Abstract
Background
States have attempted to reduce prescription opioid abuse through strengthening the regulation of pain management clinics; however, the effect of such measures remains unclear. We quantified the impact of Texas’s September 2010 “pill mill” law on opioid prescribing and utilization.
Methods
We used the IMS Health LRx LifeLink database to examine anonymized, patient-level pharmacy claims for a closed cohort of individuals filling prescription opioids in Texas between September 2009 and August 2011. Our primary outcomes were derived at a monthly level and included: (1) average morphine equivalent dose (MED) per transaction; (2) aggregate opioid volume; (3) number of opioid prescriptions; and (4) quantity of opioid pills dispensed. We compared observed values with the counterfactual, which we estimated from pre-intervention levels and trends.
Results
Texas’s pill mill law was associated with declines in average MED per transaction (−0.57 mg/month, 95% confidence interval [CI] −1.09, −0.057), monthly opioid volume (−9.99 kg/month, CI −12.86, −7.11), monthly number of opioid prescriptions (−12,200 prescriptions/month, CI −15,300, −9,150) and monthly quantity of opioid pills dispensed (−714,000 pills/month, CI −877,000, −550,000). These reductions reflected decreases of 8.1–24.3% across the outcomes at one year compared with the counterfactual, and they were concentrated among prescribers and patients with the highest opioid prescribing and utilization at baseline.
Conclusions
Following the implementation of Texas’s 2010 pill mill law, there were clinically significant reductions in opioid dose, volume, prescriptions and pills dispensed within the state, which were limited to individuals with higher levels of baseline opioid prescribing and utilization.
Keywords: Prescription pain medication, Pill mill law, Prescription drug monitoring, Time series analysis
1. Introduction
The abuse and diversion of prescription opioids has reached epidemic proportions in the United States. In 2013, death from drug overdose, of which more than one-third involved prescription opioids, was the number one cause of injury-related death in America, surpassing deaths due to motor vehicle collisions among individuals 25–64 years of age (Centers for Disease Control and Prevention CDC, 2014). Although individuals who misuse opioid pain relievers obtain them from a variety of sources, most originate from licensed prescribers (Inciardi et al., 2010). From that original source, opioids that are prescribed for appropriate indications may be misused by the patient or shared with friends and family members. However, in other cases, the original prescription is not obtained from a provider following recommended medical practices but rather is obtained from a physician in a “pill mill,” a pain management clinic whose providers operate outside the boundaries of standard medical practice by prescribing large quantities of opioids and other controlled substances with minimal medical oversight (CDC, 2012).
Historically, Texas has been among the four states in the nation with the highest concentration of pill mills (Drug Enforcement Administration, 2013). In 2009, Texas legislators addressed this problem by passing “pill mill” legislation, requiring all pain management clinics to be certified by the Texas Medical Board on a biennial basis and to be owned and operated by a licensed physician, effective September 1, 2010. Clinic owners must be present at the clinic for at least one-third of operating hours and must personally review at least one-third of all patient files. Additionally, clinic owners must annually verify the qualifications and licensure of all employees (Texas Legislature Online, 2010). Prior to 2009, the Texas Medical Board had no regulations concerning the operation or ownership of pain management clinics (Zuzek, 2013); in some cases, the process of establishing a pill mill required little more than buying property, employing minimally trained staff, and hiring a figurehead physician under whose name opioids or other controlled substances could be prescribed (Houston Chronicle, 2010). With the implementation of Texas’s pill mill law, Texas Medical Board employees are expected to inspect all pain management clinics as often as necessary to verify compliance with the law and may revoke physicians’ licenses for failure to comply with its provisions (Zuzek, 2013 and Keel, 2013).
Although we (Daubresse et al., 2015) and others (Ringwait et al., 2015; Hackman et al., 2014; Surratt et al., 2014 and Reisman et al., 2009) have used quasi-experimental methods to evaluate the effect of Prescription Drug Monitoring Programs (PDMPs), to our knowledge, no studies have empirically evaluated the unique effect of a state’s implementation of a pill mill law in absence of a concurrent PDMP. This distinction is important because these policies differ in meaningful ways. Among their uses, PDMPs address high-risk patient behaviors, such as “doctor shopping”, by enabling stakeholders such as physicians and pharmacists to access patients’ comprehensive prescription histories. These programs may also be proactively used to identify prescribers who prescribe controlled substances more frequently than their peers. In contrast, pill mill laws primarily address inappropriate controlled substance prescribing by requiring pain management clinics to abide by basic best practices, such as ensuring that physicians meet with patients (CDC, 2013). The Texas PDMP was implemented prior to our study period and is thus considered a constant. We sought to evaluate the impact of Texas’s pill mill law on opioid prescribing and utilization.
