Abstract
Seventeen laboratories participated in a study of interlaboratory reproducibility with caspofungin microdilution susceptibility testing against panels comprising 30 isolates of Candida spp. and 20 isolates of Aspergillus spp. The laboratories used materials supplied from a single source to determine the influence of growth medium (RPMI 1640 with or without glucose additions and antibiotic medium 3 [AM3]), the same incubation times (24 h and 48 h), and the same end point definition (partial or complete inhibition of growth) for the MIC of caspofungin. All tests were run in duplicate, and end points were determined both spectrophotometrically and visually. The results from almost all of the laboratories for quality control and reference Candida and Aspergillus isolates tested with fluconazole and itraconazole matched the NCCLS published values. However, considerable interlaboratory variability was seen in the results of the caspofungin tests. For Candida spp. the most consistent MIC data were generated with visual “prominent growth reduction” (MIC2) end points measured at 24 h in RPMI 1640, where 73.3% of results for the 30 isolates tested fell within a mode ± one dilution range across all 17 laboratories. MIC2 at 24 h in RPMI 1640 or AM3 also gave the best interlaboratory separation of Candida isolates of known high and low susceptibility to caspofungin. Reproducibility of MIC data was problematic for caspofungin tests with Aspergillus spp. under all conditions, but the minimal effective concentration end point, defined as the lowest caspofungin concentration yielding conspicuously aberrant hyphal growth, gave excellent reproducibility for data from 14 of the 17 participating laboratories.
Caspofungin acetate (Cancidas, formerly MK-0991 and l-743872; Merck & Co., Inc.) is an echinocandin-class antifungal agent, recently approved in the United States and Europe for the treatment of invasive aspergillosis that is refractory to other antifungal treatments, esophageal Candida infections and invasive Candida infections (11). Caspofungin inhibits synthesis of β-1,3-d-glucan in fungal cell walls, a property that results in fungicidal effects against Candida species, in which this polysaccharide is vital to cell wall integrity (8).
Susceptibility tests of caspofungin against Candida species have been done according to the National Committee for Clinical Laboratory Standards (NCCLS) method M27-A (18) or its recent second edition M27-A2 (19), the reference protocol for testing azoles, flucytosine, and polyene antifungal agents against yeasts. Quality control (QC) limits have been established for caspofungin in M27-A tests with reference isolates of Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 (3). A European reference method (EUCAST) for antifungal susceptibility testing with Candida spp. gives results that closely match those obtained by method M27 for azole antifungals (6). The EUCAST method is very similar to M27-A2 but uses a higher yeast inoculum, a shorter incubation time, flat-bottom microdilution plates, and spectrophotometric end point determinations (6).
Published caspofungin MICs for Candida species suggest interlaboratory variation in the ranges of caspofungin MICs determined by NCCLS method M27-A. For example, whereas some authors found caspofungin MIC ranges against isolates of C. albicans from 0.03 to 1 μg/ml (12, 14, 16, 21, 23, 26), other researchers have reported higher ranges of 0.25 to 4 μg/ml (5, 9, 15). For tests with C. tropicalis, the highest caspofungin MIC from some laboratories (14, 16) was at or below the lowest MIC reported from others (5, 9, 15, 21). Differences of this order may represent intrinsic differences in the susceptibility of the panels of isolates tested, or they may indicate interlaboratory disparities in susceptibility tests with caspofungin.
For Aspergillus species, agreement in published MICs is unimpressive. For filamentous fungi, the NCCLS reference method is M38-A, approved for tests with azoles, flucytosine, and polyenes (17). For 26 A. fumigatus isolates, Arikan et al. reported geometric mean MICs of caspofungin of 0.43 μg/ml after 24 h of incubation and of >16 μg/ml after 72 h of incubation (1). By comparison, 72-h geometric mean MICs versus A. fumigatus were determined to be 2.15 μg/ml by Espinel-Ingroff (9) by using method M38-A and ≤0.09 μg/ml by Del Poeta et al. (7), who used a tube dilution method. Pfaller et al. (22) reported 72-h caspofungin MICs of 0.06 and 0.12 μg/ml for 12 isolates of A. fumigatus. In all of these studies the growth medium used was RPMI 1640 buffered with morpholinepropanesulfonic acid according to NCCLS M38-A recommendations (17). The minimum effective concentration (MEC), defined as the lowest caspofungin concentration that results in growth of A. fumigatus producing conspicuously aberrant hyphae, has been found to generate more consistent susceptibility data than the MIC (1, 9, 10).
Alterations in the growth medium, pH, incubation temperature, and inoculum size were found to cause caspofungin MIC variations in microdilution tests (4) but not in macrodilution tests (12). Most investigators have found that caspofungin MICs against all fungal types are lower when determined in Antibiotic Medium 3 (AM3) than in RPMI 1640 medium (2, 4, 20, 23).
To provide a prospective evaluation of interlaboratory agreement with caspofungin susceptibility tests, 17 laboratories collaborated to test 30 isolates of Candida spp. and 20 isolates of Aspergillus spp. under a variety of different conditions. The laboratories were located in the United States, Canada, Europe, and Australia. The test plates and growth media were supplied from a central source. The MIC and MEC end points were determined by visual inspection, and MICs were additionally determined from spectrophotometric data.
MATERIALS AND METHODS
Fungi.
