Abstract
Background
Pulmonary hypertension (PH) is a common complication of idiopathic pulmonary fibrosis (IPF) that is associated with poor prognosis. Noninvasive screening for PH in IPF patients is challenging and a combination of several noninvasive determinations can improve discrimination.
Methods
We included 235 IPF patients who underwent right heart catheterization (RHC) as part of the lung transplant evaluation. We measured electrocardiographic (ECG) and echocardiographic variables as well as the pulmonary artery (PA) and ascending aorta (AA) diameters on chest CT. We recorded results of arterial blood gases (ABG), pulmonary function (PFT) and 6-min walk tests (6MWT).
Results
Several variables were predictors of PH in IPF patients in univariable models including a lower arterial oxygenation and 6MWT distance; worse right ventricular (RV) function, rightward deviation of the QRS axis and a higher FVC/DLCOc ratio, PA/AA diameter ratio, and estimated RV systolic pressure. In multivariable analysis, a worse RV function and higher PA/AA ratio remained predictors of PH (c-index 0.75 (0.65–0.84)). Similarly, a worse RV function, a higher PA/AA ratio and a rightward QRS axis deviation were independent predictors of precapillary PH (c-index 0.86 (0.76–0.92)). A combination of PA/AA diameter ratio <1.1, a QRS axis <90° and normal RV function showed a negative predictive value of 85% for precapillary PH.
Conclusions
There are significant differences in ECG, echocardiographic, chest CT, PFT and ABG parameters between IPF patients with and without PH. However, these noninvasive tests alone or combination have limited discrimination ability for PH screening in IPF.
Keywords: Idiopathic pulmonary fibrosis, pulmonary hypertension, echocardiography, electrocardiogram, computed tomography scan
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic disease that can lead to respiratory failure and death. Patients with IPF have a mean survival from diagnosis of about 3 years1,2. Pulmonary hypertension (PH) commonly occurs in advanced stages of IPF and this association has negative prognostic implications3–6. The prevalence of PH in patients with IPF varies between 14 % and 85 %3,7,8, mostly influenced by the severity of the parenchymal lung disease. Nathan et al. showed that the prevalence of PH increased from 33 to 85% as the IPF progressed7.
Pulmonary hypertension in IPF can lead to a decrease in the functional capacity and increase in oxygen requirements during rest and exercise8,9. The presence of PH in patients with advanced IPF may influence the decision to perform single versus bilateral lung transplant or use cardiopulmonary bypass10,11. More importantly, higher pulmonary artery pressures during right heart catheterization (RHC) were associated with higher mortality3,4. In fact, patients with IPF who developed PH had a 1-year mortality rate of 28 % compared to 6 % in those without PH9. Given these important implications, screening for PH in patients with IPF is of distinct importance.
Noninvasive screening for PH in IPF patients is challenging because the main methodology used for this purpose3, i.e. Doppler echocardiography, has important limitations due to the poor acoustic window12,13. In fact, several studies showed that the right ventricular systolic pressure (RVSP) estimated by echocardiography does not provide an accurate reflection of the measured values during RHC13–15. Interestingly, the accuracy of echocardiography slightly improved when the measures are combined with pulse oximetry (SpO2), pulmonary function (PFT) and six-minute walk (6MWT) tests13,15.
We hypothesized that a combination of certain determinations including 12-lead electrocardiogram (ECG)16–20, computed tomography (CT) of the chest21–24, 6MWT25, PFT26,27, arterial blood gases (ABG) and echocardiography improves the noninvasive screening for PH in patients with IPF. In the presented exploratory study, we tested whether an approach that incorporates results of several non-invasive investigations can better predict the presence of PH (diagnosed by RHC) in patients with IPF evaluated for lung transplant.
Methods
This retrospective study was approved by the Cleveland Clinic Institutional Review Board (study number 12-045). Written informed consent was waived. We included IPF patients28 evaluated for lung transplantation between March 2005 and January 2012. Patients were identified using the Cleveland Clinic Lung Transplantation Registry (EDIT). Data extracted from the EDIT database included demographics, lung allocation score (LAS) at the time of transplant29, PFT and RHC results. The patients’ electronic medical records provided any additional information. All the echocardiograms obtained during the lung transplant evaluation were reviewed. Measurements acquired included the estimated RVSP, visual right ventricular (RV) function, trans-annular plane systolic excursion (TAPSE) as well as the basal, mid and longitudinal RV dimensions30. PFT measurements included forced expiratory volume in the first second (FEV1 % predicted), forced vital capacity (FVC % predicted), FEV1/FVC, total lung capacity (TLC % predicted), corrected diffusion capacity for carbon monoxide (DLCOc % predicted), and the ratio of FVC/DLCO. We also gathered the results of the ABG analysis while breathing room air (arterial saturation (SaO2) and partial pressure of O2 (PaO2) and CO2 (PaCO2)).
We reviewed the chest CT scans and measured the pulmonary artery (PA) and ascending aorta (AA) diameters at the level of PA bifurcation. We calculated the ratio between these two measurements (PA/AA). We collected data on the 6MWT including distance walked in meters (6MWD), heart rate at the start and the end of the test, and heart rate recovery (HRR) at 1 minute31.
All 12-lead ECG (10-second recording) performed during the lung transplant evaluation were reviewed. The ECG were read by two investigators (M.B and L.A). In cases of irregular cardiac rhythm such as atrial fibrillation or flutter with variable atrioventricular conduction, we averaged at least 3 beats for each ECG determination. We excluded the ECG if the patient was on ventricular pacemaker at the time of recording or had a left bundle branch block. QT interval was corrected for heart rate by Bazett’s formula (QTc)32. We assessed whether the QRS morphology in lead V1 showed complete (RBBB) or incomplete right bundle branch block (IRBBB), qR or QS patterns. Right heart catheterization was performed following standard protocols and keeping the SpO2 at 90 % or above. Pulmonary hypertension was defined as a mean pulmonary artery pressure (mPAP) ≥ 25 mmHg3. Hemodynamic determinations were obtained at end-expiration. Pulmonary vascular resistance (PVR) was calculated by dividing the trans-pulmonary gradient (mean PAP – pulmonary artery wedge pressure) over the cardiac output obtained by thermodilution. The investigators that reviewed the echocardiograms, CT and ECG were blinded to the clinical and hemodynamic data.
Statistical Analysis
Continuous variables were summarized using mean ± standard deviation or median and interquartile range (IQR) when appropriate. We compared numerical variables using t-test and categorical variables with Chi-square test. Agreement was tested with Bland-Altman plot and expressed as mean difference with 95% limit of agreement33.
