Abstract
An online questionnaire was developed to explore respiratory protective device (RPD) prevalence in U.S. health care facilities. The survey was distributed to professional nursing society members in 2014 and again in 2015 receiving 322 and 232 participant responses, respectively. The purpose of this study was to explore if the emergency preparedness climate associated with Ebola virus disease changed the landscape of RPD use and awareness. Comparing response percentages from the two sampling time frames using bivariate analysis, no significant changes were found in types of RPDs used in health care settings. N95 filtering facepiece respirators continue to be the most prevalent RPD used in health care facilities, but powered air-purifying respirators are also popular, with regional use highest in the West and Midwest. Understanding RPD use prevalence could ensure that health care workers receive appropriate device trainings as well as improve supply matching for emergency RPD stockpiling.
Keywords: respiratory protective devices, pandemic preparedness, personal protective equipment (PPE), health care workers, prevalence survey, emergency response
The emergence of novel, global, infectious diseases such as severe acute respiratory syndrome (SARS), Influenza A H1N1, and most recently Ebola virus disease (EVD), highlights the need for more focus on respiratory protection pandemic planning (Frieden, Damon, Bell, Kenyon, & Nichol, 2014; Iskander, Strikas, Gensheimer, Cox, & Redd, 2013). Central to the emergency preparedness paradigm is the accessibility of personal protective equipment (PPE), specifically respiratory protective devices (RPDs). More than 11 million health care workers (HCWs) are expected to benefit from the use of RPDs during an infectious respiratory pandemic (Cooley et al., 2010). One report found national emergency planning hampered by lack of information on current PPE supplies in hospitals, variability in regional distribution, and the number and types of respiratory PPE needed for emergency capacity (Association of State and Territorial Health Officials [ASTHO], 2014). It is estimated that U.S. acute care hospitals collectively hold 60 million N95 filtering facepiece respirators (N95 FFRs), a commonly used type of RPD, with state holdings varying from 14,000 to 32 million (ASTHO, 2014). A projected 1.7 to 7.3 billion respirators would be needed by health care and emergency services in a respiratory pandemic, more than 30 times the current local holdings (Carias et al., 2015).
Engineering and administrative controls are the preferred methods of minimizing HCW infection risk; however, these types of controls are not always possible to maintain in health care facilities. Respiratory protective devices and other PPE are a last line of defense when exposures cannot be reduced to an acceptable level using other control methods (National Institute for Occupational Safety and Health [NIOSH], Occupational Safety & Health Administration [OSHA], 2015). The Department of Labor’s OSHA respiratory protection standards can be met using many different types, makes, and models of RPDs (OSHA, 2011). Three major types of RPDs are used to protect HCWs: disposable N95 FFRs, powered air-purifying respirators (PAPRs), and elastomeric half-facepiece respirators (EHFRs; NIOSH, OSHA, 2015). All N95 FFRs are approved by NIOSH; some, known as “surgical N95 respirators,” are cleared by the U.S. Food and Drug Administration (FDA) as medical devices (FDA, 2015). Other respirators such as PAPRs and EHFRs are approved by NIOSH but are not currently evaluated or cleared by the FDA as medical devices. All RPDs, even those cleared by the FDA as a medical device, must be used in an OSHA respiratory protection program.
Applying Research to Practice.
N95 filtering facepiece respirators (N95 FFRs) continue to be the most prevalent respiratory protective device (RPD) used by health care workers (HCWs); however, increasing use of powered air-purifying respirators in health care indicates the need for targeted education based on regional trends tailored to the types of RPDs used in health care facilities. Regional pandemic planning agencies must work closely with health care facilities to understand which RPDs are being used, and for FFRs and elastomeric half-facepiece respirators fit tested, in the field so product matching best protects HCWs in emergency situations. It is important that health care organizations improve communication with HCWs to provide them with accessible information about the types and models of RPDs available for their use.
In addition to local facility stockpiles, hospitals can receive PPE supply assistance from state and federal programs. The Centers for Disease Control and Prevention (CDC) Strategic National Stockpile (SNS), responsible for national emergency preparedness, holds large quantities of health care supplies, including respirators, for distribution in the event of a public health emergency severe enough to exhaust local supplies (CDC, 2015b). Several studies suggest that stockpiling multiple types of respirators (i.e., FFR, PAPR, and EHFR) could be a valuable strategy in terms of cost, use, and quality (Baracco, Eisert, Eagan, & Radonovich, 2015; Carias et al., 2015). However, to date, little data on the actual makes and models of RPDs used in health care have been reported.
During the 2009 H1N1 pandemic, 75% of the SNS’s supply of FFRs were deployed to health care facilities. In many cases, the FFRs delivered from the SNS were not the same model for which HCWs had been fit tested. Consequently, valuable time was used for just-in-time fit testing (U.S. Department of Health and Human Services [HHS], 2012). Fit testing is required to ensure that when donned properly, the selected brand and size of respirator fits adequately to protect the wearer (NIOSH, OSHA, 2015). Even more troublesome, many HCWs reported that FFRs were not readily available at their facility throughout the 2009 pandemic (Beckman et al., 2013; Lautenbach, Saint, Henderson, & Harris, 2010). A study conducted by Rebmann, Wang, Swick, Reddick, and delRosario (2013) found that almost half of the sampled hospitals did not have enough stockpiled N95 FFRs. To address this problem, the Office of the Assistant Secretary for Preparedness and Response (ASPR), part of the HHS, recommended regional PPE cache inventory supplies be deployed to match the models used by health care organizations to promote proper use and compliance as well as reduce the need for additional fit testing (ASPR, Hospital Preparedness Program, 2012).