A panel of 30 isolates of Candida spp. and 20 Aspergillus spp. (Table 1) was assembled by Merck Research Laboratories (Rahway, N.J.). A blinded code number was assigned to each isolate, and the panels of fungi were distributed to the 17 participating laboratories. C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were included among the 30 yeast isolates as NCCLS-recommended QC strains for antifungal susceptibility testing by method M27-A2 (19). A. flavus ATCC 204304 and A. fumigatus ATCC 204305 were included among the 20 mould isolates as reference strains for testing by method M38-A (17). The panel of yeast isolates also contained two strains known to be heterozygous for a mutation in the FKS1 gene, the caspofungin target, and six strains known to be homozygous for mutations in FKS1 (8). All eight of these yeasts have reduced susceptibility to caspofungin in vitro; the two heterozygous mutants less so than the six homozygous mutants.
TABLE 1.
Details of the panel of fungi tested in this studya
| Isolate | Species | Description | Isolate | Species | Description | |
|---|---|---|---|---|---|---|
| Y101 | C. dubliniensis | Rex collection 34-504-032 | A201 | A. flavus | Nares isolate from Houston, Tex. | |
| Y102 | C. albicans | Biopsy sample from Colombia | A202 | A. flavus | Sputum sample from Tucson, Ariz. | |
| Y103 | C. albicans | Merck collection MY1055 | A203 | A. flavus | Bone fragment from Brazil | |
| Y104 | C. albicans | CAI-4 (8) | A204 | A. flavus | Sputum sample from Houston, Tex. | |
| Y105* | C. albicans | CAI-4-R1 (8) | A205 | A. fumigatus | Sputum sample from Belgium | |
| Y106* | C. albicans | NR4 (8) | A206 | A. fumigatus | BAL sample from Lexington, Ky. | |
| Y107* | C. albicans | NR3 (8) | A207 | A. fumigatus | Sputum sample from France | |
| Y108* | C. albicans | T25 (8) | A208 | A. fumigatus | Sputum sample from Rochester, N.Y. | |
| Y109* | C. albicans | Merck collection strain 212 | A209 | A. fumigatus | Tracheal secretions sample from France | |
| Y110 | C. albicans | Rex collection 34-032-001 | A210 | A. fumigatus | Rinaldi collection 02-1835 | |
| Y111* | C. albicans | Blood isolate from Montreal, Quebec, Canada | A211 | A. niger | Sputum sample from Rochester, Minn. | |
| Y112* | C. albicans | Tissue isolate from Montreal, Quebec, Canada | A212 | A. niger | BAL sample from Rochester, Minn. | |
| Y113 | C. glabrata | Blood isolate from Australia | A213 | A. niger | BAL sample from Lexington, Ky. | |
| Y114 | C. glabrata | Blood isolate from Chile | A214 | A. nidulans | Perlin collection CLF12 | |
| Y115 | C. guilliermondii | Blood isolate from Belgium | A215 | A. nidulans | Perlin collection CLF14 | |
| Y116 | C. guilliermondii | Blood isolate from Russia | A216 | A. terreus | Lung (post mortem) sample from Tucson, Ariz. | |
| Y117 | C. krusei | Blood isolate from Buffalo, N.Y. | A217 | A. terreus | Left breast sample from Australia | |
| Y118* | C. krusei | Stool isolate from Germany | A218 | A. terreus | Left toe sample from Australia | |
| Y119 | C. parapsilosis | Blood isolate from Houston, Tex. | A219 | A. fumigatus | ATCC 204305 (NCCLS reference isolate) | |
| Y120 | C. parapsilosis | Blood isolate from Belgium | A220 | A. flavus | ATCC 204304 (NCCLS reference isolate) | |
| Y121 | C. parapsilosis | Biopsy isolate from Peru | ||||
| Y122 | C. parapsilosis | Blood isolate from Brazil | ||||
| Y123 | C. Iusitaniae | |||||
| Y124 | C. tropicalis | Blood isolate from Canada | ||||
| Y125 | C. tropicalis | Blood isolate from Brazil | ||||
| Y126 | C. rugosa | Blood isolate from Canada | ||||
| Y127 | C. lipolytica | Oropharyngeal swab from Peru | ||||
| Y128 | C. kefyr | Biopsy isolate from Guatemala | ||||
| Y129 | C. krusei | ATCC 6258 (NCCLS QC isolate) | ||||
| Y130 | C. parapsilosis | ATCC 22019 (NCCLS QC isolate) |
Yeast isolates of known low susceptibility to caspofungin are indicated by an asterisk.
For all isolates, inocula of yeasts and Aspergillus conidia were prepared by standard methods (17, 19) and diluted appropriately to the specified initial concentrations. The growth media and 96-well microdilution plates containing antifungal agents were prepared by a commercial supplier (Trek Diagnostic Systems, Inc., Cleveland, Ohio). The media used in the tests were morpholinepropanesulfonic acid-buffered RPMI 1640 (RPMI) (17, 19), RPMI 1640 with a 2% glucose concentration instead of the usual 0.2% (RP-G), and AM3.
Experimental design.
For the 30 Candida spp. isolates, duplicate plates were set up with caspofungin dilutions from 32 to 0.032 μg/ml tested in RPMI and RP-G with starting yeast concentrations of 1 to 5 × 103 CFU/ml and in RP-G with a starting yeast concentration of 1 × 105 to 5 × 105 CFU/ml. For tests in AM3, the caspofungin dilution range was 8 to 0.008 μg/ml, and the initial yeast concentration was 103 CFU/ml. As a reference standard, fluconazole dilutions from 64 to 0.063 μg/ml were tested in RPMI with an initial yeast concentration of 103 CFU/ml (19).