We used binary logistic regression (forward- variable selection) to test the variables that either alone or in combination could predict the presence of PH (mean PAP ≥ 25 mmHg) or precapillary PH (PH with PVR > 3 Wood units)34. For the univariable analysis we used the demographic, functional (6MWT), gasometric, spirometric, electrocardiographic, echocardiographic and radiographic variables presented in table 1 to 3. Results are given as odds ratio (OR) with 95% confidence interval. We performed correlation analyses and tested variance inflation factor (VIF) of the predictors to prevent collinearity and multicollinearity, respectively. We excluded variables that showed p values ≥ 0.10 in univariable logistic regression. We also excluded variables with VIF higher than 5, correlation value ≥ 0.8 or that measured similar effects (e.g. PaO2 and SaO2, 6MWD in meters and percentage of predicted, PA diameter or PA/AA ratio, etc). In the latter situation we kept the variables with the OR that departed the furthest from 1. Linearity assumption for logistic regression was met. We also explored non-linear relationships and reported the model accuracy and c-index when appropriate. Accuracy represents the proportion of the total number of model predictions that were correct. The c-index (concordance index) examines how well the models discriminate between groups of interest. For our binary outcomes (presence of PH or precapillary PH) the c-index represents the probability that a measure is higher or lower for a case than for a non-case35. For instance, in our data we found a probability of 64% that a PA/AA ratio is higher in IPF patient with PH than those without PH (c-index 0.64). In general, c-index values of 0.7 to 0.8, 0.8 and 0.9 and ≥ 0.9 indicate acceptable, excellent and outstanding discrimination, respectively36.
Table 1.
Patient characteristics
| All patients Mean ± SD or n (%) |
No PH Mean ± SD or n (%) |
PH Mean ±SD or n (%) |
P (T-test / Fisher’s exact test)^ |
|
|---|---|---|---|---|
| N | 235 | 116 (49.4) | 119 (50.6) | |
| Age (year) | 59.3 ± 8.9 | 60.7 ± 8.5 | 57.9 ± 9.1 | 0.02 |
| Male gender | 167 (71.1) | 79 (68.1) | 88 (73.9) | 0.32 |
| Lung allocation score29 | 49.3 ± 18.8 | 44.1 ± 15.2 | 54.0 ± 20.4 | <0.001 |
| Hemodynamic data | ||||
| -Systolic ABP (mm Hg) | 129.4 ± 19.0 | 131.1 ± 20.0 | 128.4 ± 18.7 | 0.67 |
| -Diastolic ABP (mm Hg) | 83.9 ± 17.2 | 83.4 ± 9.9 | 84.2 ± 20.7 | 0.89 |
| -RA pressure (mm Hg) | 6.2 ± 4.3 | 4.4 ± 2.7 | 7.9 ± 4.6 | <0.001 |
| -Systolic PAP (mm Hg) | 43.8 ± 16.8 | 32.6 ± 6.8 | 54.8 ± 16.4 | <0.001 |
| -Diastolic PAP (mm Hg) | 17.9 ± 8.6 | 12.6 ± 4.7 | 23.1 ± 8.4 | <0.001 |
| -Mean PAP (mm Hg) | 27.1 ± 10.8 | 19.2 ± 4.0 | 34.7 ± 9.7 | <0.001 |
| -PAWP (mm Hg) | 10.7 ± 6.0 | 8.6 ± 4.4 | 12.8 ± 6.6 | <0.001 |
| -CO (L/min) | 5.4 ± 1.5 | 5.3 ± 1.4 | 5.5 ± 1.6 | 0.33 |
| -CI (L/min/m2) | 2.8 ± 0.7 | 2.9 ± 0.7 | 2.8 ± 0.7 | 0.44 |
| -PVR (Wood Units) | 3.4 ± 2.4 | 2.2 ± 1.1 | 4.6 ± 2.8 | <0.001 |
| Echocardiographic data | ||||
| Left ventricular EF (%) | 56.4 ± 10.5 | 56.8 ± 6.3 | 57.5 ± 6.5 | 0.93 |
| RVSP (mmHg)* | 49.1 ± 20.4 | 43.8 ± 15.0 | 54.1 ±23.5 | 0.001 |
| TAPSE (cm)¶ | 1.4 ± 0.4 | 1.40 ± 0.4 | 1.3 ± 0.4 | 0.31 |
| RV diameter (cm)‡ | ||||
| -Basal | 4.1 ± 0.8 | 3.9 ± 0.7 | 4.2 ± 0.8 | 0.01 |
| -Mid | 3.7 ± 0.8 | 3.5 ± 0.7 | 3.8 ± 0.9 | 0.02 |
| -Longitudinal | 7.0 ± 1.1 | 6.8 ± 1.1 | 7.1 ± 1.1 | 0.04 |
| RV dysfunction | ||||
| -Not available | 50 (21.3) | |||
| -Normal | 111 (47.2) | 66 (75.0) | 45 (46.4) | |
| -Mild | 34 (14.5) | 13 (14.8) | 21 (21.6) | <0.001 |
| -Moderate | 18 (7.7) | 6 (6.8) | 12 (12.4) | |
| -Severe | 22 (9.4) | 3 (3.4) | 19 (19.6) | |
Abbreviations: ABP: arterial blood pressure, CI: cardiac index, CO: cardiac output, EF: ejection fraction, PAWP: pulmonary artery wedge pressure, PAP: pulmonary artery pressure, PVR: pulmonary vascular resistance, RA: right atrium, RV: right ventricle, RVSP: right ventricular systolic pressure, TAPSE: tricuspid annular plane systolic excursion.
Data available in 159 patients.
Data available in 166 patients.
Data available in 159 patients.
p values are given for reference only and have not been adjusted for multiple comparison.
Table 3.