2. Methods
2.1. Data
We analyzed prescription claims data obtained from the IMS Health LifeLink LRx Anonymized Longitudinal Prescription database. These data represent de-identified, patient-level transactions from retailers throughout the United States, representing approximately 65% of retail prescription transactions in the country. IMS Health receives weekly data from food stores, mass merchandisers, and independent as well as chain drug stores. These data include information about prescribers (specialty and zip code), patients (age, sex, and date of first pharmacy transaction), and transactions (product type, National Drug Code [NDC], dosage, days’ supply and quantity of pills dispensed, out-of-pocket and total costs), pharmacy type, and source of payment (Medicare, Medicaid, commercial insurance and cash). The use and analysis of this data were deemed exempt from review by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
2.2. Time segments and subjects
We divided our 24-month observation period into a 12-month pre-intervention period preceding the policy intervention (September, 2009 through August, 2010) and a 12-month post-intervention period following the policy intervention (September, 2010 through August, 2011).
We derived a closed cohort of patients who had claims activity throughout the 24-month observation period. To do so, we first excluded observations from stores that did not have consistent reporting of data to IMS Health throughout the entire period of interest. Next, we derived a closed cohort of patients by selecting those who had any prescription claims activity 3-months before and after the first and last month of observation, thus allowing us to minimize the bias introduced from patients entering into and exiting out of our cohort. We also excluded spurious observations, such as those with zero or negative values, or that represented extreme values, such as those with a morphine equivalent dose (MED) per transaction greater than 360 mg (see Supplementary Table 3) (Ohio State Board of Pharmacy, 2013). Such values, which can arise during claims processing and adjudication, accounted for 0.01% of the raw data and are unlikely to have affected the accuracy of the analysis. Our final data set consisted of 8.3 million patients, approximately one-third of the total population of Texas in 2010. As expected, our subjects were more likely to be female and more likely to be older, with a median age of 44, than those of a representative population of Texans based on the 2010 Census.
2.3. Analysis
We examined four outcomes, each of which was calculated on a monthly basis and derived twice to represent prescriber-and patient-level transactions. The first outcome we examined was average morphine equivalent dose (MED) per transaction, which represents a standardized metric for comparing opioids after accounting for prescription dose and potency using an opioid conversion table created by the CDC (Ohio State Board of Pharmacy, 2013). The morphine equivalent daily dose (MEDD), which is equivalent to the MED divided by the days’ supply, is extensively used by medical organizations, such as the American Academy of Pain Medicine and the Centers for Medicare and Medicaid Services, in order to define threshold opioid doses above which prespecified clinical action should be taken. The MED, expressed in milligrams, is calculated as the product of the number of opioid pills dispensed, the strength of the opioid prescribed (in milligrams), and the equianalgesic morphine conversion factor. The morphine conversion factor is derived from prior work characterizing long-term opioid therapy for noncancer pain (Von Korff et al., 2008). These conversion factors account for differences in oral, transdermal, and transmucosal preparations based on assumptions of the bioavailability of different formulations. Higher MED is associated with increased risk for opioid overdose-related mortality and is thus an important measure to characterize (Bohnert et al., 2011; Gomes et al., 2011). Second, we examined the total opioid volume across all transactions. This measure quantifies the total amount of opioids dispensed each month by combining the total MED across all transactions to provide market-level information about the volume of opioid utilization in each state while accounting for differences in molecule type and strength. Third, we examined the total number of opioid prescriptions filled per month, since changes in the first two outcomes could occur independently of changes in the number of prescription transactions each month. Finally, we examined the quantity of opioid pills dispensed, since as with the number of transactions, pill-volume could change independently of the other measures examined. We chose to include opioids such as tramadol and tapentadol, however we excluded claims for codeine cough syrups and similar preparations.
We used a segmented linear regression, which assumes normally distributed regression coefficients (and thus mean values that are equal to median values), to quantify the impact of the policy intervention on each outcome of interest (Wagner et al., 2002). Each segment of the time series was defined by two parameters: the level, defined as the value of the outcome at the beginning of the time segment, and the trend, defined as the rate of change in the outcome over a given time segment. In the regression model, we specified the level by an indicator variable denoting before or after the intervention and we specified the trend by a continuous variable detailing the time in months before or since the intervention. The overall effect of the intervention was derived as a comparison between the observed values and the counterfactual value, estimated by extrapolating the model fit in the baseline period out into the post-law period. Each model was checked for autocorrelation across time using the generalized Durbin–Watson test and corrected using the appropriate autocorrelation order (proc autoreg command with nlag function in SAS version 9.4). The adjusted models had R2 values between 80 and 95%, indicating that the models explained much of the variability in the data.