Inoculated microplates were incubated at 35°C in a humid environment. After 24 h the plates were carefully shaken to distribute yeast growth evenly, and the MICs of caspofungin for Candida spp. were recorded according to two visual criteria: MIC0, the lowest caspofungin concentration that supported no visible growth (a clear well), and MIC2, the lowest caspofungin concentration that caused a significant diminution of growth below control growth levels (19). Each lab then used a microplate spectrophotometer to determine the turbidity (optical density [OD]) of each well at 530 to 500 nm. For each of the three growth media tested, one caspofungin plate was set up with uninoculated medium for determination of the mean background OD values. These values were subtracted from the experimental OD values, and two MIC end points were determined from the spectrophotometric data: IC50, the lowest caspofungin concentration that reduced turbidity to <50% of the mean control turbidity, and IC90, the lowest caspofungin concentration that reduced turbidity to <90% of the control.
For the 20 Aspergillus spp. isolates, duplicate microdilution plates contained a caspofungin concentration series from 16 to 0.016 μg/ml, tested in RPMI, RP-G, and AM3, all inoculated to an initial concentration of 1 × 104 to 5 × 104 CFU/ml as recommended by the NCCLS for mold testing (17). Itraconazole concentrations from 8 to 0.008 μg/ml were also tested as a reference controls. Visual and spectrophotometric MIC and IC readings were made as for the Candida spp., except that the plates were not shaken before they were read. In addition, the caspofungin MEC was recorded at 24 h; this concentration was defined by reference to a photograph as the lowest that led to the growth of small, rounded, compact colonies compared to the florid hyphal growth seen in controls.
After 48 h of incubation, the MIC0, MIC2, IC90, and IC50 values were read for all fungi in the same way as at 24 h. After 48 h, MIC2 was read for fluconazole, and the MIC0 was read for itraconazole. The protocol also called for measurement of minimum fungicidal concentrations of caspofungin against Candida spp. at 48 h, but a high level of protocol violation in this test led to its being discounted for further analysis.
Analysis of data.
All visually read susceptibility end points were typed into standardized result spreadsheets, and all spectrophotometric data were pasted into a standardized analysis template distributed to all participants. MIC and IC values that were off-scale were recorded as the lowest test concentration when all wells showed growth inhibition and as the next-highest concentration above the highest in a series when no well reached the end point being tested.
For each test condition the minimum, maximum, and modal MIC or IC value was determined. For each isolate tested, the number and percentage of results within ±1 dilution of the modal MIC or IC value was determined. “Excellent agreement” was defined as ≥90% of results or more within the mode ± one dilution range. “Moderate agreement” was defined as ≥80% of results within the mode ± one dilution range.
RESULTS
Data returns, technical problems, and protocol violations.
All 17 participating laboratories, designated A through Q here, returned data spreadsheets. The visually read MICs were complete according to protocol for all 17 laboratories, with the sole exception of laboratory P, which provided no caspofungin MEC readings for molds tested in RP-G and AM3. For the spectrophotometric data, the results from laboratory K contained many omissions and inexplicable inaccuracies and were rejected from analysis. Laboratory F provided spectrophotometric results only for one of each of the duplicate plates set up with Candida spp. and no spectrophotometric data for the Aspergillus spp.
Two complaints of technical problems arose commonly among the participants. One was of yeast isolates where growth was inhibited in wells in the central portion of the dilution series but returned at the two or three highest concentrations (spectrophotometric data verified that regrowth at the highest test concentrations occurred often for some of the isolates tested [see below]). The second was of the appearance of air bubbles in the plates, which hampered the reading of end points, particularly by spectrophotometry. In some laboratories that experienced air bubble formation in the plates the plates were centrifuged to disperse bubbles before they were read. Where participants felt unable to judge a visual MIC end point (commonly because of inadequate control growth), the result was recorded as a blank for purposes of analysis.
Intralaboratory data agreement.
With very few exceptions, the results showed excellent agreement for duplicate results obtained within each laboratory. For the 30 yeast isolates across 17 laboratories, 1.8 to 3.6% of the results showed intralaboratory differences beyond one twofold dilution, depending on the test conditions; these percentages were calculated from >1,000 data per test condition. For the 20 mold isolates across the 17 laboratories, 0.0 to 5.1% of results showed intralaboratory differences beyond one twofold dilution.
Fluconazole MICs for Candida spp.
For QC strain C. krusei ATCC 6258, the current fluconazole QC limits according to the NCCLS are 16 to 128 μg/ml (19). From the 17 participating laboratories, the fluconazole modal MIC for this strain was 32 μg/ml, and all laboratories reported this value or 16 or 64 μg/ml, which is in perfect agreement with the reference MIC range. For QC strain C. parapsilosis ATCC 22019 the reference fluconazole MIC range is 1.0 to 4.0 μg/ml (19). In the present study the modal MIC for fluconazole was 2.0 μg/ml, and 30 of 33 (90.9%) of results fell within ±1 dilution of this mode and thus within the NCCLS specified limits. One laboratory reported duplicate off-range results of 8 μg/ml for ATCC 22019; however, exclusion of data from this laboratory had no effect on the results of the analyses of interlaboratory variation for fluconazole or caspofungin MICs.