PFT, ABG and chest CT determinations.
| All patients Mean ± SD or n (%) |
No PH Mean ± SD or n (%) |
PH Mean ±SD or n (%) |
P (t-test) (T-test) |
|
|---|---|---|---|---|
| Spirometry (n=225) | ||||
| FVC (% of predicted) | 47.5 ± 16.2 | 45.2 ± 13.8 | 49.8 ± 18.0 | 0.03 |
| FEV1 (% of predicted) | 51.5 ± 14.8 | 50.5 ± 14.4 | 52.3 ± 15.4 | 0.37 |
| FEV1/FVC | 0.84 ± 0.10 | 0.85 ± 0.11 | 0.83 ± 0.1 | 0.09 |
| TLC (% of predicted) | 54.5 ± 14.0 | 52.1 ± 10.9 | 56.8 ± 16.1 | 0.02 |
|
DLCOc (% of predicted) |
27.3 ± 10.4 | 28.9 ± 11.3 | 25.1 ± 9.0 | 0.004 |
| FVC / DLCOc | 2.1 ± 1.0 | 1.8 ± 0.8 | 2.3 ± 1.1 | 0.002 |
| Six-minute walk test (n=199) | ||||
| Baseline HR (bpm) | 87.8 ± 15.6 | 88.1 ± 15.0 | 87.5 ± 16.1 | 0.79 |
| Distance walked (m) | 318.8 ± 101.2 | 342.6 ± 107.9 | 295.3 ± 88.5 | 0.001 |
|
Distance walked (% predicted) |
60.7 ± 21.1 | 66.8 ± 21.6 | 54.0 ± 18.4 | <0.001 |
| HRR (1 min) | 14.2 ± 9.2 | 13.6 ± 9.3 | 14.7 ± 9.2 | 0.51 |
| Arterial blood gases on room air (n=205) | ||||
| PaO2 (mmHg) | 56.9 ± 13.1 | 60.4 ± 12.1 | 53.5 ± 13.2 | <0.001 |
| PaCO2 (mmHg) | 39.5 ± 5.8 | 40.3 ± 4.8 | 39.0 ± 6.5 | 0.11 |
| Computed tomography (n=189) | ||||
| Aortic diameter (mm) | 34.7 ± 3.8 | 34.9 ± 4.0 | 34.5 ± 3.6 | 0.46 |
| PA diameter in (mm) | 32.2 ± 5.2 | 31.0 ± 4.8 | 33.2 ± 5.3 | 0.003 |
|
Ratio of PA/AA diameters |
0.93 ± 0.15 | 0.89 ± 0.14 | 0.97 ± 0.16 | 0.001 |
Abbreviations: AA: ascending aorta, DLCOc: corrected carbon monoxide diffusion capacity, FEV1: forced expiratory volume in 1 sec, FVC: forced-vital capacity, HR: heart rate, HRR: heart rate recovery, PA: pulmonary artery, PaO2: partial arterial pressure of O2, PaCO2: partial arterial pressure of CO2, TLC: total lung capacity.
We developed models using CART (classification and regression tree) binary recursive partitioning37 in which the parent nodes are split into two nodes in a recursive manner until each terminal node is assigned to a class outcome. CART looks at all possible splits for all variables included in the analysis. The chosen split is based on maximizing classification and minimizing error. Splitting stops when a node has few cases or all cases belong to one group. Once a maximal tree is grown, smaller trees are examined by pruning away branches. We included categorical and continuous data in the CART models. The whole cohort was used to build the trees presented.
V-fold cross-validation38 with 10 partitions was used to estimate the error rate of a sub-tree, in where the total sample is divided 9/10 for learning and 1/10 for testing. This is repeated 10 times, an in each iteration a unique 1/10 of the total sample is used for testing and the remaining 9/10 for learning. There is no overlap in the testing samples, but there is considerable overlap in the learning samples. When summed, the test partitions are equal to the entire original training data. Importantly, each sub-tree has the same distribution of patients with and without the condition of interest (e.g. PH and no PH). Random forest, a nonparametric tree-based ensemble machine learning tool, was used to rank variables that best discriminate between groups39.
All the p values were reported as two tailed. A p value of <0.05 was pre-specified as indicative of statistical significance. The statistical analyses were performed using the statistical package SPSS version 17 (IBM; Armonk, N.Y., USA), MedCalc version 14.10.2 (MedCalc Software bvba, Ostend, Belgium) and CART version 7.0 (Salford Systems, California, USA).
Results
a) Patient characteristics
We included 235 IPF patients with a mean age of 60 ± 9 years. One hundred and sixty-seven (71 %) were male. Pulmonary hypertension was present in 119 patients (51 %). Patients with PH had similar gender, were slightly younger and had higher LAS than patients without PH. In PH patients, mean PAP and PVR were 35 ± 10 mmHg and 4.6 ± 2.8 Woods units, respectively. Three quarters of the patients (n=185) had an echocardiogram performed around the time of RHC. The median (IQR) time difference in weeks between RHC and ECG, PFT, chest CT, ABG, 6MWT and echocardiogram were 0 (−1, +14), −3 (−6, −1), −2 (−6, 0), −3 (−6, 0) and −3 (−45, 17), respectively.
Echocardiography showed RV dysfunction (mild to severe) in 22 (25%) IPF patients without PH and 52 (54%) with PH (p<0.001) (Table 1). The RV size appears larger in patients with PH (Table 1). TAPSE was decreased in IPF patients both with and without PH. Using Bland-Altman analysis the agreement (95% limit of agreement) between the systolic PAP measured during RHC and the RVSP estimated with echocardiogram was −3.9 mmHg (+34.6 and −42.3 mmHg).
b) Comparison between IPF patients with and without PH
Most of the patients had normal sinus rhythm (98%). In IPF patients with PH, we noticed a rightward deviation in both the P and QRS axes and leftward deflection of the T wave axis. Significant differences were noticed in other electrocardiographic signs as shown in Table 2.
Table 2.
Electrocardiographic determinations.