The need for RPDs may be affected by hospital emergency planning and recommendations for outbreaks (e.g., SARS and H1N1 influenza). The Veterans Health Administration, after the 2009 H1N1 outbreak, stockpiled three models of EHFRs (3M™ 7501, Scott 7421, and Sperian Survivair 1050) to be used if a shortage of N95 FFRs occurs (Bessesen, Adams, Radonovich, & Anderson, 2015). Most recently, the EVD epidemic spurred an evaluation of the emergency preparedness protocols developed for HCW safety, including RPD recommendations (Frieden et al., 2014). The CDC recommends the use of a PAPR or a disposable NIOSH-approved N95 FFR with a face shield for EVD patient care to protect eyes, mouth, and nose from contact and aerosol exposure. Although airborne transmission of Ebola has not been documented, aerosol-generating procedures (e.g., endotracheal intubation) may create mechanically generated infectious aerosols. The CDC recommends HCWs wear respiratory protection to limit exposure during these procedures that may be required unexpectedly during lifesaving EVD patient care (CDC, 2015a). Although the CDC offers recommendations for hospital PPE, employers are ultimately responsible for providing hazard-free workplaces (NIOSH, OSHA, 2015). Therefore, exact PPE for specific EVD patients may vary based on availability, staff, and facility (Hewlett et al., 2015). In 2014, U.S. hospitals across the country took stock of PPE supplies and modified standing protocols to match CDC recommendations for providing care to EVD patients; however, PPE often remained the most frequent concern (Polgreen et al., 2015). Many hospitals chose to use PAPRs as part of HCW PPE ensembles. This shift to PAPR use in hospitals may have affected the prevalence of RPD types available to HCWs.
The NIOSH in collaboration with the American Association of Occupational Health Nurses (AAOHN) conducted an exploratory study to determine the prevalence of RPDs in U.S. health care facilities. Originally designed to explore if, and how often PAPRs were being used in health care settings, the study became an opportunity to monitor changes in the landscape of RPD use and awareness from 2014 to 2015. The aim of this study was to answer the following questions about hospital preparedness:
Has the prevalence of RPD use changed from 2014 to 2015, before and after Ebola PPE preparations?
Are there regional differences in RPD use?
What are the most common RPD types and models?
Were respondents aware of types and models of RPDs used in their health care facility?
This project was deemed non-research by the NIOSH Human Subjects Review Board as responses were anonymous.
Method
In November 2013, an 11-question survey was developed by AAOHN and NIOSH. AAOHN is a professional association of licensed nurses engaged in the practice of occupational and environmental health nursing. The association represents the largest group of occupational health professionals, with more than 6,000 members, delivering health and safety services to employees, employee populations, and community groups in a variety of workplace settings (AAOHN, 2012). AAOHN staff and members served as both subject matter experts and hosts for the survey. Distribution of the anonymous survey was facilitated using the website SurveyMonkey® (Palo Alto, CA) with participant recruitment of AAOHN members working in hospitals and other health care facilities.
Survey questions were derived from past studies about RPD use in health care settings, a review of EHFR and PAPR models sold by top medical supply distributors, and N95 FFR models known to have been in the SNS during the H1N1 pandemic (Ciconte & Danyluk, 2013; FDA, 2010; Peterson, 2013; Radonovich, Yanke, Cheng, & Bender, 2010; Tompkins & Kerchberger, 2010). Pilot testing resulted in minor survey changes, including adding pictures of each type of RPD, additional answer options, and a respondent question or comments section. The survey instrument asked respondents to provide their health care facility state location, RPD types used at their facility, number of employees using these devices, and commonly used models for each type of RPD (Table 4). Respondents were asked to report information from the past 12 months, including RPD training exercises and fit testing. Type and size of health care facility as well as the circumstance for RPD use (i.e., patient care vs. non-patient care use) were not captured. The 2015 survey instrument did not change except to update the listing of RPD model names if manufacturers made changes after the 2014 survey. No models were removed from the list, and participants could write in an RPD model if a particular model was not listed.
Table 4.