Fluconazole modal MICs for the 30 Candida spp. isolates ranged from 0.13 to 32 μg/ml, showing that a full range of fluconazole susceptibilities was represented in the test panel. Across the 30 isolates, reproducibility within a three-dilution range varied from 62.9 to 100%. Excellent agreement was achieved for only 14 (47%) of the isolates, but moderate agreement was achieved for 25 (83%) of the isolates. Excellent agreement was less common (36.4% of 11 isolates) for C. albicans than for other Candida species (52.6% of 19 isolates), but this difference was not statistically significant (χ2 test).
Caspofungin MICs for Candida spp.
Levels of agreement for MIC and IC values obtained with caspofungin for the 30 Candida isolates tested in 17 laboratories for all growth media, inoculum sizes, incubation times, and end points are summarized in Table 2. For visual readings, the interlaboratory reproducibility of the MIC estimates was poor under most of the test conditions. Excellent interlaboratory agreement was achieved for ≥60% of the isolates tested only for MIC2 in RPMI read at 24 h, MIC0 in RP-G with 105 cells/ml read at 48 h, and MIC2 in AM3 read at 24 h. Moderate agreement was achieved with visual end points for 93% of the Candida isolates with MIC2 read at 24 h in RPMI and for 77% of the isolates with MIC0 or MIC2 read at 24 h in AM3 (details not shown).
TABLE 2.
Interlaboratory agreement for caspofungin susceptibility data with 30 isolates of Candida spp.a
| Test conditions | Endpoint | Visual readings
|
Spectrophotometer results
|
||
|---|---|---|---|---|---|
| Agreement range (%) | Excellent agreement (%) | Agreement range (%) | Excellent agreement (%) | ||
| RPMI, 24 h | MIC0 or IC90 | 37.5-100.0 | 33.3 | 36.6-93.3 | 3.3 |
| MIC2 or IC50 | 64.7-100.0 | 73.3 | 26.6-100.0 | 53.3 | |
| RPMI, 48 h | MIC0 or IC90 | 44.1-100.0 | 36.7 | 33.3-86.7 | 0.0 |
| MIC2 or IC50 | 26.4-100.0 | 43.3 | 40.0-96.7 | 20.0 | |
| RP-G, 103 yeasts/ml, 24 h | MIC0 or IC90 | 29.4-94.1 | 20.0 | 33.3-100.0 | 16.7 |
| MIC2 or IC50 | 53.1-97.0 | 50.0 | 40.0-100.0 | 53.3 | |
| RP-G, 103 yeasts/ml, 48 h | MIC0 or IC90 | 41.1-94.1 | 40.0 | 26.6-90.0 | 10.0 |
| MIC2 or IC50 | 38.2-100.0 | 26.7 | 36.6-96.7 | 36.7 | |
| RP-G, 105 yeasts/ml, 24 h | MIC0 or IC90 | 38.2-100.0 | 36.7 | 30.0-100.0 | 20.0 |
| MIC2 or IC50 | 41.1-94.1 | 26.7 | 53.3-100.0 | 40.0 | |
| RP-G, 105 yeasts/ml, 48 h | MIC0 or IC90 | 35.2-100.0 | 60.0 | 46.6-96.7 | 16.7 |
| MIC2 or IC50 | 40.6-100.0 | 16.7 | 30.0-100.0 | 30.0 | |
| AM3, 24 h | MIC0 or IC90 | 56.2-100.0 | 50.0 | 40.0-92.9 | 6.7 |
| MIC2 or IC50 | 43.7-100.0 | 60.0 | 42.8-93.3 | 33.3 | |
| AM3, 48 h | MIC0 or IC90 | 48.3-100.0 | 40.0 | 43.3-96.7 | 6.7 |
| MIC2 or IC50 | 18.7-100.0 | 56.7 | 20.0-96.7 | 16.7 | |
Agreement range, percentage of results within excellent agreement range across all 30 isolates; excellent agreement, percentage of isolates with ≥90% interlaboratory agreement.
For the two QC isolates, excellent agreement with published MIC ranges (3) was achieved under NCCLS-recommended conditions (MIC2 read at 48 h in RPMI). For C. parapsilosis ATCC 22019 all data, except for a single result of 0.25 μg/ml fell in the QC range of 0.5 to 4.0 μg/ml (mode 1.0 μg/ml). For C. krusei ATCC 6258, 16 laboratories reported results within the QC range of 0.25 to 1.0 μg/ml (mode = 0.5 μg/ml), with one laboratory reporting 0.13 μg/ml.
Under many of the test conditions, the interlaboratory disparities in caspofungin visual MIC results for all 30 yeast isolates were considerable. MICs reported for some individual isolates included the high and low extremes of the concentration ranges tested. However, this effect was exacerbated by a minority of laboratories whose results occasionally differed substantially from those of the other participants. Scrutiny of the modal MICs for the panel of 30 Candida spp. isolates from each laboratory indicated outlying data (more than two dilutions higher or lower than the mode of values for all of the laboratories) under most test conditions. The results from four laboratories—C, D, F, and L—differed most often from the remainder by this assessment, but there was no consistent discrepancy, suggesting that data from any individual laboratories were persistently higher or lower than the rest to justify their exclusion from analysis.