| All patients Mean ± SD or n (%) |
No PH Mean ± SD or n (%) |
PH Mean ± SD or n (%) |
P (T-test / Fisher’s exact test) |
|
|---|---|---|---|---|
| N | 228 | 111 | 117 | |
| Rhythm | 0.62 | |||
| -Sinus | 222 (97.8) | 110 (99.1) | 112 (96.6) | |
| -Atrial Fibrillation | 1 (0.4) | 0 (0) | 1 (0.9) | |
| -Nodal | 4 (1.8) | 1 (0.9) | 3 (2.6) | |
| Heart rate (bpm) | 81.5 ± 16.6 | 80.5 ± 15.5 | 82.5 ± 17.5 | 0.35 |
| P wave axis (degrees) | +38.8 ± 19.0 | +34.8 ± 18.9 | +42.7 ± 18.5 | 0.002 |
| P wave amplitude V1 (mA) | 0.14 ± 0.06 | 0.13 ± 0.06 | 0.14 ± 0.07 | 0.11 |
| P wave amplitude lead II (mA) | 0.14 ± 0.06 | 0.13 ± 0.05 | 0.15 ± 0.06 | 0.02 |
| PR interval (ms) | 152.2 ± 24.0 | 153.3 ± 22.7 | 151.1 ± 25.3 | 0.51 |
| QRS complex (ms) | 92.2 ± 16.2 | 90.9 ± 14.7 | 93.4 ± 17.4 | 0.24 |
| QRS axis (degrees) | +23.2 ± 46.2 | +10.5 ± 30.9 | +35.2 ± 54.4 | <0.001 |
| R wave lead V1 (mA) | 0.29 ± 0.26 | 0.26 ± 0.21 | 0.32 ± 0.29 | 0.13 |
| S wave lead V1 (mA) | 0.81 ± 0.47 | 0.87 ± 0.45 | 0.75 ± 0.48 | 0.07 |
| R/S ratio lead V1 | 0.59 ± 1.2 | 0.48 ± 1.0 | 0.71 ± 1.3 | 0.15 |
| QTc interval (ms) | 442.7 ± 38.5 | 439.1 ± 35.4 | 446.2 ± 41.1 | 0.17 |
| T wave axis (degrees) | +23.8 ± 34.6 | +28.9 ± 29.5 | +18.9 ± 38.4 | 0.03 |
| RBBB | 10 (4.4) | 4 (3.6) | 6 (5.1) | 0.75 |
| IRBBB | 34 (14.9) | 10 (9.0) | 24 (20.5) | 0.02 |
| qR complex in lead V1 | 6 (2.6) | 0 (0) | 6 (5.1) | 0.03 |
| QS complex in lead V1 | 6 (2.6) | 1 (0.9) | 5 (4.3) | 0.02 |
| Negative T wave in V1–V3 | 140 (61.4) | 64 (57.7) | 76 (65.0) | 0.28 |
| Negative T wave in inferior leads | 113 (49.6) | 50 (45.0) | 63 (53.8) | 0.19 |
| ST segment depression in V1–V3 | 27 (11.8) | 7 (6.3) | 20 (17.1) | 0.01 |
|
ST segment depression in inferior leads |
5 (2.2) | 0 (0) | 5 (4.3) | 0.06 |
Abbreviations: bpm: beats-per-minute, IRBBB: incomplete right bundle branch block, RBBB: right bundle branch block, RHC: right heart catheterization.
Patients with IPF and PH have higher FVC and TLC, but lower DLCOc; therefore the ratio of FVC/DLCOc was higher in this subgroup of patients. IPF patients with PH had more hypoxemia and a larger diameter of PA and ratio between the PA / AA. Furthermore, IPF patients with PH covered less distance during 6MWT than patients without PH (Table 3).
c) Univariable and multivariable analyses to predict PH
Several variables were predictors of PH in univariable logistic regression models (Table 4). The discriminatory ability of the tested variables was modest and similar for PaO2, SaO2, FVC/DLCOc ratio, PA/AA diameter ratio, 6MWD, QRS axis and RVSP (Table 4). In multivariable analysis, not including echocardiographic variables, only SaO2 (OR for every 10% increase: 0.35, 95% CI: 0.18–0.69, p=0.002) and PA/AA diameter ratio (OR for every 0.1 increase: 1.38, 95% CI: 1.04–1.83, p=0.03) remained significant predictors of PH, with a c-index of 0.71 (95% CI: 0.63–0.79) and accuracy of 64.2%. When including echocardiographic determinations, RV function (OR for every unit of worsening: 2.79, 95% CI: 1.38–5.64, p=0.004) and PA/AA ratio (OR for every 0.1 increase: 1.45, 95% CI: 1.04–2.03, p=0.03) remained predictors of PH with a c-index of 0.75 (95% CI: 0.65–0.84) and accuracy of 71.4%.
Table 4.
Univariable logistic regression to predict patients with PH (mPAP ≥25 mmHg)
| Variables (incremental change) | OR | 95%CI | p | C-index | 95%CI |
|---|---|---|---|---|---|
| Age (every 10 years) | 0.69 | 0.51–0.94 | 0.02 | 0.40 | 0.33–0.47 |
| PaO2 (every 10 mmHg) | 0.64 | 0.50–0.81 | <0.001 | 0.33 | 0.26–0.40 |
| SaO2 (every 10 %) | 0.36 | 0.22–0.58 | <0.001 | 0.31 | 0.24–0.38 |
| FVC (every 10 %) | 1.20 | 1.01–1.42 | 0.04 | 0.56 | 0.48–0.63 |
| TLC (every 10 %) | 1.29 | 1.03–1.61 | 0.03 | 0.57 | 0.49–0.65 |
| DLCOc (every 10 %) | 0.63 | 0.45–0.86 | 0.004 | 0.37 | 0.28–0.46 |
| FVC/DLCO ratio (every 0.1) | 1.77 | 1.20–2.61 | 0.004 | 0.65 | 0.56–0.73 |
| PA diameter (every 1 cm) | 2.41 | 1.32–4.39 | 0.004 | 0.61 | 0.53–0.69 |
| PA/AA ratio (every 0.1) | 1.42 | 1.15–1.74 | 0.001 | 0.64 | 0.56–0.72 |
| 6MWD (every 10 m) | 0.95 | 0.92–0.98 | 0.001 | 0.36 | 0.28–0.44 |
| 6MWD (every 10 %) | 0.73 | 0.61–0.87 | 0.001 | 0.33 | 0.24–0.42 |
| P axis (every 10 degrees) | 1.26 | 1.09–1.46 | 0.002 | 0.60 | 0.53–0.68 |
| P wave in D2 (every 0.1 mV) | 1.73 | 1.07–2.79 | 0.03 | 0.58 | 0.51–0.66 |
| QRS axis (every 10 degrees) | 1.14 | 1.07–1.22 | <0.001 | 0.62 | 0.55–0.70 |
| T axis (every 10 degrees) | 0.91 | 0.84–0.99 | 0.03 | 0.41 | 0.33–0.48 |
| V1–V3 ST depression (Yes) | 3.06 | 1.24–7.57 | 0.02 | ||
| IRBBB (Yes) | 2.61 | 1.18–5.74 | 0.02 | ||
| RVSP (every 10 mmHg) | 1.32 | 1.10–1.57 | 0.002 | 0.63 | 0.54–0.72 |
| RV dilation (every 1 grade^) | 1.96 | 1.31–2.93 | 0.001 | ||
| RV function (every 1 grade^) | 2.24 | 1.52–3.31 | <0.001 | ||
| RV basal diameter (per 1 cm) | 1.71 | 1.11–2.64 | 0.02 | 0.60 | 0.51–0.69 |
| RV mid diameter (per 1 cm) | 1.59 | 1.05–2.39 | 0.03 | 0.57 | 0.48–0.66 |
|
RV longitudinal dimension (per 1 cm) |
1.36 | 1.01–1.83 | 0.04 | 0.59 | 0.50–0.68 |
Abbreviations: 6MWD: distance walked in six minute walk test, AA: ascending aorta, DLCOc: corrected diffusion lung capacity of carbon monoxide, FVC: forced vital capacity, IRBBB: incomplete right bundle branch block, PA: pulmonary artery, PaO2: partial arterial pressure of O2, RV: right ventricle, RVSP: right ventricular systolic pressure, SaO2: arterial O2 saturation.