Sample Prevalence Instrument
| Survey questions | Answers |
|---|---|
| 1. Which state is your health care facility located in? | (select one state) |
Below is an example of an N95 (courtesy of the OSHA). 2. Have any of your employees used an N95 FFR at least once in the past year, including training exercises or fit testing? |
Yes No |
| 3. Approximately how many of your employees have used an N95 FFR in the past year, including training exercises or fit testing? | 10 or less 11-100 101-500 More than 500 |
| 4. What are the three most common types of N95 FFRs used in your health care facility? If not listed, please provide the name of the manufacturer and model number. | 3M™ 1860/1860S 3M™ Aura™ 1870+ 3M™ 8210/8110S 3M™ Aura™ 9210+ 3M™ VFlex™ 1805/1805S Gerson® 1730 Gerson® 2735 Kimberly-Clark™ PFR95-170 (46727)/PFR95174 (46827) Kimberly-Clark™ PFR N95 (62355)/PFR N95 (62126) Moldex® 1500 Moldex® 2200 series Moldex® 3000 series Safe Life® B130 Safe Life® B150 Other (please specify) |
Below are examples of PAPRs (courtesy of OSHA). 5. Have any of your employees used a PAPR at least once in the past year, including training exercises or fit testing? |
Yes No |
| 6. Approximately how many of your employees have used a PAPR in the past year, including training exercises or fit testing? | 10 or less 11-100 101-500 More than 500 |
| 7. What are the three most common types of PAPRs used in your health care facility? If not listed, please provide the name of the manufacturer and model number. | 3M™AirMate™ 3M™ Breathe Easy™ 3M™ Versa Flo™ Bullard™ PA20™ Bullard™ PA30™ Bullard™ EVA™ Syntech International MAXAIR® ILC Dover Sentinel series Other (please specify) |
Below is an example of an elastomeric half-mask respirator (courtesy of OSHA). 8. Have any of your employees used an EHFR at least once in the past year, including training exercises or fit testing? |
Yes No |
| 9. Approximately how many of your employees have used an EHFR in the past year, including training exercises or fit testing? | 10 or less 11 to 100 101 to 500 More than 500 |
| 10. What are the three most common types of EHFRs used in your health care facility? If not listed, please provide the name of the manufacturer and model number. | 3M™ 6000 series 3M™ 7500 series Honeywell Sperian® Survivair® Blue 1 Hversion Honeywell Sperian® Survivair® Blue 1 Sseries North® by Honeywell 5500 series Half-Mask North® by Honeywell 7700 series Half-Mask MSA Comfo Classic® Half-Mask MSA Advantage® series Half-Mask Other (please specify) |
| 11. Do you have any comments, questions, or concerns about the material covered in this survey? If you would like a response, please provide your contact information. | (free response space) |
Note. OSHA = Occupational Safety and Health Administration; FFR = filtering facepiece respirators; PAPR = powered air-purifying respirators; EHFR = elastomeric half-facepiece respirators.
Participant recruitment was conducted via email invitation to AAOHN members who indicated they worked in a health care setting from January to March 2014. To capture a wider audience, AAOHN invited the participation of other professional HCW organizations, including Association of Occupational Health Professionals in Healthcare (AOHP), American Nurses Association (ANA), and the American Board of Occupational Health Nurses (ABOHN). Weekly email reminders were sent to invited participants. The 2015 survey was online from August to October 2015, using the same 2014 recruitment method. Social media, including Facebook and LinkedIn, was used to encourage participation.
Statistical Analysis
Due to missing responses and skip patterns within the survey, each question had a different number of responses; each analysis included only those respondents who answered the question of interest. This method was used because not all respondents answered each question and some questions required multiple answers. All free response answers (i.e., “other” categories, comment/question section) were quantified and recoded prior to analysis. Regional comparisons used U.S. census regions for equal population distribution defined as Northeast (CT, ME, MA, NJ, NH, NY, PA, RI, VT), Midwest (IL, IN, IA, KS, MI, MN, MO, NE, ND, OH, SD, WI), South (AL, AR, DE, DC, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY; U.S. Census Bureau, 2015). Analytical focus relied on descriptive statistics and bivariate analyses using SAS Version 9.3 (Cary, NC). Participants’ use of an RPD, estimates of how many workers in their facilities used RPDs, and common models were compared by type within and between the surveys. In addition, RPD use by region, use of multiple types of RPDs, and comments received were analyzed for patterns.
Results
The survey garnered 554 survey responses: 322 respondents in 2014 and 232 respondents in 2015. Most of the participants (2014/2015, respectively) worked for health care facilities in the Midwest (35%/28%) or South (28%/29%) census regions and were affiliated with AAOHN (53%/66%) or AOHP (34%/23%). Table 1 describes the number of participants that answered “yes” to each type of respirator with the denominator being the total number of responses to that particular question. Both samples reported a majority of facilities used N95 FFRs (94%/95%) and PAPRs (78%/77%) in the past year; only 31%/26% reported the use of EHFRs. When looking at multiple types of respirators used and comparing the initial survey with the 2015 survey, 78%/79% reported using at least two types of RPDs in the workplace, the majority of which (48%/53%) reported using N95 FFRs as well as PAPRs in the past year. A smaller percentage (26%/21%) reported use of all three types of RPDs (N95 FFR, PAPR, and EHFR) within the past year. Only 3%/2% reported no RPD use; the exclusive use of EHFRs was not reported in either sample.
Table 1.