In addition to analyzing the aggregate dataset, we performed regression analyses on stratified subsets of the data, based on pre-intervention prescribing and utilization levels of prescribers and patients, respectively. First, we ranked all prescribers and all patients by their baseline total opioid volume prescription and utilization, respectively. We then stratified each group into quartiles and top percentiles, including the top 90th, 95th, 97th, and 99th percentiles. We then conducted analyses within each strata of patient and prescriber to examine whether the impact of the pill mill law varied among different subpopulations of these prescribers and patients.
2.4. Sensitivity analyses
We tested the stability of our estimates through multiple sensitivity analyses. First, we varied the observation period from 24 months to 12 months (March, 2010 through February, 2011) and 36 months (March, 2009 through February, 2012). Next, we converted our closed cohort into an open cohort by removing the requirement for patients to have claims activity throughout the entire observation period. We then repeated our main analyses, including outliers to account for all claims representing high-risk utilization or diversion. Finally, we repeated our main analyses excluding oxycontin to account for its tamper-resistant reformulation in 2010.
3. Results
3.1. Patient, prescriber, and pharmacy characteristics and baseline prescribing and utilization
Our final data set included 8.3 million patients, 737,123 providers, and 37,260 stores. Between September 2009 and August 2011, there were 724 million prescription transactions observed, of which 10.5% were for opioids.
Table 1 presents the baseline levels and trends of our four outcome measures. For example, twelve months prior to the implementation of the pill mill law, the baseline average MED per transaction was 54.6 mg/month (95% CI 52.1, 57.2), with a non-statistically significant declining trend of −0.11 mg/month (95% CI−0.43, 0.20).
Table 1.
Baseline opioid prescribing and utilization.
| Morphine equivalent dose, mean (mg) | Opioid volume (kg)
|
No. of opioid prescriptions (thousand)
|
Quantity of opioid pills dispensed (million)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | Value | 95% CI | Value | 95% CI | |
| Level | 54.6*** | 52.1, 57.2 | 181*** | 166,196 | 233*** | 217, 249 | 15.0*** | 14.2, 15.9 |
| Trend | −0.11 | −0.43, 0.20 | 5.66*** | 3.66, 7.66 | 7.29*** | 5.11, 9.47 | 0.46*** | 0.35, 0.58 |
p < 0.05.
p < 0.01.
3.2. Effect of pill mill law
Fig. 1 depicts the observed monthly opioid volume and MED in the entire time period as well as the corresponding counterfactuals had the pill mill law not been implemented. For example, the effect on average MED per transaction appeared to lag by 1 month, after which there was a steady two-month decline followed by a steady state through the remainder of the observation period. In contrast, total opioid volume steadily declined for the first 5 months after the policy intervention, followed by a small one-month increase and an approximate steady state for the following 5 months. The trend in total number of prescriptions written and quantity of opioid pills dispensed following the pill mill law displayed a pattern similar to that of the total opioid volume in that each of these outcomes declined for the first 5 months and then reached a relative steady state.
Fig. 1.
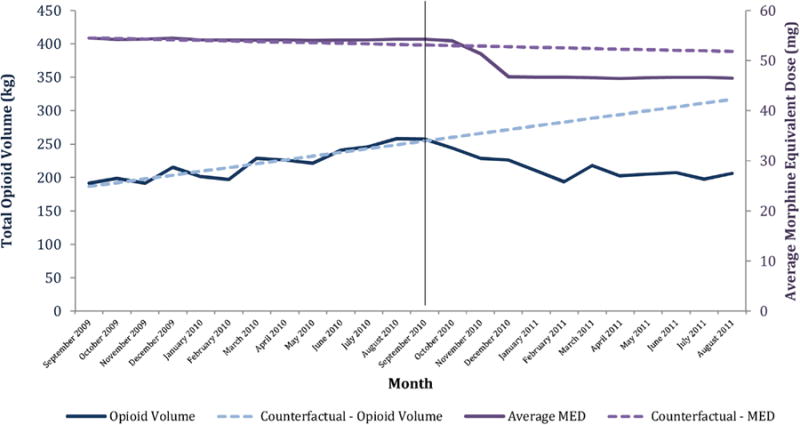
Total opioid volume and average morphine equivalent dose, September 2009–August 2011.
Table 2 depicts the quantitative magnitude of these changes, which were statistically significant across all outcomes. For example, the average MED per transaction declined by −0.57 mg/month (95% CI −1.09, −0.06), while the total opioid volume across all transactions declined by approximately −9.99 kg/month (95% CI −12.9, −7.11), which, if applied evenly across all patients in the cohort, is equivalent to a monthly reduction of approximately −1.2 mg MED per patient per month. Relative to the baseline upward trend of 0.68 mg MED per patient per month (95% CI 0.44, 0.92), which represents the month-to-month increase in the average MED per patient during the 12 months prior to the intervention and is derived from the β1 term of the segmented regression for opioid volume divided by the number of patients in the cohort (Wagner et al., 2002), the post-intervention downward trend of −1.2 mg MED per patient per month represents a 276% (95% CI 230%, 373%) decline in the monthly opioid volume trend. Similarly, the total number of prescriptions written in the state decreased by −12,200 prescriptions/month (95% CI −15,300, −9,150) and the quantity of opioid pills dispensed fell by −714,000 pills/month (95% CI −877,000, 550,000).