a change from normal to mild, mild to moderate or moderate to severe.
d) Univariable and multivariable analyses to predict patients with precapillary PH (PVR > 3 Wood units)
Several variables were predictors of precapillary PH in univariable analyses (Table 5). The variables that provided the best discrimination were SaO2, FVC/DLCOc ratio, PA/AA diameter ratio, 6MWD, QRS axis and RVSP (Table 5). In a multivariable analysis, not including echocardiographic variables, SaO2 (OR every 10% increase: 0.50, 95% CI: 0.25–0.99, p=0.04), PA/AA diameter ratio (OR every 0.1 increase: 1.76, 95% CI: 1.27–2.46, p< 0.001) and QRS axis (OR every 10° increase: 1.19, 95%: 1.06–1.34, p=0.004) remained significant predictors of precapillary PH (c-index 0.83 (95% CI: 0.75–0.89), accuracy of 80.9 %).
Table 5.
Univariable analysis to predict patients with precapillary PH (PVR > 3 Wood Units)
| Variables (incremental change) | OR | 95%CI | p | C-index | 95%CI |
|---|---|---|---|---|---|
| PaO2 (every 10 mmHg) | 0.62 | 0.47–0.82 | 0.001 | 0.32 | 0.24–0.40 |
| SaO2 (every 10 %) | 0.39 | 0.25–0.62 | <0.001 | 0.30 | 0.21–0.38 |
| TLC (every 10 %) | 1.32 | 1.06–1.67 | 0.02 | 0.58 | 0.49–0.68 |
| DLCOc (every 10 %) | 0.52 | 0.35–0.78 | 0.001 | 0.34 | 0.25–0.43 |
| FVC/DLCOc (every 0.1) | 2.20 | 1.47–3.32 | <0.001 | 0.69 | 0.59–0.78 |
| PA diameter (every 1 cm) | 2.84 | 1.50–5.40 | 0.001 | 0.65 | 0.56–0.74 |
| PA/AA diameter ratio (every 0.1) | 1.60 | 1.27–2.00 | <0.001 | 0.69 | 0.60–0.77 |
| 6MWD (every 10 m) | 0.96 | 0.92–0.99 | 0.006 | 0.38 | 0.29–0.46 |
|
6MWD %predicted (every 10 %) |
0.70 | 0.57–0.87 | 0.001 | 0.31 | 0.21–0.41 |
| P axis (every 10 degrees) | 1.22 | 1.03–1.44 | 0.02 | 0.60 | 0.51–0.68 |
| P wave in lead II (every 0.1 mV) | 2.26 | 1.35–3.79 | 0.002 | 0.65 | 0.57–0.73 |
| QRS axis (every 10 degrees) | 1.21 | 1.12–1.30 | <0.001 | 0.69 | 0.61–0.77 |
| T axis (every 10 degrees) | 0.90 | 0.82–1.00 | 0.04 | 0.40 | 0.32–0.49 |
| V1–V3 ST depression (Yes) | 3.60 | 1.58–8.21 | 0.002 | ||
| IRBBB (Yes) | 4.19 | 1.95–9.03 | <0.001 | ||
| RVSP (every 10 mmHg) | 1.46 | 1.21–1.76 | <0.001 | 0.68 | 0.58–0.78 |
| RV dilation (every 1 grade^) | 2.39 | 1.59–3.61 | <0.001 | ||
| RV function (every 1 grade^) | 2.85 | 1.92–4.23 | <0.001 | ||
| RV basal diameter (per 1 cm) | 1.68 | 1.08–2.60 | 0.02 | 0.61 | 0.52–0.71 |
| RV mid diameter (per 1 cm) | 1.81 | 1.18–2.78 | 0.007 | 0.62 | 0.52–0.72 |
Abbreviations: 6MWD: distance walked in six minute walk test, AA: ascending aorta, DLCOc: corrected diffusion lung capacity of carbon monoxide, IRBBB: incomplete right bundle branch block, PA: pulmonary artery, PaO2: pulmonary artery pressure of O2, PVR: pulmonary vascular resistance, RV: right ventricle, RVSP: right ventricular systolic pressure, SaO2: arterial O2 saturation, TLC: total lung capacity.
a change from normal to mild, mild to moderate or moderate to severe.
In a multivariable analysis that included echocardiographic variables, PA/AA (OR every 0.1 increase: 2.01, 95% CI: 1.30–3.12, p=0.002), QRS axis (OR every 10° increase: 1.22, 95% CI: 1.04–1.42, p=0.02) and RV function (OR for every unit of worsening: 2.36, 95% CI: 1.04–5.39, p=0.04) were independent predictors of precapillary PH (c-index 0.86 (95% CI: 0.76–0.92), accuracy of 84.2%). Interestingly, a combination of PA/AA diameter ratio < 1.1, a QRS axis < 90° and a normal RV function encompassed 57% of the patients and had a sensitivity of 73%, specificity of 70% and negative predictive value of 85% for the presence of precapillary PH. The cut-offs selected for PA/AA diameter ratio and QRS axis were a priori-planned and based on the literature20,24,40.