The interlaboratory agreement for caspofungin IC50 and IC90 values determined from spectrophotometric data are also summarized in Table 2. When all data were included in the analysis the interlaboratory agreement for the spectrophotometric data was of a similar order as for visually read MICs. Excellent agreement for more than half of the test isolates was seen only in RPMI and in RP-G with the 103 yeasts/ml initial concentration.
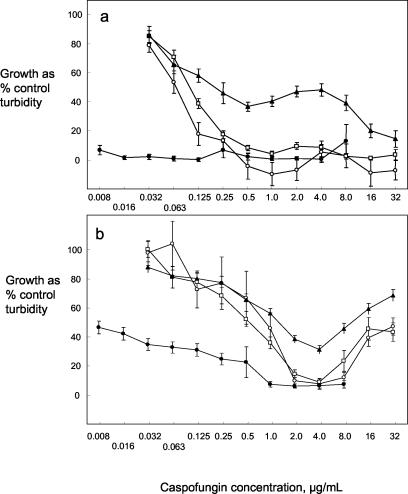
Figure 1 illustrates typical characteristics of the dose-response curves obtained from spectrophotometric means of growth turbidity relative to control growth in caspofungin dilution series under different conditions. For C. albicans isolate Y102 after 24 h of incubation (Fig. 1a), the dose-response curves in RPMI and RP-G with 103 cells/ml were similar. For RP-G with the higher inoculum, the curve showed an inflexion at middle-range caspofungin concentrations and then fell to <20% of control at 32 μg/ml. The curve in AM3 indicated considerable growth inhibition at all caspofungin concentrations. Interlaboratory agreement was good after 24 h, with low standard errors of the mean at all caspofungin concentrations. For C. albicans Y106 after 48 h (Fig. 1b) the dose-response curves showed a nonsigmoid appearance. In all three RPMI 1640-based media the curves indicated growth inhibition to be <40% of the control with caspofungin at 4 μg/ml and then regrowth at higher concentrations. The curve in AM3 showed no regrowth of the yeast at high caspofungin concentrations.
FIG. 1.
Dose-response curves for caspofungin growth inhibition constructed from individual spectrophotometric data presented by 16 laboratories. Curves show growth of a C. albicans isolate as mean percent control in the presence of a caspofungin concentration series, with error bars showing the standard error of the mean (n = 32). Cultures were in RPMI (○), RP-G inoculated to 103/ml (□), RP-G inoculated to 105/ml (▴), and AM3 (•). (a) Y102 after 24 h of incubation; (b) Y106 after 48 h of incubation.
Test conditions to distinguish Candida isolates of high and low caspofungin susceptibility.
The panel of yeast isolates included eight yeasts known to have mutations in the FKS1 gene that result in lower than normal caspofungin susceptibility. To determine whether the conditions that gave highest interlaboratory test reproducibility for caspofungin MIC and IC values could also distinguish the two yeast susceptibility groups, the results obtained by MIC2 and IC50 in RPMI and AM3 after 24 h were analyzed (Table 3). The distribution of MIC results reported from the 17 laboratories for the isolates with normal and reduced caspofungin susceptibility were analyzed by the χ2 test. The larger the value of χ2 for each paired data set of high versus low susceptibility, the greater the differentiation between the susceptibility groups (all of the values in Table 4 showed a statistically significant difference in the MIC distributions for high- and low-susceptibility isolates). The results suggested that the visually determined MIC2 values discriminated better between the two groups than the spectrophotometrically determined IC50 values and that the data from tests in AM3 discriminated better than the data from tests in RPMI.
TABLE 3.
Percentages of MIC2 or IC50 results recorded in 17 laboratories for 8 Candida spp. isolates with known low susceptibility to caspofungin and 22 isolates with normal susceptibility
| Caspofungin concn (μg/ml) | % MIC2 or IC50a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| RPMI (MIC2)
|
RPMI (IC50)
|
AM3 (MIC2)
|
AM3 (IC50)
|
|||||
| Normal (n = 739) | Low (n = 265) | Normal (n = 535) | Low (n = 193) | Normal (n = 733) | Low (n = 270) | Normal (n = 513) | Low (n = 198) | |
| 0.008 | 0.0 | 0.0 | 0.0 | 0.0 | 56.5 | 6.7 | 71.2 | 8.6 |
| 0.016 | 0.0 | 0.0 | 0.0 | 0.0 | 8.9 | 0.4 | 9.6 | 2.5 |
| 0.032 | 4.6 | 0.4 | 9.3 | 3.6 | 22.0 | 1.5 | 11.1 | 2.5 |
| 0.063 | 21.2 | 0.0 | 26.5 | 1.6 | 7.6 | 1.9 | 5.8 | 2.5 |
| 0.13 | 20.7 | 0.0 | 23.6 | 5.7 | 2.7 | 3.3 | 0.4 | 5.1 |
| 0.25 | 18.4 | 1.9 | 18.1 | 2.1 | 0.5 | 7.4 | 0.0 | 4.5 |
| 0.5 | 21.1 | 2.3 | 14.6 | 3.6 | 0.1 | 21.5 | 0.4 | 15.2 |
| 1 | 11.1 | 14.0 | 6.5 | 13.5 | 0.0 | 28.1 | 0.0 | 33.3 |
| 2 | 1.8 | 46.8 | 1.1 | 37.8 | 0.0 | 17.8 | 0.0 | 15.7 |
| 4 | 0.0 | 25.3 | 0.0 | 20.2 | 0.0 | 6.7 | 0.0 | 6.1 |
| 8 | 0.1 | 4.5 | 0.0 | 5.7 | 0.0 | 0.7 | 0.2 | 2.0 |
| 16 | 0.5 | 2.3 | 0.2 | 4.7 | 1.6 | 4.1 | 1.4 | 2.0 |
| 32 | 0.4 | 1.5 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| >32 | 0.0 | 1.1 | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 |
n = the number of results from 17 laboratories. The χ2 values for RPMI (MIC2), RPMI (IC50), AM3 (MIC2), and AM3 (IC50) were 727, 467, 784, and 529, respectively.