Table 2.
Overall impact of Texas’s pill mill law on monthly opioid prescribing and utilization.
| Morphine equivalent dose, mean(mg)
|
Opioid volume (kg)
|
No. of opioid prescriptions (thousand)
|
Quantity of opioid pills dispensed (thousand)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | Value | 95% CI | Value | 95% CI | |
| Impact on level | −0.007 | −2.32, 2.31 | −4.40 | −23.7,14.9 | −4.46 | −25.8,16.9 | 11.5 | −1150,1170 |
| Impact on trend | −0.57** | −1.09, −0.06 | −9.99*** | −12.9, −7.11 | −12.2*** | −15.3, −9.15 | −714*** | −877, −550 |
p < 0.05.
p < 0.01.
Table 3 depicts reductions in each of the four outcome measures during the first 6 months, second 6 months, and cumulatively for 12 months after the policy intervention by quantifying the difference between the observed and predicted values of each measure. For example, 12 months after the pill mill law was implemented, the observed average MED per transaction was 48.2 mg/month, 8.1% lower than the expected 52.5 mg/month based on the pre-intervention levels and trends. Similarly, one year after the intervention, the state-wide total opioid volume, total number of opioid prescriptions, and total quantity of opioid pills dispensed were 24.3%, 22.8%, and 19.5% lower than what would have been expected, respectively.
Table 3.
Difference between actual values and values predicted without policy intervention, first 6 months, second 6 months, and cumulatively 1 year after policy implementation.
| First 6 months
|
Second 6 months
|
One year
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Observed | Predicted | Difference (%) | Observed | Predicted | Difference (%) | Observed | Predicted | Difference (%) | |
| Morphine equivalent dose, mean (mg) | 49.9 | 52.8 | −5.43 | 46.5 | 52.1 | −10.7 | 48.2 | 52.5 | −8.06 |
| Opioid volume (kg) | 227 | 269 | −15.7 | 206 | 303 | −31.9 | 216 | 286 | −24.3 |
| No. of opioid prescriptions (thousands) | 296 | 346 | −14.5 | 272 | 389 | −30.2 | 284 | 368 | −22.8 |
| Quantity of opioid pills dispensed (millions) | 19.6 | 22.3 | −11.9 | 18.5 | 25.1 | −26.3 | 19.0 | 23.7 | −19.5 |
Source: IMS Health LifeLink LRx Database®, 2009–2011.
3.3. Policy effect stratified by prescribers’ baseline opioid prescription volume
Table 4 and Fig. 2 demonstrate that the effects of the policy were greatest among prescribers with the highest baseline opioid prescribing volume. For example, among prescribers with the highest quartile opioid volume at baseline (N = 85,354), the policy was associated with statistically and clinically significant reductions in MED (−0.56 mg/month), opioid volume (−9.92 kg/month), number of opioid prescriptions (−11,800 prescriptions/month), and quantity of opioid pills dispensed (−707,000/month). In contrast, among prescribers with the lowest quartile opioid volume at baseline (N = 80,454), the policy was not associated with any statistically significant changes in the outcomes examined.
Table 4.
Impact of Texas’s pill mill law on prescribers stratified by baseline opioid prescribing levels.
| Quartiles of baseline opioid prescribing
|
Highest baseline opioid prescribing (percentile)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0–25th | 26–50th | 51–75th | 76–100th | 90th | 95th | 97th | 99th | |
| Morphine equivalent dose, mean (mg) | ||||||||
| Impact on level | −0.42 | −0.96*** | −0.48 | −0.007** | 0.17 | 0.47 | −0.37 | −0.02*** |
| Impact on trend | −0.01 | −0.52 | −0.47 | −0.56 | −0.88** | −0.88** | −0.58 | −0.33 |
| Opioid volume (kg) | ||||||||
| Impact on level | −0.01 | −0.06 | −0.22 | −4.14*** | 0.14 | −0.20*** | −0.82*** | −1.50*** |
| Impact on trend | −0.002 | −0.005 | −0.05 | −9.92 | −0.99*** | −1.00 | −2.12 | −4.99 |
| Number of opioid prescriptions (thousands) | ||||||||
| Impact on level | −0.15 | −0.34 | −0.95*** | −3.00*** | −0.69*** | 0.03 | 0.34 | −0.32*** |
| Impact on trend | 0.02 | 0.01 | −0.45 | −11.8 | −1.97 | −1.44*** | −2.44*** | −3.50 |
| Quantity of opioid pills dispensed (thousands) | ||||||||
| Impact on level | −2.26 | −7.64 | −17.8 | 38.6 | −4.31*** | 21.0 | 66.6 | −5.54 |
| Impact on trend | 0.36 | 1.14 | −5.87 | −707*** | −82.4 | −82.8*** | −170*** | −297*** |
p < 0.05.
p < 0.01.