e) Classification and regression tree analyses
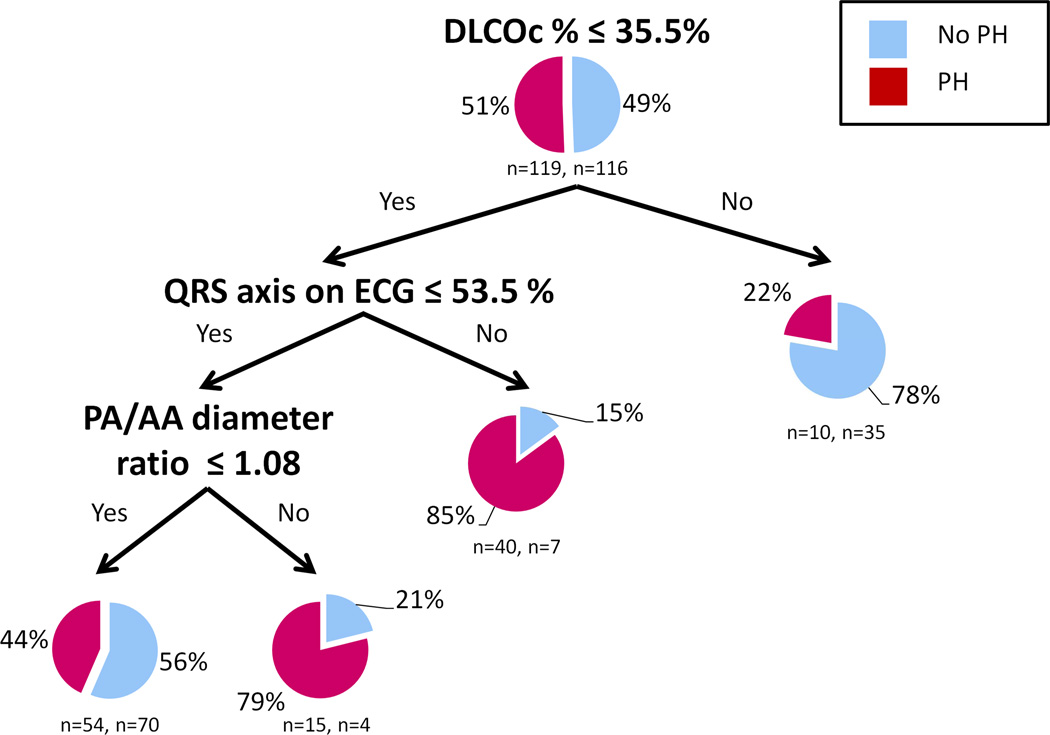
Random forests analysis identified DLCOc, P axis, QRS axis and PA/AA ratio as the variables with the highest importance for adequate discrimination between IPF patients with and without PH. CART analysis is shown in Figure 1. In the testing sample, the model misclassified 58 out of 119 (48.7%) PH subjects and 47 out of 116 (40.5%) non PH patients, with an overall adequate discrimination of 55.3%. Receiver operating characteristics (ROC) for the testing cohort showed an area under the curve of 0.56. When echocardiographic data were allowed in the model, RV function and RVSP were used in distal nodes, but they minimally improved the overall discrimination and made the overall model more complex.
Figure 1. CART analysis to discriminate between IPF patients with and without PH.
Each terminal node provides a pie chart with the percentage of IPF patients with PH (mean PAP ≥ 25 mmHg) and without PH (mean PAP < 25 mmHg). The number of patient with and without PH is provided below the pie chart.
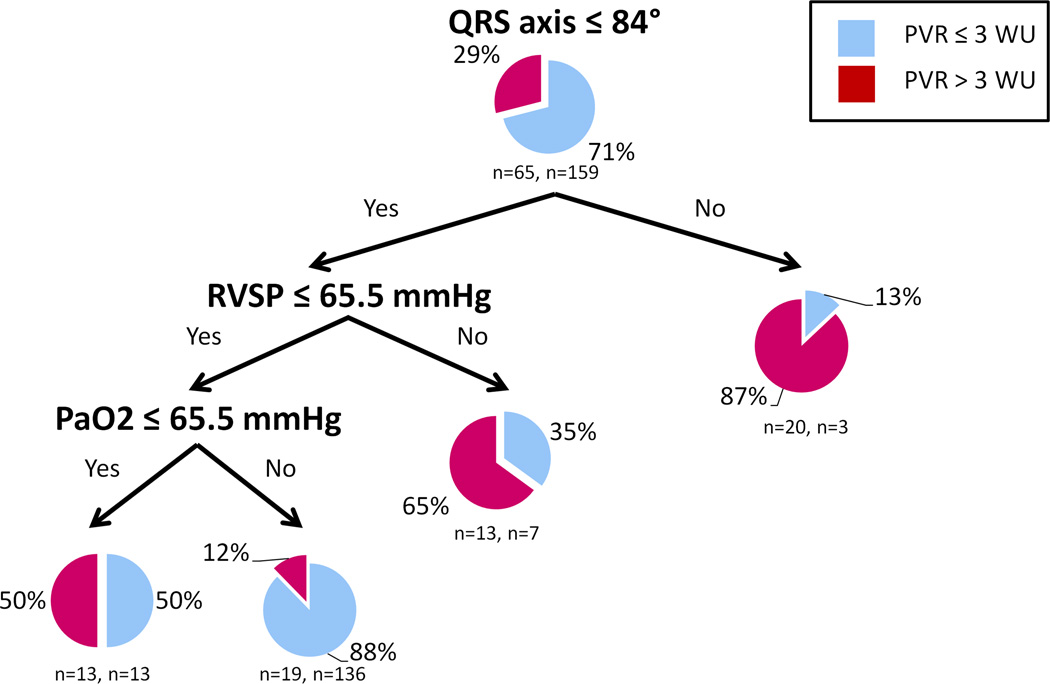
The variables with the highest importance to discriminate IPF patients with and without precapillary PH (PVR <3 Wood units) were QRS axis and RVSP. The CART analysis is shown in Figure 2. In the testing sample, the percentages of misclassification were 20.1 % and 53.9% in the PVR ≤ 3 and PVR > 3 Wood unit groups, with an overall prediction success of 70.1 % and ROC area under the curve of 0.64.
Figure 2. CART analysis to discriminate between IPF patients with and without precapillary PH.
Each terminal node provides the number and percentage of IPF patients with precapillary PH (mean PAP ≥ 25 mmHg and PVR >3 Wood units) and without precapillary PH (PVR ≤3 Wood units). The number of patient with and without precapillary PH is provided below the pie chart.
Discussion
The identification of PH is of great importance in IPF; however, the traditional noninvasive tools used for PH screening have limitations particularly in patients with parenchymal lung diseases. We tested whether a distinct non-invasive methodology or a combination of tests is particularly useful in discriminating IPF patients with or without PH. We found that a large number of non-invasive variables derived from ABG, ECG, chest CT, PFT and echocardiography were significant predictors of PH. However, none of these tests either alone or in combination correctly classified PH patients with a percentage higher than 71 %.