Number of Respondents Reporting RPD Use in Their Facilities in the Past Year by Type, Including During Training/Fit Testing
| 2014 n/N (%)a | 2015 n/N (%)a | |
|---|---|---|
| Used an N95 FFR in past year | 296/314 (94.3) | 221/232 (95.3) |
| Used a PAPR in past year | 234/299 (78.3) | 173/225 (76.9) |
| Used an EHFR in past year | 89/290 (30.7) | 57/220 (25.8) |
Note. RPD = respiratory protective devices; FFR = filtering facepiece respirators; PAPR = powered air-purifying respirators; EHFR = elastomeric half-facepiece respirators.
n = the number of respondents for that question, participants responded for each respirator type separately (see Table 4, Questions 2, 5, and 8).
Respondents were asked to estimate how many workers in their facilities used each type of RPD: less than 10 employees, 11 to 100 employees, 101 to 500 employees, or more than 500 employees. Similar to the overall prevalence of RPDs, respondents indicated that more employees in their facilities used N95 FFRs (more than 500 employees; 54%/57%) than PAPRs (11–100 employees; 56%/55%) or EHFRs (less than 10 employees; 58%/66%) in the past year. Very few participants indicated that more than 500 workers used a PAPR (6%/7%) or EHFR (1%/0%) in the prior reporting year. No significant difference was found between the 2014 and 2015 survey responses for this question.
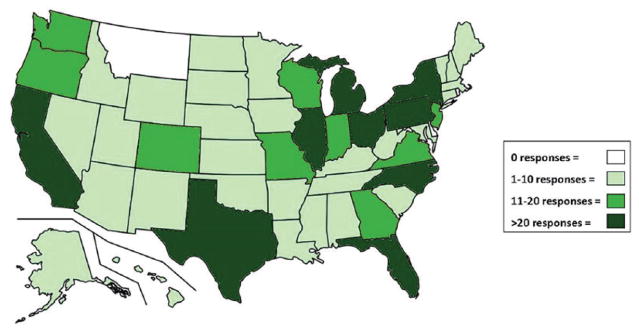
Study participants from 47 states responded to the 2014 survey, with no participation from HCWs in Delaware, Montana, or North Dakota. The 2015 survey respondents practiced in 45 states, with no participation from Delaware, Hawaii, Montana, Vermont, or Wyoming (Figure 1; Table 4, Question 1.). No significant difference between the 2014 and 2015 surveys were found for number of respondents per state.
Figure 1.
Combined 2014 and 2015 response map of participant health care facility location (see Table 4, Question 1).
As shown in Table 2, the range of reported respirator usage among the four census regions (Northeast, Midwest, South, and West) was fairly small for N95 FFRs (92%–98% in 2014, 92%–100% in 2015) and EHFRs (25%–35% in 2014, 21%–35% in 2015); the largest range was for PAPRs (65%–88% in 2014, 68%–87% in 2015). These RPD category results were not exclusive of each other as participants were asked to describe all RPDs used at their facility. Therefore, in Table 2, columns compare a type of respirator between regions while the rows compare respirator types within a region for the two sampled time frames. Bivariate analysis determined that in both samples, a significant difference in respirator usage was found among the four census regions for PAPR use (χ2 = 17.04, p < .001 in 2014 and χ2 = 9.06, p = .03 in 2015). Highest PAPR use was in the Midwest in 2014 and the West in 2015; lowest, in both samples in the Northeast. The range of use for both N95 FFRs and EHFRs was not significantly different among the four regions in either sample, indicating that the use of these RPDs was not dependent on regional factors.
Table 2.
RPD Type Used in Health Care Facilities by Census Region
| 2014 n (%)a | 2015 n (%)a | |||||||
|---|---|---|---|---|---|---|---|---|
| Total in region (n = 322) | N95 FFR (n = 314) | PAPR (n = 299) | EHFR (n = 290) | Total in region (n = 232) | N95 FFR (n = 232) | PAPR (n = 225) | EHFR (n = 220) | |
| Northeast | 47 (14.6) | 45 (97.8) | 28 (65.1) | 14 (35.0) | 50 (21.6) | 50 (100) | 34 (69.4) | 17 (35.4) |
| Midwest | 113 (35.1) | 102 (91.9) | 95 (88.0) | 33 (31.1) | 64 (27.6) | 60 (93.8) | 53 (84.1) | 16 (25.8) |
| South | 89 (27.6) | 84 (96.6) | 54 (67.5) | 20 (25.0) | 68 (29.3) | 66 (97.1) | 45 (68.2) | 13 (20.6) |
| West | 73 (22.7) | 65 (92.9) | 57 (83.8) | 22 (34.4) | 50 (21.6) | 45 (91.8) | 41 (87.2) | 11 (23.4) |
| Total | 322 (100) | 296 (94.3) | 234 (78.3) | 89 (30.7) | 232 (100) | 230 (99.6) | 173 (76.9) | 57 (25.9) |
| p value | .34 | .0007b | .576 | .18 | .03b | .34 | ||
Note. RPD = respiratory protective devices; FFR = filtering facepiece respirators; PAPR = powered air-purifying respirators; EHFR = elastomeric half-facepiece respirators.
n = the number of respondents for that question, participants responded for each respirator type separately (see Table 4, Questions 2, 5, and 8).
Significant.