Fig. 2.
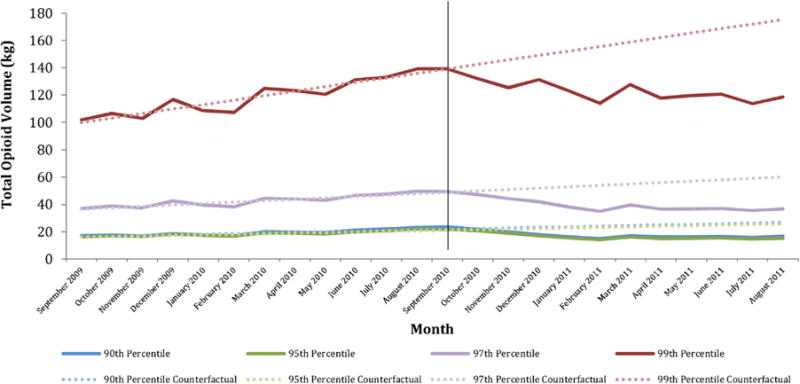
Total opioid volume, by top prescriber percentile*, September 2009–August 2011.
3.4. Policy effect on opioid sales stratified by patients’ baseline opioid use
Table 5 and Fig. 3 depict similar information for patients stratified by their volume of baseline opioid use. As was observed for prescribers, patients with the highest baseline opioid utilization experienced the greatest reductions in outcome measures following the intervention. Patients in the highest quartile of baseline opioid utilization experienced statistically significant and clinically meaningful reductions in MED (−0.51 mg/month), total opioid volume (−8.07 kg/month), number of opioid prescriptions (−6350 prescriptions/month), and quantity of opioid pills dispensed (−510,000 pills/month). In contrast, patients in the lowest quartile of baseline opioid utilization experienced statistically significant reductions in only three of the outcomes examined, with magnitudes of changes that were not clinically significant. Among these patients, total opioid volume declined by −0.14 kg/month, number of opioid prescriptions declined by −1120 prescriptions/month, and quantity of opioid pills dispensed declined by −23,800 pills/month.
Table 5.
Impact of Texas’s Pill mill law on patients stratified by baseline opioid utilization levels.
| Quartiles of baseline opioid utilization
|
Highest baseline opioid utilization (percentile)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0–25th | 26–50th | 51–75th | 76–100th | 90th | 95th | 97th | 99th | |
| Morphine equivalent dose, mean (mg) | ||||||||
| Impact on level | 0.07 | 0.05 | 0.17 | −0.11** | −0.08 | −0.48*** | −0.57*** | 1.46*** |
| Impact on trend | −0.14 | −0.83** | −0.83 | −0.51 | −0.43 | −0.67 | −0.66 | −1.52*** |
| Opioid volume (kg) | ||||||||
| Impact on level | −0.05*** | −0.16** | 0.40 | −4.41*** | −1.23*** | −0.91*** | −0.93*** | −0.35 |
| Impact on trend | −0.14 | −0.37 | −1.28** | −8.07 | −1.51 | −0.87 | −1.40 | −1.75*** |
| Number of opioid prescriptions, thousands | ||||||||
| Impact on level | −0.80*** | −1.37*** | −0.53*** | −1.73*** | −0.87*** | −0.31*** | −0.43*** | −0.27*** |
| Impact on trend | −1.12 | −1.40 | −3.16 | −6.35 | −1.28 | −0.56 | −0.65 | −0.36 |
| Quantity of opioid pills dispensed, thousands | ||||||||
| Impact on level | −3.85*** | −15.9** | 25.0 | −6.62*** | −22.8*** | −24.7*** | −27.0*** | −20.3*** |
| Impact on trend | −23.8 | −37.1 | −125*** | −510 | −119 | −58.7 | −66.9 | −39.7 |
p < 0.05.
p < 0.01.
Fig. 3.
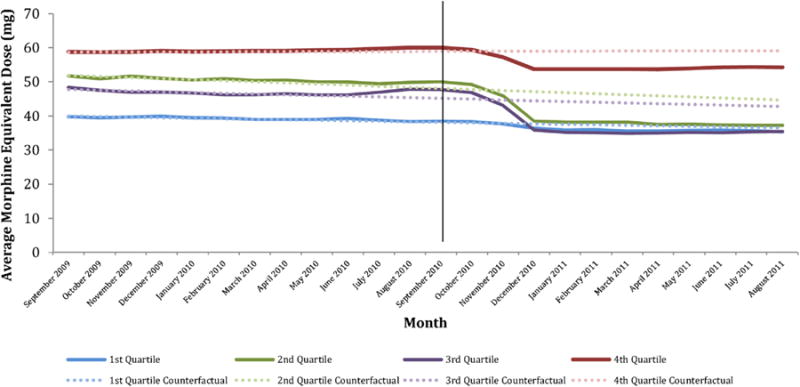
Average morphine equivalent dose per transaction, by patient quartile*, September 2009–August 2011.