Echocardiography is commonly used in screening of PH. However, this test has limited accuracy in the setting of IPF12,13,15. In fact, Arcasoy et al. showed that 48% of IPF patients were misclassified as having PH; moreover, echocardiography was unable to estimate the RVSP in 44% of patients due to poor acoustic window12. We found that the echocardiographic determinations that included RVSP, TAPSE and RV dimensions were of limited value in discriminating IPF patients with and without PH. Similarly to results reported by other authors, we found a limited utility in combining non-invasive investigations to increase the ability of detecting PH in IPF13,26,27. Interestingly, 22 out of 88 (25%) IPF patients without PH by RHC had some degree of RV dysfunction by echocardiography, both by visual estimation and TAPSE. Fourteen of the 22 patients with RV dysfunction underwent lung transplantation and in all the RV function returned to normal. The origin of the RV dysfunction in the absence of PH during RHC is unclear but likely represents and early involvement of the RV function in IPF due to systemic inflammation, endothelial dysfunction and the result of small increases in mean PAP (below 25 mmHg)41.
In the present study, we found several ECG variables associated with PH. These include larger P wave amplitude in DII and rightward deflection of both the QRS and P axes because of right atrial and ventricular dilation. Other studies showed that P wave amplitude, QRS and P axes were significantly different in PH patients and they correlated with worse prognosis18,42,43. Ahearn, et al. found that QRS axis deviation was the ECG sign that correlated best with hemodynamic parameters and right ventricular size in PH16. We have previously reported that the QRS axis deviates to the right from the time between PAH diagnosis and death20.
On chest CT the PA and PA/AA diameter ratio were significantly larger in patients with PH24. These CT scan measurements usually predict PH with good sensitivity and specificity44,45. However, in pulmonary fibrosis, the predictive value of these CT measurements is inadequate23,24,46,47. In a prospective study, Alhamad, et al. found a poor correlation between mPAP and PA diameter in patients with IPF21. It has been proposed that pulmonary fibrotic changes cause traction and dilation of the pulmonary artery wall, thus, blunting the relationship between dilated PA and PH24.
In our study we found that SaO2 was an independent predictor of PH and precapillary PH in IPF patients. Several determinations obtained from ABG and PFT have been associated with the presence of PH in IPF9,26,27,48,49. Patients with IPF and PH have a lower DLCO that the one expected for their degree of parenchymal lung disease26,27,48. Lettieri, et al. noted that a DLCO < 40 % of predicted was a strong predictor of PH in IPF patients9. Since FVC/DLCO ratio and SaO2 were significantly associated with PH26,27, a formula that uses these two variables was proposed for estimating the mean PAP in IPF patients26,27. In our study, DLCOc was lower in PH patients but the FVC/DLCOc was higher. FVC/DCLOc showed one of the highest c-index to predict PH in univariable analysis; however this variable was not selected in multivariable analyses.
In the 6MWT, we showed that a shorter distance walked was associated with the presence of PH in IPF patients. Heart rate recovery (HRR) in 1 minute50 was not significantly different between patients with and without PH, possibly because all the patients had advanced IPF and in this context, HRR might lose its predictive ability. In other studies, HRR was predictive of mortality and the development of PH in IPF patients25,51.
None of the investigations tested, including echocardiography, was good enough either alone or in combination to accurately screen IPF patients for the presence of PH. A quarter of IPF patients were misclassified, even when combining the information provided by several non-invasive tests. Therefore, RHC remains necessary to confirm the diagnosis of PH in IPF. Nevertheless, the combination of a low PA/AA diameter ratio, a normal QRS axis and good RV function has a high negative predictive value for the presence of precapillary PH, which has evolved as a useful parameter in predicting mortality in parenchymal lung diseases52,53.
Our study has limitations: a) all of the patients had advanced IPF evaluated for lung transplantation; therefore our results might not apply to the milder forms of IPF or other interstitial lung diseases, b) given the retrospective nature of our study, external validation of our results is necessary. Nevertheless, we included a large number of IPF patients who underwent RHC and several non-invasive determinations which are traditionally used for PH screening. Overall, this study highlights the challenges of diagnosing PH by noninvasive methodologies in patients with IPF.
Conclusion
There are significant differences in ECG, echocardiographic chest CT, PFT and ABG parameters between IPF patients with and without PH. However, these noninvasive tests alone or combination are not accurate enough for PH screening in IPF patients.
-
-
Recognition of pulmonary hypertension (PH) is important in idiopathic pulmonary fibrosis (IPF)
-
-
We found that several non-invasive determinations are predictors of PH or precapillary PH in IPF
-
-
Worse right ventricular (RV) function and higher pulmonary artery to aorta ratio predict PH
-
-
The value to detect PH of non-invasive determinations either alone or in combination was limited
-
-
A combination of certain determinations and cut-offs provides adequate negative predictive value
Acknowledgments
Funding sources: This publication was made possible by CTSA KL2 (Grant #RR024990) (A.R.T.) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Abbreviations
- 6MWT
six minute walk test
- ABG
arterial blood gases
- AA
ascending aorta
- AUC
area under curve
- CART
classification and regression tree
- CI
confidence interval
- CT
computed tomography
- DLCOc
corrected diffusion lung capacity of carbon monoxide
- ECG
electrocardiography
- FEV1
forced expiratory volume in the first second
- FVC
forced vital capacity
- IPF
idiopathic pulmonary fibrosis
- IQR
inter-quartile range
- IRBBB
incomplete right bundle branch block
- HR
heart rate
- HRR
heart rate recovery
- LAS
lung allocation score
- mPAP
mean pulmonary artery pressure
- PA
pulmonary artery
- PaCO2
partial arterial pressure of CO2 in arterial blood
- PaO2
partial arterial pressure of O2 in arterial blood
- PAWP
pulmonary artery wedge pressure
- PAP
pulmonary artery pressure
- PFT
pulmonary function test
- PH
pulmonary hypertension
- PVR
pulmonary vascular resistance
- RBBB
right bundle branch block
- RHC
right heart catheterization
- RV
right ventricle
- RVSP
right ventricular systolic pressure
- SaO2
pulse oximetry
- TAPSE
tricuspid annular plane systolic excursion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions of authors:
Laith Alkukhun MD: Participated in the design of the study, data collection, interpretation of the results, writing and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Xiao-Feng Wang, PhD: Participated in the statistical analysis, interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Mostafa Ahmed MD: Participated in the data collection, interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Manfred Baumgartner MD: Participated in the data collection, interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Marie Budev DO: Participated in the design of the study, writing and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Raed A. Dweik MD: Participated in the interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Adriano R. Tonelli MD: Participated in the design of the study, data collection, statistical analysis, interpretation of the results, writing and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted. Dr. Tonelli is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article.