Although fewer HCWs responded to the second survey, similar results were reported for common RPD models used in health care facilities (Table 3). Participants were asked to report the top three RPD models used in their facilities (Table 4, Questions 4, 7, 10). The manufacturer 3M™ was the most prevalent of all three types of RPDs. Prior to implementation of the second survey, the 3M™ 1870 was discontinued and replaced by the 3M™ Aura™ 1870+. Many respondents may have still been using the 3M™ 1870 at the time of the second survey; responses were recoded to represent the same product. Similarly, the N95 3M™ 9210 became the 3M™ Aura™ 9210+. Another challenge with product names was that participants chose “other” for the PAPR questions indicating they used the CAPR® (Controlled Air-Purifying Respirator) system by Syntech International MAXAIR. This product is a specific PAPR design from the manufacturer, but is still considered a PAPR and was thus recoded as a MAXAIR PAPR product. This recoding was required for 22%/28% of the PAPRs reported.
Table 3.
Survey Responses by RPD Type, Manufacturer, and Model
| Most common N95 FFR models | 2014 n (%)a | 2015 n (%)a |
|---|---|---|
| 3M™ 1860/1860S | 202 (71.9) | 147 (70.7) |
| 3M™ 1870 or 3M™ Aura™ 1870+ | 133 (47.3) | 83 (39.9) |
| Kimberly-Clark™ PFR95-170 (46727)/PFR95-174 (46827) | 77 (27.4) | 46 (22.1) |
| Kimberly-Clark™ PFR95 N95 (62355)/PFR N95 (62126) | 67 (23.8) | 48 (23.1) |
| Moldex® 1500 series | 17 (6.0) | 11 (5.3) |
| Otherb | 87 (31.0) | 76 (36.5) |
| Do not know | 1 (0.4) | 0 |
| Total number of respondents | n = 281 | n = 208 |
| Most common PAPR models | 2014 n (%)a | 2015 n (%)a |
| 3M™ Air-Mate™ | 107 (51.2) | 80 (50.6) |
| 3M™ Breathe Easy™ | 34 (16.3) | 30 (19.0) |
| 3M™ Versa Flo™ | 16 (7.7) | 16 (10.1) |
| ILC Dover Sentinel series | 14 (6.7) | N/Ab |
| Syntech International MAXAIR | 46 (22.1) | 44 (27.9) |
| Otherb | 12 (5.7) | 12 (7.6) |
| Do not know | 9 (4.3) | 4 (2.5) |
| Total number of respondents | n = 209 | n = 158 |
| Most common EHFR models | 2014 n (%)a | 2015 n (%)a |
| 3M™ 6000 series | 38 (48.7) | 20 (41.7) |
| 3M™ 7500 series | 24 (30.8) | 17 (35.4) |
| North® by Honeywell 5500 series Half-Mask | N/Ab | 4 (8.3) |
| North® by Honeywell 7700 series Half-Mask | 7 (9.0) | 3 (6.3) |
| MSA Comfo Classic® Half-Mask | N/Ab | 3 (6.3) |
| MSA Advantage® series Half-Mask | N/Ab | 3 (6.3) |
| Otherb | 7 (9.0) | 3 (6.3) |
| Do not know | 8 (10.3) | 3 (6.3) |
| Total number of respondents | n = 78 | n = 48 |
Note. Table 4, Questions 4, 7, and 10. RPD = respiratory protective devices; FFR = filtering facepiece respirators; PAPR = powered air-purifying respirators; EHFR = elastomeric half-facepiece respirators.
More than one response permitted; numbers and percentages may sum to more than total number of respondents or more than 100%.
“Other” category includes models that represent less than 5% of the sample.
The majority of the sample (79%/81%) reported using an FDA-cleared N95 FFR (i.e., surgical N95 respirator), although more than one third of respondents used PAPRs or EHFRs, which are not currently cleared by the FDA (CDC, National Personal Protective Technology Laboratory [NPPTL], 2015). In the initial survey, 27 unique N95 FFR models were reported, 15 of which were FDA-cleared. However, six of the 27 models represented less than 5% of the total number of responses (categorized as “other” in Table 3) indicating that the wide variation of models reported may be due to a handful of respondents using uncommon FFRs. In the second survey, 30 different models were reported, 18 of which were FDA-cleared; eight of these models represented less than 5% of the total sample.
In the “other” category for common RPD models, respondents included RPD models not listed. Also in this section, in 2014, one answer equating to do not know was received for N95 FFR models, nine do not know answers for PAPR models, and eight for EHFR models (Table 3). In the same section of the 2015 survey, zero do not know answers were listed for N95 FFR, four for PAPR models, and three for EHFR models. Thirty-eight participants wrote free response comments at the end of the survey; 21 in 2014 and 17 in 2015. The most frequently cited comments related to barriers of completing proper fit testing.
Discussion
Despite widespread, heightened pandemic PPE awareness and the overall belief that hospital preparedness was on the rise during the EVD epidemic, this study found that RPD use did not significantly change between 2014 and 2015. A poll by the Association for Professionals in Infection Control and Epidemiology (APIC) found that nearly all (92%) infection control leaders believed their facilities were better prepared for an emergency like Ebola, but 55% reported that hospitals had not reallocated resources for infection prevention and control (APIC, 2015).