3.5. Results of sensitivity analyses
Varying the observation period from 24 months to 12 and 36 months provided results that were similar in trend but different in magnitude (Supplementary Table 1). For example, estimates of reductions in the quantity of opioid pills dispensed varied from −1.3 million [M] pills/month (95% CI −1.7 M, −0.90 M) using a 12-month window to −0.54 M pills/month (95% CI −0.63 M, −0.45 M) using a 36-month window. Use of an open cohort yielded findings that varied somewhat across measures. For example, estimates of the decline in average MED per transaction were larger (−0.65 mg/month, 95% CI −1.21, −0.072) and estimates of the decline in total opioid volume were smaller (−3.62 kg/month, 95% CI −6.49, −0.76), while there was no statistically significant effect of the policy on quantity of opioid pills dispensed or number of opioid prescriptions (Supplementary Table 2). However, using an open cohort reduced our ability to make direct comparisons between the pre- and post-policy periods as the estimates reflect differences in the population with prescription transactions across time, in addition to differences attributable to the policy change. Analyses including outliers were similar to our main analyses in trend and statistical significance, but increased in magnitude (−9.99 kg/month vs. −10.66 kg/month). Exclusion of oxycontin did not result in substantive differences from our main analyses.
4. Discussion
We evaluated the impact of Texas’s 2010 pill mill law on opioid prescribing and utilization. At one year following its implementation, we identified statistically and clinically significant reductions attributable to the policy in each of the outcomes examined. For example, there was an approximate 8% reduction in average morphine equivalent dose per transaction, 24% reduction in total opioid volume, 23% reduction in the total number of opioid prescriptions, and 20% reduction in total quantity of opioid pills dispensed. These reductions were concentrated among patients and prescribers with the highest baseline opioid use and prescribing.
To our knowledge, our study represents one of the first rigorous analyses evaluating the effect of pill mill law implementation (Haegerich et al., 2014). This is important because of the magnitude of the opioid epidemic and the increasing number of states that have implemented pill mill laws. The magnitude of reductions we examined was substantial. For example, we estimate that by one year following implementation of the law, the volume of opioids that was taken out of circulation is equivalent to the volume needed to supply every 45–49 year old in Texas with a week’s supply of continuous, round-the-clock Vicodin (CDC, 2015).
Our study contributes to the growing body of research assessing the effectiveness of state-level interventions aimed at reducing the opioid abuse and diversion epidemic. States have taken many approaches toward addressing this problem, ranging from education outreach programs and clinical guidelines to prescription drug monitoring programs; however, the evidence assessing the effectiveness of many of these interventions remains limited (Haegerich et al., 2014). A longitudinal analysis examining the impact of state-monitored triplicate prescription forms on benzodiazepine utilization suggests state-level interventions decrease use of prescribed controlled substances (Simoni-Wastila et al., 2004). Given the high implementation and opportunity costs of such interventions, additional evidence to inform the optimal approaches that states can use is particularly valuable (Finklea et al., 2014).
Our study raises several questions for further investigation. It is unknown what effect the changes that we document have had on opioid-related injuries and deaths. An evaluation of Florida’s 2010 PDMP and pill mill law found that the number of drug overdose deaths decreased by 16.7% in the 2 years following the policy interventions (Johnson et al., 2012). In addition, prior studies have demonstrated the association between changes in opioid prescribing and opioid-related mortality (Bohnert et al., 2011; Dunn et al., 2010; Gwira Baumblatt et al., 2014; Daubresse et al., 2013). Additionally, it is important to characterize changes in pain treatment patterns following pill mill law enactment, such as the use of non-opioid analgesics and non-pharmacologic treatments for pain (Sauber-Schatz et al., 2013). It is also unknown what impact pill mill laws have on the trafficking of illicit opioids and whether specific features of these laws, such as variations in the consequences for noncompliance, influence their impact.