TABLE 4.
Interlaboratory agreement for caspofungin susceptibility data with 20 isolates of Aspergillus spp.a
| Test conditions | Endpoint | Visual readings
|
Spectrophotometer results
|
||
|---|---|---|---|---|---|
| Agreement range (%) | Excellent agreement (%) | Agreement range (%) | Excellent agreement (%) | ||
| RPMI, 24 h | MIC0 or IC90 | 73.5-100.0 | 75.0 | 34.4-93.1 | 5.0 |
| MIC2 or IC50 | 46.8-91.2 | 5.0 | 27.5-86.2 | 0.0 | |
| MEC | 47.0-90.6 | 5.3 | |||
| MEC (modified)b | 91.1-100.0 | 100.0 | |||
| RPMI, 48 h | MIC0 or IC90 | 38.2-79.4 | 0.0 | 48.0-96.6 | 20.0 |
| MIC2 or IC50 | 50.0-96.4 | 10.0 | 20.0-72.0 | 0.0 | |
| RP-G, 24 h | MIC0 or IC90 | 82.3-100.0 | 70.0 | 37.9-93.1 | 5.0 |
| MIC2 or IC50 | 37.5-96.9 | 15.0 | 24.1-82.8 | 0.0 | |
| MEC | 50.0-96.9 | 0.0 | |||
| MEC (modified) | 85.2-100.0 | 75.0 | |||
| RP-G, 48 h | MIC0 or IC90 | 52.9-88.2 | 0.0 | 52.1-95.7 | 55.0 |
| MIC2 or IC50 | 57.6-100.0 | 30.0 | 24.0-80.0 | 0.0 | |
| AM3, 24 h | MIC0 or IC90 | 57.6-100.0 | 70.0 | 41.3-93.1 | 35.0 |
| MIC2 or IC50 | 32.3-94.1 | 10.0 | 31.0-89.7 | 0.0 | |
| MEC | 60.0-96.7 | 15.0 | |||
| MEC (modified) | 81.2-100.0 | 85.0 | |||
| AM3, 48 h | MIC0 or IC90 | 26.4-91.2 | 10.0 | 44.8-93.1 | 5.0 |
| MIC2 or IC50 | 69.2-100.0 | 65.0 | 37.9-89. | 0.0 | |
High levels of agreement reflect predominantly off-scale readings. Agreement range, percentage of results within excellent agreement range across all 30 isolates; excellent agreement, percentage of isolates with ≥90% interlaboratory agreement.
MEC (modified), data from laboratories E, H, and J were excluded from analysis.
Itraconazole MICs for Aspergillus spp.
The itraconazole MICs for the panel of 20 Aspergillus spp. isolates tested in the 17 participating laboratories were read as the MIC0 after 48 h growth of the molds. The data varied only over a narrow range of values, from 0.13 to 1.0 μg/ml, with a modal MIC for most isolates of 0.5 μg/ml. For the two NCCLS reference isolates, A. flavus ATCC 204304 and A. fumigatus ATCC 204305, the modal itraconazole MICs of 0.50 and 1.0 μg/ml fell within the prescribed reference ranges for method M38-A (17). The percentage of data within the prescribed ranges was 90.9% (30 of 33 readings) and 100% for the two strains, respectively.
Caspofungin MICs for Aspergillus spp.
Agreement between 17 laboratories for MIC and IC values of caspofungin for the 20 Aspergillus isolates for all growth media, inoculum sizes, incubation times, and end points is summarized in Table 4. The agreement between results obtained with the spectrophotometer was impossibly poor under all conditions. For the visually read end points, the best agreement was obtained (≥70% of isolates showing excellent agreement) for MIC0 readings at 24 and 48 h for all growth media. However, this is a misleading observation, since almost all of the isolates were scored as resistant to the highest concentration of caspofungin tested (MIC > 16 μg/ml) in terms of MIC0 in most laboratories, and agreement with so many off-scale data was to be expected. The interlaboratory agreement for the tests with Aspergillus spp. isolates was therefore interpreted as poor under all conditions. Spectrophotometric IC determinations with the molds were unhelpful with the Aspergillus spp. isolates.
Caspofungin MEC values for Aspergillus spp.
Measurement of the 24-h MECs did not enhance the poor interlaboratory reproducibility of the Aspergillus susceptibility data; results tended to parallel those obtained with the MIC2 readings (Table 4). However, MEC data from laboratories E, H, and J tended to be much higher than those from other laboratories, suggesting possible problems with MEC readings from these three participants. Removal of these sets of data from the analysis improved the interlaboratory agreement under all three test conditions (Table 4). The percentage of 20 isolates for which excellent agreement was achieved rose from only 5 to 15%, with all results included, to 100, 75, and 85% in RPMI, RP-G, and AM3, respectively, with the data from laboratories E, H, and J omitted.