Interest in PAPRs began to increase in 2003 when their use became widespread in some areas during the SARS outbreak (Khoo et al., 2005). The ASTHO estimated that in 2014, on average, 21 PAPRs per hospital were available with PAPR purchasing in hospitals increasing from 131,387 purchased in 2011 to more than four million purchased the following year (ASTHO, 2014). Increased usage of PAPRs was discussed in a 2014 Institute of Medicine (IOM) workshop on the use and effectiveness of PAPRs in health care (IOM, 2015). Pillai et al. (2015) found among infectious disease physicians that 60% reported PAPRs availability at their facilities and 10% reported EHFRs availability. These trends indicate that PAPR use is on the rise in a market currently dominated by N95 FFRs. However, in this study, no significant change in PAPR usage between the 2014 and 2015 surveys was reported. Because the two surveys were administered only 1 year apart, not enough time may have elapsed to capture long-term trends toward increased PAPR usage.
N95 FFRs continue to be the most common RPDs used in health care facilities, with 94%/95% of respondents indicating they used N95 FFRs in the past year. However, the use of PAPRs and EHFRs in health care is also significant when considering practical and legal implications associated with FDA-cleared (i.e., surgical N95 respirators) versus non-FDA-cleared devices (PAPRs and EHFRs), especially during a pandemic.
The limited number of responses about EHFRs in this study makes interpretation difficult, other than to say that EHFRs do not appear to be widely used by these respondents. Some HCWs may find the devices burdensome and difficult to clean or disinfect, which may be why EHFR use remains low within the health care sector (Ciconte & Danyluk, 2013).
Regional differences in PAPR use was the only statistically significant finding between samples. In the 2014 and 2015 surveys, the West and Midwest reported more PAPR use than the South or Northeast. The State of California, Department of Industrial Relations, Division of Occupational Safety and Health, better known as Cal/OSHA, has worker safety laws that require higher safety standards than federal regulations, which may be an explanation as to why the West respondents reported more PAPR use (Cal/OSHA, 2012). N95 FFR and EHFR use did not show a regional trend, indicating that overall use of these types of RPDs in health care did not change due to EVD during this time.
Survey participants in 2014 and 2015 reported similar models for each type of RPD in their health care facilities: N95 FFRs—3M™ 1860/1860S and 3M™ 1870/Aura™ 1870+; PAPRs—3M™ Air-Mate™ and Syntech International MAXAIR; and EHFRs—3M™ 6000 series and 3M™ 7500 series. It is important for emergency planning agencies to account for variations when considering supplying HCWs with FFRs as users should only wear the specific brand, model, and size respirators that they wore during successful fit tests (CDC, NPPTL, 2015). To better prepare end users, targeted education and training should be tailored to the types of RPDs regularly used. In addition, pandemic planning agencies must understand which RPDs are used in the field so products can be matched to best protect HCWs in emergency situations and facilitate ease of use within such contexts.
Survey respondents appeared to have some difficulty identifying the types and models of RPDs used in their facility (e.g., commenting on self-contained breathing apparatus [SCBA] RPDs in the PAPR section or writing “I’m not sure” for the RPD model name.) Fewer “I’m not sure” responses were documented in the 2015 survey (Table 3; n = 18 for 2014, and n = 7 for 2015), which may indicate increased awareness of RPD types used in their workplaces. Respiratory protection program administrators, unit managers, and employees should be knowledgeable about N95 FFR models to ensure the correct model is used for fit testing and training.
Peterson, Novak, Stradtman, Wilson, and Couzens (2015) found that many HCWs were unclear about when and how to use respiratory protection in acute care hospitals and which type of protection was needed in specific situations. This lack of awareness may be due to HCWs either not using these devices themselves or using them but not understanding the differences in various respirators. These findings suggest that HCWs may need more education and training on proper respiratory protection practices. Adequate knowledge of respiratory protection practices and guidelines will better protect workers during daily tasks as well as during a pandemic. Both managers and frontline workers who use respiratory protection must be knowledgeable about RPDs, the differences between types and models, and intended uses in health care facilities; employees must be able to identify the specific RPD device and model assigned to them.
The number of respondents who chose to answer each set of questions about the three RPD types varied; N95 FFRs and general yes/no questions received the most responses and EHFRs and specific model type questions the least responses. Based on anecdotal evidence, this pattern may be due to respondents being unable to find the information needed to complete the survey; several pilot survey respondents noted that these data are not readily available in health care facilities including how RPDs were used (e.g., during patient care, while cleaning patient rooms) or which employees were using RPDs (e.g., registered nurses, environmental services staff).
This study relied on cross-sectional, self-reported data. The findings are not generalizable to all U.S. health care facilities because of the nonprobability, convenience sampling used for this study. A non-response bias, both from those who did not participate as well as those who elected to answer only some of the questions could have also affected study results. It is also possible that some respondents incorrectly self-identified their work environment to the organizations that disseminated the survey. Due to the mass email distribution by multiple professional societies, an accurate response rate could not be determined.