Our study has several limitations. First, we did not have access to diagnostic or other clinical information that would have allowed us to have greater insight regarding the clinical appropriateness of the prescribing and utilization observed. Similarly, because we did not have mortality data, we were not able to correlate trends in opioid prescribing with trends in opioid-related mortality. Second, our data source does not capture transactions occurring through non-retail channels such as hospitals or long-term care facilities, although these settings are not the focus of pill mill laws. Third, we did not choose to investigate whether the pill mill law impacted the extent to which overlapping opioids are prescribed. For this reason, we derived the monthly average MED per transaction using the method used by the CDC and other organizations (Ohio State Board of Pharmacy, 2013) rather than deriving an average MED per patient using a method that would account for overlapping prescriptions among individual patients. Fourth, the results of our sensitivity analyses differed from the primary analysis in magnitude and statistical significance; despite this variation, the direction of effect and clinical interpretation of the results remained the same. Fifth, we limited our main analyses to observations provided by stores that consistently reported data throughout the study period; this limited the number of observations available for analysis but insured that our results reflected differences attributable to the policy change rather than differences caused by changes in the source of prescription data. Sixth, our analysis did not include a comparison state to control for any changes that may have occurred on a national level. This decision was made because neighboring states had implemented their own policy changes in close proximity to Texas’s pill mill law, making direct comparisons difficult; however, we are unaware of any national-level changes that would have influenced prescription transactions during our observation period. Seventh, due to the lack of detailed data regarding the implementation and enforcement of the pill mill law in Texas, our study was not designed to assess the quality of its implementation. Finally, we were unable to rule out other events in Texas that may have contributed to the noted declines. However, we do not know of any other state-level policies occurring in Texas over the observation period that could have affected our results. Sensitivity analyses accounting for the reformulation of oxycodone in 2010 supported findings from our main analysis.
The abuse and diversion of prescription opioids represents a complex and multifaceted public health problem. Our analysis contributes to an understanding of the extent to which the regulation of pill mills plays a role in addressing this epidemic. Our analysis demonstrates that the enactment of Texas’s pill mill law resulted in substantial reductions in average morphine equivalent dose per transaction, opioid volume, number of opioid prescriptions, and quantity of opioid pills among providers and patients with the highest baseline levels of opioid prescribing and utilization, respectively.
Supplementary Material
Acknowledgments
None.
Role of funding source
This manuscript was supported by the Centers for Disease Control and Prevention (CDC) under Cooperative Agreement U01CE002499. CDC personnel participated in the analysis and interpretation of the data, as well as preparation of the manuscript and final approval of the manuscript prior to publication. The opinions and conclusions expressed are solely of the author(s) and should not be construed as representing the opinions of CDC or any agency of the Federal Government.
This manuscript was additionally supported by the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number TL1TR001078 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS or NIH.
The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the following IMS Health Incorporated information service(s): LifeLink LRx Database®, 2009–2011. All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are those of the authors, and are not necessarily those of IMS Health Incorporated or any of its affiliated or subsidiary entities, or of the institutions with whom the authors are affiliated.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.drugalcdep.2015.12.025.
Footnotes
Contributors
Tatyana Lyapustina was responsible for design, analysis, and manuscript preparation. Hsien-Yen Chang and Alim F. Ramji were responsible for statistical support. Matthew Daubresse was responsible for data acquisition. Lainie Rutkow, Mark Faul, and Elizabeth A. Stuart were responsible for critical revision. G. Caleb Alexander was responsible for project oversight and critical revision. All authors have approved the final manuscript and have approved of its submission.
Conflict of interest
Dr. Alexander is Chair of the FDA’s Peripheral and Central Nervous System Advisory Committee; serves as a paid consultant to PainNavigator, a mobile startup to improve patient self-management of pain; serves as a paid consultant to IMS Health; and serves on an IMS Health scientific advisory board. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies.