MEC results also produced the lowest susceptibility readings for the Aspergillus isolates. In RPMI, RP-G, and AM3, respectively, 93, 91, and 87% of the reported MECs were <1 μg/ml, rising to 100, 98, and 98%, respectively, when the results from laboratories E, H, and J were omitted.
DISCUSSION
This study involved analysis of more than 52,000 individual datum points determined from 17 laboratories located in North America, Europe, and Australasia and represents one of the most extensive surveys of methodology for susceptibility testing with an antifungal agent ever undertaken. The results provide reasons for both optimism and concern over susceptibility testing with caspofungin in vitro. We set our criterion for excellent reproducibility as a requirement for ≥90% of MIC or IC data for a single fungal isolate to be within a mode ± one dilution range. This matches the levels of agreement obtained in, for example, NCCLS QC studies with antifungal agents (3). It may be argued that the criterion is too stringent, but it represents the normally accepted standard.
A positive finding of our study is that two test conditions, MIC2 or IC50 read at 24 h for cultures in RPMI 1640 medium or AM3, gave excellent interlaboratory agreement for a majority of the panel of 30 isolates of Candida spp., both by visual and spectrophotometric end point determination. Under these conditions a high level of discrimination was achieved between strains known to have lowered caspofungin susceptibility and normally susceptible isolates. These conditions therefore emerge as strong contenders for refinement of methodology to give as-near-as-possible acceptable interlaboratory reproducibility for caspofungin testing—an obvious prerequisite before clinically relevant resistance breakpoints can be set for this new antifungal agent.
However, it is a matter of concern that the reproducibility in caspofungin susceptibility tests with isolates of Aspergillus spp. was of such a low order. Under all conditions tested, some growth of these molds occurred in the presence of all concentrations of caspofungin, so that a susceptibility test end point based on complete inhibition of growth is impractical, as has been observed previously (1, 10, 13). In contrast, an MIC end point based on “prominent growth reduction relative to control” (MIC2) gave results at the very low end of the caspofungin concentration range tested, suggesting a high susceptibility of Aspergillus spp. to this agent. However, even for MIC2 data, the proportion of isolates for which 90% of the results fell within the range of mode ± one dilution was unacceptably low. The MEC, an end point proposed in previous studies that also measures partial, rather than complete growth inhibition (1, 10, 13), greatly improved interlaboratory reproducibility over MIC readings in the present study when data from 3 of the 17 participating laboratories were excluded. The reason why three laboratories that would generally be regarded as experienced with antifungal susceptibility testing should produce aberrant data is unclear. The MEC end point requires some caution in its interpretation, as it represents a subjective assessment of the appearance of growth, but our findings generally confirm the utility of this end point for testing caspofungin against Aspergillus spp.
For both the Candida and the Aspergillus spp. tested, the data for fluconazole or itraconazole against QC and reference isolates fell almost perfectly within expected limits. This finding confirmed the competence of the 17 laboratories to undertake antifungal susceptibility testing. Although it was possible to define MEC data from 3 of 17 laboratories as unacceptably divergent, the same was not true for caspofungin MICs against Candida or Aspergillus spp., where apparently outlying data sets were typically seen as occasional idiosyncracies under just one of the conditions tested, rather than as consistently “maverick” results from one or more laboratories.
The results for tests with fluconazole versus Candida spp. gave a higher level of interlaboratory discordance than anticipated. These tests were done by the reference microdilution methodology prescribed by the NCCLS (19); yet, for 5 of the 30 isolates tested, fewer than 80% of the results from the 17 laboratories agreed within a mode ± one dilution range. A recent New York State proficiency testing study found an overall level of agreement with reference values of just 75% for fluconazole MICs determined in seven laboratories with five isolates of Candida spp. (24). No Candida isolate from an infected patient is routinely subjected to multiple-laboratory testing, and the possibility of over- or underestimation of susceptibility in a single laboratory test appears high enough to justify future attention.
Susceptibility testing with any microorganism is a procedure with a high level of inherent run-to-run variability, and any prescribed set of test conditions represents a compromise between choices of test variables. Some isolates of fungi seem to generate consistent MIC data regardless of variations in test conditions, whereas others show high vulnerability even to relatively minor variations (4, 25). The objective of the design of susceptibility test conditions should be to devise a methodology that is least affected by small differences in technical parameters. Our study shows that, for tests with caspofungin against Candida spp., the present NCCLS method M27-A gives reasonable interlaboratory reproducibility, provided MICs are read as MIC2 after 24 h of incubation. However, there is still room for improvement in the methodology to raise the level of interlaboratory agreement to a higher standard. For caspofungin testing with Aspergillus spp., the MEC currently offers an end point that can give generally reproducible results in many laboratories, but the finding of 3 laboratories of 17 generating aberrant data even by MEC suggests that further refinement of caspofungin test methodology for Aspergillus spp. is still required.
Acknowledgments
This study was supported by a grant from Merck & Co., Inc.
