-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huycke, M. M., D. F. Sahm, and M. S. Gilmore. 1998. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg. Infect. Dis. 4:239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hynes, R. O. 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69:11-25. [DOI] [PubMed] [Google Scholar]
- 20.Isenmann, R., M. Schwarz, E. Rozdzinski, R. Marre, and H. G. Beger. 2000. Aggregation substance promotes colonic mucosal invasion of Enterococcus faecalis in an ex vivo model. J. Surg. Res. 89:132-138. [DOI] [PubMed] [Google Scholar]
- 21.Kreft, B., R. Marre, U. Schramm, and R. Wirth. 1992. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect. Immun. 60:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libby, E. D., and J. W. Leung. 1996. Prevention of biliary stent clogging: a clinical review. Am. J. Gastroenterol. 91:1301-1308. [PubMed] [Google Scholar]
- 23.Magi, G., R. Capretti, C. Paoletti, M. Pietrella, L. Ferrante, F. Biavasco, P. E. Varaldo, and B. Facinelli. 2003. Presence of a vanA-carrying pheromone response plasmid (pBRG1) in a clinical isolate of Enterococcus faecium. Antimicrob. Agents Chemother. 47:1571-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAllister, E. W., L. C. Carey, P. G. Brady, R. Heller, and S. G. Kovacs. 1993. The role of polymeric surface smoothness of biliary stents in bacterial adherence, biofilm deposition, and stent occlusion. Gastrointest. Endosc. 39:422-425. [DOI] [PubMed] [Google Scholar]
- 25.Molinari, G., V. Pugliese, G. C. Schito, and C. A. Guzman. 1996. Bacteria involved in the blockage of biliary stents and their susceptibility to antibacterial agents. Eur. J. Clin. Microbiol. Infect. Dis. 15:88-92. [DOI] [PubMed] [Google Scholar]
- 26.Olmsted, S. B., G. M. Dunny, S. L. Erlandsen, and C. L. Wells. 1994. A plasmid-encoded surface protein on Enterococcus faecalis augments its internalization by cultured epithelial cells. J. Infect. Dis. 170:1549-1556. [DOI] [PubMed] [Google Scholar]
- 27.Smit, J. M., M. M. Out, A. K. Groen, K. Huibregtse, P. L. Jansen, J. van Marle, and G. N. Tytgat. 1989. A placebo-controlled study on the efficacy of aspirin and doxycycline in preventing clogging of biliary endoprostheses. Gastrointest. Endosc. 35:485-489. [DOI] [PubMed] [Google Scholar]
- 28.Speer, A. G., P. B. Cotton, J. Rode, A. M. Seddon, C. R. Neal, J. Holton, and J. W. Costerton. 1988. Biliary stent blockage with bacterial biofilm. A light and electron microscopy study. Ann. Intern. Med. 108:546-553. [DOI] [PubMed] [Google Scholar]
- 29.Sussmuth, S. D., A. Muscholl-Silberhorn, R. Wirth, M. Susa, R. Marre, and E. Rozdzinski. 2000. Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect. Immun. 68:4900-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weaver, K. E. 2000. Enterococcal genetics, p. 259-271. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, D.C.
- 31.Wells, C. L., E. A. Moore, J. A. Hoag, H. Hirt, G. M. Dunny, and S. L. Erlandsen. 2000. Inducible expression of Enterococcus faecalis aggregation substance surface protein facilitates bacterial internalization by cultured enterocytes. Infect. Immun. 68:7190-7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu, J. L., R. Andersson, and A. Ljungh. 1996. Protein adsorption and bacterial adhesion to biliary stent materials. J. Surg. Res. 62:69-73. [DOI] [PubMed] [Google Scholar]