References
- Bohnert ASB, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, Blow FC. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- CDC. Menu of Pain Management Clinic Regulation. Centers for Disease Control and Prevention; 2012. http://www.cdc.gov/phlp/docs/menu-pmcr.pdf. [Google Scholar]
- CDC. Addressing prescription drug abuse in the United States: current activities and future opportunities. Centers for Disease Control and Prevention; 2013. http://www.cdc.gov/HomeandRecreationalSafety/pdf/HHS_Prescription_Drug_Abuse_Report_09.2013.pdf. [Google Scholar]
- CDC. Prescription drug overdose data. Centers for Disease Control and Prevention; 2014. http://www.cdc.gov/drugoverdose/data/overdose.html. [Google Scholar]
- CDC. Prescription drug overdose in the United States: fact sheet. Centers for Disease Control and Prevention; 2015. http://www.cdc.gov/homeandrecreationalsafety/overdose/facts.html. [Google Scholar]
- Daubresse M, Chang HY, Yu Y, Viswanathan S, Shah ND, Stafford RS, Kruszewski SP, Alexander GC. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000–2010. Med Care. 2013;51:870–878. doi: 10.1097/MLR.0b013e3182a95d86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubresse M, Rutkow L, Chang HY, Webster D, Stuart E, Alexander GC. Effect of Florida’s Prescription Drug Monitoring Program and Pill Mill Law on Opioid Prescribing and Utilization. Poster Session Presented at the Meeting of the International Society for Pharmacoepidemiology; Boston, Massachusetts. 2015. [Google Scholar]
- Drug Enforcement Administration, 2013. National Drug Threat Assessment Summary. Drug Enforcement Administration; 2013. http://www.dea.gov/resource-center/DIR-017-13%20NDTA%20Summary%20final.pdf. [Google Scholar]
- Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finklea K, Sacco L, Bagalman E. Prescription drug monitoring programs. Congressional Research Service; 2014. http://fas.org/sgp/crs/misc/R42593.pdf. [Google Scholar]
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686–691. doi: 10.1001/archinternmed.2011.117. [DOI] [PubMed] [Google Scholar]
- Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174:796–801. doi: 10.1001/jamainternmed.2013.12711. [DOI] [PubMed] [Google Scholar]
- Hackman DT, Green MS, Fernandex TJ, Brown AM, Wright ER, Chambers ER. Prescription drug monitoring program inquiry in psychiatric assessment: detection of high rates of opioid prescribing to a dual diagnosis population. J Clin Psychiatry. 2014;75:750–756. doi: 10.4088/JCP.14m09020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM. What we know, and don’t know: about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014;145:34–47. doi: 10.1016/j.drugalcdep.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston Chronicle. Texas ‘pill mill’ law puts 217 pain clinics on radar. Houston Chronicle; 2010. http://www.beaumontenterprise.com/news/article/Texas-pill-mill-law-puts-217-pain-clinics-on-698445.php. [Google Scholar]
- Inciardi JA, Surratt HL, Cicero TJ, Rosenblum A, Ahwah C, Bailey JE, Dart RC, Burk JJ. Prescription drugs purchased through the internet: who are the end users? Drug Alcohol Depend. 2010;110:21–29. doi: 10.1016/j.drugalcdep.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H, Paulozzi L, Porucznik C, Mack K, Herter B. Decline in drug overdose deaths after state policy changes-Florida 2010–2012. MMWR. 2012;63(26):569–574. [PMC free article] [PubMed] [Google Scholar]
- Keel J. An Audit Report On Pain Management Clinic Registration At The Texas Medical Board. State Auditor’s Office; 2013. http://www.sao.state.tx.us/reports/report.aspx?reportnumber=13-037. [Google Scholar]
- Ohio State Board of Pharmacy. Attention Pharmacists: Major change in the OARRS report to address the MED Ohio Initiative. 2013 http://pharmacy.ohio.gov/Documents/Pubs/Special/OARR/Changes%20Coming%20to%20OARRS%20for%20Opioid%20Guidelines%20-%2010.11.2013.pdf.
- Reisman RM, Shenoy PJ, Atherly AJ, Flowers CR. Prescription opioid usage and abuse relationships: an evaluation of state prescription drug monitoring program efficacy. Subst Abuse. 2009;1:41–51. doi: 10.4137/sart.s2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringwait C, Garrettson M, Alexandridis A. The effects of North Carolina’s prescription drug monitoring program on the prescribing behaviors of the state’s providers. J Prim Prev. 2015;36:131–137. doi: 10.1007/s10935-014-0381-0. [DOI] [PubMed] [Google Scholar]
- Sauber-Schatz EK, Mack KA, Diekman ST, Paulozzi LJ. Associations between pain clinic density and distributions of opioid pain relievers, drug-related deaths, hospitalizations, emergency department visits, and neonatal abstinence syndrome in Florida. Drug Alcohol Depend. 2013;133:161–166. doi: 10.1016/j.drugalcdep.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Simoni-Wastila L, Ross-Degnan D, Mah C, Gao X, Brown J, Cosler L, Fanning T, Gallagher P, Salzman C, Soumerai SB. A retrospective data analysis of the impact of the New York triplicate prescription program on benzodiazepine use in medicaid patients with chronic psychiatric and neurologic disorders. Clin Ther. 2004;26:322–336. doi: 10.1016/s0149-2918(04)90030-6. [DOI] [PubMed] [Google Scholar]
- Surratt HL, O’Grady C, Kurtz SP, Stivers Y, Cicero TJ, Dart RC, Chen M. Reductions in prescription opioid diversion following recent legislative interventions in Florida. Pharmacoepidemiol Drug Saf. 2014;23:314–320. doi: 10.1002/pds.3553. [DOI] [PubMed] [Google Scholar]
- Texas Legislature Online. Bill: SB 911. Texas Legislature Online; 2010. http://www.legis.state.tx.us/billlookup/text.aspx?LegSess=81R&Bill=SB911. [Google Scholar]
- Von Korff M, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter CM, Silverberg MJ, Banta-Green C, Weisner C. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- Zuzek C. Feds and State crack down. Tex Med. 2013;109:18–24. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