Lack of surveillance data regarding the specific types of HCWs using each type of RPD, and the circumstances in which they use them, continues to be a problem. This type of information was beyond the scope of this study, but would be beneficial in developing informational products and interventions to improve respiratory protection practices among HCWs. Future studies should address facility characteristics such as hospital size, quantity of RPDs used annually, and onsite supply caches to better understand how RPDs are used by HCWs in real-time.
Conclusion
Despite increased awareness of PPE and emergency preparations after the EVD epidemic, no significant change in the type, models, or number of HCWs using RPDs in U.S. health care settings occurred between 2014 and 2015. N95 FFRs remain the most prevalent RPD. The use of PAPRs varied by region of the country with the highest use found in the Midwest and West. Regional planning is an essential part of emergency preparedness, and this study reinforced the need for targeted education and training so that HCWs are best prepared to use the devices available to them. In addition, findings from this study provide preliminary information to assist pandemic planning agencies for stockpiling RPD models used in the field to ensure that HCWs receive RPDs in emergency situations that match their training.
Acknowledgments
The authors thank Annette Byrd and Shelah Fletcher for their dedication to this project.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Biographies
Kerri Wizner is an Association of Schools and Programs of Public Health, Centers for Disease Control and Prevention Fellow, National Institute for Occupational Safety and Health—National Personal Protective Technology Laboratory, Pittsburgh, PA.
Lindsay Stradtman was an Association of Schools and Programs of Public Health, Centers for Disease Control and Prevention Fellow, National Institute for Occupational Safety and Health—National Personal Protective Technology Laboratory, Pittsburgh, PA.
Debra Novak is a Senior Service Fellow, National Institute for Occupational Safety and Health—National Personal Protective Technology Laboratory, Pittsburgh, PA.
Ronald Shaffer is a Branch Chief, National Institute for Occupational Safety and Health—National Personal Protective Technology Laboratory, Pittsburgh, PA.
Footnotes
Mention of a product or brand does not constitute product endorsement by the National Institute for Occupational Safety and Health (NIOSH). Findings and conclusions of this article are those of the authors and do not necessarily represent the views of NIOSH.
Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- American Association of Occupational Health Nurses. AAOHN fact sheet. 2012 Feb; Retrieved from http://aaohn.org/about-us/aaohn-vision-and-mission/aaohn-fact-sheet.html.
- Association for Professionals in Infection Control and Epidemiology. Ebola preparedness one year later: A poll of APIC members. 2015 Nov; Retrieved from http://www.apic.org/Resource_/TinyMceFileManager/Topic-specific/APIC_Ebola_Survey_Results_November_2015.pdf.
- Association of State and Territorial Health Officials. Assessment of respiratory personal protective equipment in U.S. acute care hospitals–2012. 2014 Report. Vol October 24 2014 ). Retrieved from http://www.astho.org/Preparedness/Respiratory-PPE-Assessment-Report/
- Baracco G, Eisert S, Eagan A, Radonovich L. Comparative cost of stockpiling various types of respiratory protective devices to protect the health care workforce during an influenza pandemic. Disaster Medicine and Public Health Preparedness. 2015;9:313–318. doi: 10.1017/dmp.2015.12. [DOI] [PubMed] [Google Scholar]
- Beckman S, Materna B, Goldmacher S, Zipprich J, D’Alessandro M, Novak D, Harrison R. Evaluation of respiratory protection programs and practices in California hospitals during the 2009–2010 H1N1 influenza pandemic. American Journal of Infection Control. 2013;41:1024–1031. doi: 10.1016/j.ajic.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessesen M, Adams J, Radonovich L, Anderson J. Disinfection of reusable elastomeric respirators by health care workers: A feasibility study and development of standard operating procedures. American Journal of Infection Control. 2015;43:629–634. doi: 10.1016/j.ajic.2015.02.009. [DOI] [PubMed] [Google Scholar]
- California/Occupational Safety & Health Administratoin. Cal/OSHA respiratory protection standard. 2012 Retrieved from http://www.cdph.ca.gov/programs/ohb/Pages/atdstd.aspx.
- Carias C, Rainisch G, Shankar M, Adhikari B, Swerdlow D, Bower W, … Koonin L. Potential demand for respirators and surgical masks during a hypothetical influenza pandemic in the United States. Clinical Infectious Diseases. 2015;60(Suppl 1):S42–S51. doi: 10.1093/cid/civ141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Guidance on personal protective equipment (PPE) to be used by healthcare workers during management of patients with confirmed Ebola or persons under investigation (PUIs) for Ebola who are clinically unstable or have bleeding, vomiting, or diarrhea in U.S. hospitals, including procedures for donning and doffing PPE. 2015a Aug; Retrieved from http://www.cdc.gov/vhf/ebola/healthcare-us/ppe/guidance.html.
- Centers for Disease Control and Prevention. Strategic National Stockpile (SNS) 2015b Apr; Retrieved from http://www.cdc.gov/phpr/stockpile/stockpile.htm.
- Centers for Disease Control and Prevention, National Personal Protective Technology Laboratory. Respirator trusted-source information. 2015 Jun; Retrieved from http://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/respsource3healthcare.html.