REFERENCES
- 1.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2002. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob. Agents Chemother. 46:245-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry, A. L., M. A. Pfaller, S. D. Brown, A. Espinel-Ingroff, M. A. Ghannoum, C. Knapp, R. P. Rennie, J. H. Rex, and M. G. Rinaldi. 2000. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J. Clin. Microbiol. 38:3457-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartizal, C., and F. C. Odds. 2003. Influence of methodologic variables on susceptibility testing with caspofungin against Candida species and Aspergillus fumigatus. Antimicrob. Agents Chemother. 47:2100-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartizal, K., C. J. Gill, G. K. Abruzzo, A. M. Flattery, L. Kong, P. M. Scott, J. G. Smith, C. E. Leighton, A. Bouffard, J. F. Dropinski, and J. Balkovec. 1997. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2326-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuenca-Estrella, M., W. Lee-Yang, M. A. Ciblak, B. A. Arthington-Skaggs, E. Mellado, D. W. Warnock, and J. L. Rodriguez-Tudela. 2002. Comparative evaluation of NCCLS M27-A and EUCAST broth microdilution procedures for antifungal susceptibility testing of Candida species. Antimicrob. Agents Chemother. 46:3644-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Poeta, M., W. A. Schell, and J. R. Perfect. 1997. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob. Agents Chemother. 41:1835-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas, C. M., J. A. Dippolito, G. J. Shei, M. Meinz, J. Onishi, J. A. Marrinan, W. Li, G. K. Abruzzo, A. Flattery, K. Bartizal, A. Mitchell, and M. B. Kurtz. 1997. Identification of the fks1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 41:2471-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole Sch56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinel-Ingroff, A. 2003. Evaluation of broth microdilution testing parameters and agar diffusion Etest procedure for testing susceptibilities of Aspergillus spp. to caspofungin acetate (MK-0991). J. Clin. Microbiol. 41:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keating, G. M., and B. Jarvis. 2001. Caspofungin. Drugs 61:1121-1129. [DOI] [PubMed] [Google Scholar]
- 12.Krishnarao, T. V., and J. N. Galgiani. 1997. Comparison of the in vitro activities of the echinocandin LY303366, the pneumocandin MK-0991, and fluconazole against Candida species and Cryptococcus neoformans. Antimicrob. Agents Chemother. 41:1957-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtz, M. B., G. Abruzzo, K. Bartizal, J. A. Marrinan, W. Li, J. Milligan, K. Nollstadt, and C. M. Douglas. 1996. Characterization of echinocardin-resistant mutants of Candida albicans—genetic, biochemical, and virulence studies. Infect. Immun. 64:3244-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laverdiere, M., C. Restieri, and F. Habel. 2002. Evaluation of the in vitro activity of caspofungin against bloodstream isolates of Candida species from cancer patients: comparison of Etest and NCCLS reference methods. Int. J. Antimicrob. Agents 20:468-471. [DOI] [PubMed] [Google Scholar]
- 15.Lozano-Chiu, M., P. W. Nelson, V. L. Paetznick, and J. H. Rex. 1999. Disk diffusion method for determining susceptibilities of Candida spp. to MK-0991. J. Clin. Microbiol. 37:1625-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marco, F., M. A. Pfaller, S. A. Messer, and R. N. Jones. 1998. Activity of MK-0991 (L-743,872), a new echinocandin, compared to those of LY303366 and four other antifungal agents tested against blood stream isolates of Candida spp. Diagn. Microbiol. Infect. Dis. 32:33-37. [DOI] [PubMed] [Google Scholar]
- 17.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. NCCLS, Wayne, Pa.
- 18.NCCLS. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 2nd ed. NCCLS, Wayne, Pa.
- 20.Nelson, P. W., M. Lozano-Chiu, and J. H. Rex. 1997. In vitro growth-inhibitory activity of pneumocandins L-733,560 and L-743,872 against putatively amphotericin B- and fluconazole-resistant Candida isolates: influence of assay conditions. J. Med. Vet. Mycol. 35:285-287. [PubMed] [Google Scholar]
- 21.Pfaller, M. A., R. N. Jones, G. V. Doern, A. C. Fluit, J. Verhoef, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, and R. J. Hollis. 1999. International surveillance of blood stream infections due to Candida species in the European SENTRY program: species distribution and antifungal susceptibility including the investigational triazole and echinocandin agents. Diagn. Microbiol. Infect. Dis. 35:19-25. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller, M. A., F. Marco, S. A. Messer, and R. N. Jones. 1998. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn. Microbiol. Infect. Dis. 30:251-255. [DOI] [PubMed] [Google Scholar]
- 23.Pfaller, M. A., S. A. Messer, K. Mills, A. Bolmstrom, and R. N. Jones. 2001. Evaluation of Etest method for determining caspofungin (MK-0991) susceptibilities of 726 clinical isolates of Candida species. J. Clin. Microbiol. 39:4387-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramani, R., and V. Chaturvedi. 2003. Proficiency testing program for clinical laboratories performing antifungal susceptibility testing of pathogenic yeast species. J. Clin. Microbiol. 41:1143-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rambali, B., J. A. Fernandez, L. Van Nuffel, F. Woestenborghs, L. Baert, D. L. Massart, and F. C. Odds. 2001. Susceptibility testing of pathogenic fungi with itraconazole: a process analysis of test variables. J. Antimicrob. Chemother. 48:163-177. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez, J. A., M. Lynch, D. Boikov, and J. D. Sobel. 1997. In vitro activity of a new pneumocandin antifungal, L-743,872, against azole-susceptible and -resistant Candida species. Antimicrob. Agents Chemother. 41:1612-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]