- Ciconte R, Danyluk Q. Assessment and determination of practical considerations for wide-scale utilization of EHFRs during a pandemic or outbreak situation (Report No. RS2011-IG13) Richmond, Canada: Work Safe BC; 2013. Retrieved from: http://216.176.54.70/contact_us/research/funding_decisions/assets/pdf/2011/RS2011-IG13.pdf. [Google Scholar]
- Cooley P, Lee B, Brown S, Cajka J, Chasteen B, Ganapathi L, … Burke D. Protecting health care workers: A pandemic simulation based on Allegheny County. Influenza and Other Respiratory Viruses. 2010;4:61–72. doi: 10.1111/j.1750-2659.2009.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden T, Damon I, Bell B, Kenyon T, Nichol S. Ebola 2014-new challenges, new global response and responsibility. New England Journal of Medicine. 2014;371:1177–1180. doi: 10.1056/NEJMp1409903. [DOI] [PubMed] [Google Scholar]
- Hewlett A, Varkey J, Smith P, Ribner B. Ebola virus disease: Preparedness and infection control lessons learned from two biocontainment units. Current Opinion Infections Diseases. 2015;28:343–348. doi: 10.1097/QCO.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. The use and effectiveness of powered air purifying respirators in health care: Workshop summary. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- Iskander J, Strikas R, Gensheimer K, Cox N, Redd S. Pandemic influenza planning, United States, 1978–2008. Emerging Infectious Diseases. 2013;19:879–885. doi: 10.3201/eid1906.121478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo K, Leng P, Ibrahim I, Lim T. The changing face of healthcare worker perceptions on powered air-purifying respirators during the SARS outbreak. Respirology. 2005;10:107–110. doi: 10.1111/j.1440-1843.2005.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenbach E, Saint S, Henderson D, Harris A. Initial response of health care institutions to emergence of H1N1 influenza: Experiences, obstacles, and perceived future needs. Clinical Infectious Diseases. 2010;50:523–527. doi: 10.1086/650169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health, Occupational Safety & Health Administration. Hospital respiratory protection program toolkit. 2015 May; Retrieved from http://www.cdc.gov/niosh/docs/2015-117/pdfs/2015-117.pdf.
- Occupational Safety & Health Administration. Respiratory protection—1910.134. 2011 Jun; Retrieved from https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=12716.
- Office of the Assistant Secretary for Preparedness and Response, Hospital Preparedness Program. Healthcare preparedness capabilities: National guidance for healthcare system preparedness. 2012 Retrieved from http://www.phe.gov/Preparedness/planning/hpp/reports/Documents/capabilities.pdf.
- Peterson K. Hospital adherence to respiratory protection guidelines by healthcare workers and hospitals: REACH 2 evaluation. 2013 Retrieved from https://apha.confex.com/apha/141am/webprogramadapt/Paper277372.html.
- Peterson K, Novak D, Stradtman L, Wilson D, Couzens L. Hospital respiratory protection practices in 6 U.S. states: A public health evaluation study. American Journal of Infection Control. 2015;43:63–71. doi: 10.1016/j.ajic.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S, Beekmann S, Babcock H, Pavia A, Koonin L, Polgreen P. Clinician beliefs and attitudes regarding use of respiratory protective devices and surgical masks for influenza. Health Security. 2015;13:274–280. doi: 10.1089/hs.2015.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgreen P, Santibanez S, Koonin L, Rupp M, Beekmann S, Del Rio C. Infectious disease physician assessment of hospital preparedness for Ebola virus disease. Open Forum Infectious Diseases. 2015;2:1–6. doi: 10.1093/ofid/ofv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonovich L, Yanke R, Cheng J, Bender B. Diminished speech intelligibility associated with certain types of respirators worn by healthcare workers. Journal of Occupational and Environmental Hygiene. 2010;7:63–70. doi: 10.1080/15459620903404803. [DOI] [PubMed] [Google Scholar]
- Rebmann T, Wang J, Swick Z, Reddick D, delRosario J. Business continuity and pandemic preparedness: US health care versus non-health care agencies. American Journal of Infection Control. 2013;41:e27–e33. doi: 10.1016/j.aijc.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Tompkins B, Kerchberger J. Special article: Personal protective equipment for care of pandemic influenza patients: A training workshop for the powered air purifying respirator. Anesthesia & Analgesia. 2010;111:933–945. doi: 10.1213/ANE.0b013e3181e780f8. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. Geographic terms and concepts-census divisions and census regions. 2015 Retrieved from https://www.census.gov/geo/reference/gtc/gtc_census_divreg.html.
- U.S. Department of Health and Human Services. 2009 H1N1 influenza improvement plan. 2012 May; Retrieved from http://www.phe.gov/Preparedness/mcm/h1n1-retrospective/Documents/2009-h1n1-improvementplan.pdf.
- U.S. Food and Drug Administration. Summary fact sheet for disposable respirators for use during the swine flu emergency. 2010 Retrieved from http://www.fda.gov/MedicalDevices/Safety/EmergencySituations/ucm161612.htm.
- U.S. Food and Drug Administration. Medical devices: Personal protective equipment for infection control. 2015 Jul; Retrieved from http://www.fda.gov/medicaldevices/productsandmedicalprocedures/generalhospitaldevicesandsupplies/personalprotectiveequipment/default.htm.