Disclosures: The authors have no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Conflict of interest statements:
Laith Alkukhun MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Mostafa Ahmed: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Manfred Baumgartner MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Marie Budev DO: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Raed A. Dweik MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Adriano R. Tonelli MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Contributor Information
Laith Alkukhun, Email: alkukhl@ccf.org, Department of Internal Medicine, Cleveland Clinic, Cleveland, OH, USA.
Xiao-Feng Wang, Email: wangx6@ccf.org, Respiratory Institute Biostatistics Core. Quantitative Health Sciences. Cleveland Clinic, Cleveland, OH, USA.
Mostafa Ahmed, Email: Ahmedm2@ccf.org, Department of Pulmonary, Allergy and Critical Care Medicine. Respiratory Institute, OH, USA. Assistant Lecturer, Department of Chest Diseases, Faculty of Medicine, Assiut University, Assiut, Egypt.
Manfred Baumgartner, Email: mbaumgartner@billingsclinic.org, Department of Pulmonary, Allergy and Critical Care Medicine. Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA.
Marie Budev, Email: budevm@ccf.org, Department of Pulmonary, Allergy and Critical Care Medicine. Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA.
Raed A. Dweik, Email: dweikr@ccf.org, Pulmonary Vascular Diseases Program, Department of Pulmonary, Allergy and Critical Care Medicine. Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA.
Adriano R. Tonelli, Email: tonella@ccf.org, Department of Pulmonary, Allergy and Critical Care Medicine. Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA.
References
- 1.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. The New England journal of medicine. 2001;345(7):517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 2.Araki T, Katsura H, Sawabe M, Kida K. A clinical study of idiopathic pulmonary fibrosis based on autopsy studies in elderly patients. Internal medicine. 2003;42(6):483–489. doi: 10.2169/internalmedicine.42.483. [DOI] [PubMed] [Google Scholar]
- 3.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Hamada K, Nagai S, Tanaka S, et al. Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest. 2007;131(3):650–656. doi: 10.1378/chest.06-1466. [DOI] [PubMed] [Google Scholar]
- 5.Nadrous HF, Pellikka PA, Krowka MJ, et al. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128(4):2393–2399. doi: 10.1378/chest.128.4.2393. [DOI] [PubMed] [Google Scholar]
- 6.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. American journal of respiratory and critical care medicine. 2001;164(7):1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 7.Nathan SD, Shlobin OA, Ahmad S, et al. Serial development of pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Respiration; international review of thoracic diseases. 2008;76(3):288–294. doi: 10.1159/000114246. [DOI] [PubMed] [Google Scholar]
- 8.Shorr AF, Wainright JL, Cors CS, Lettieri CJ, Nathan SD. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. The European respiratory journal. 2007;30(4):715–721. doi: 10.1183/09031936.00107206. [DOI] [PubMed] [Google Scholar]
- 9.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129(3):746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 10.Bando K, Keenan RJ, Paradis IL, et al. Impact of pulmonary hypertension on outcome after single-lung transplantation. The Annals of thoracic surgery. 1994;58(5):1336–1342. doi: 10.1016/0003-4975(94)91908-9. [DOI] [PubMed] [Google Scholar]
- 11.Whelan TP, Dunitz JM, Kelly RF, et al. Effect of preoperative pulmonary artery pressure on early survival after lung transplantation for idiopathic pulmonary fibrosis. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005;24(9):1269–1274. doi: 10.1016/j.healun.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167(5):735–740. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 13.Modrykamien AM, Gudavalli R, McCarthy K, Parambil J. Echocardiography, 6-minute walk distance, and distance-saturation product as predictors of pulmonary arterial hypertension in idiopathic pulmonary fibrosis. Respiratory care. 2010;55(5):584–588. [PubMed] [Google Scholar]
- 14.Homma A, Anzueto A, Peters JI, et al. Pulmonary artery systolic pressures estimated by echocardiogram vs cardiac catheterization in patients awaiting lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2001;20(8):833–839. doi: 10.1016/s1053-2498(01)00274-1. [DOI] [PubMed] [Google Scholar]
- 15.Nathan SD, Shlobin OA, Barnett SD, et al. Right ventricular systolic pressure by echocardiography as a predictor of pulmonary hypertension in idiopathic pulmonary fibrosis. Respiratory medicine. 2008;102(9):1305–1310. doi: 10.1016/j.rmed.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahearn GS, Tapson VF, Rebeiz A, Greenfield JC., Jr Electrocardiography to define clinical status in primary pulmonary hypertension and pulmonary arterial hypertension secondary to collagen vascular disease. Chest. 2002;122(2):524–527. doi: 10.1378/chest.122.2.524. [DOI] [PubMed] [Google Scholar]
- 17.Bossone E, Butera G, Bodini BD, Rubenfire M. The interpretation of the electrocardiogram in patients with pulmonary hypertension: the need for clinical correlation. Italian heart journal : official journal of the Italian Federation of Cardiology. 2003;4(12):850–854. [PubMed] [Google Scholar]
- 18.Kanemoto N. Electrocardiogram in primary pulmonary hypertension. European journal of cardiology. 1981;12(3–4):181–193. [PubMed] [Google Scholar]
- 19.Alkukhun L, Baumgartner M, Budev M, Dweik RA, Tonelli AR. Electrocardiographic differences between COPD patients evaluated for lung transplantation with and without pulmonary hypertension. COPD. 2014;11(6):670–680. doi: 10.3109/15412555.2014.898047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tonelli AR, Baumgartner M, Alkukhun L, Minai OA, Dweik RA. Electrocardiography at diagnosis and close to the time of death in pulmonary arterial hypertension. Ann Noninvasive Electrocardiol. 2014;19(3):258–265. doi: 10.1111/anec.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alhamad EH, Al-Boukai AA, Al-Kassimi FA, et al. Prediction of pulmonary hypertension in patients with or without interstitial lung disease: reliability of CT findings. Radiology. 2011;260(3):875–883. doi: 10.1148/radiol.11103532. [DOI] [PubMed] [Google Scholar]
- 22.Devaraj A, Wells AU, Meister MG, Corte TJ, Hansell DM. The effect of diffuse pulmonary fibrosis on the reliability of CT signs of pulmonary hypertension. Radiology. 2008;249(3):1042–1049. doi: 10.1148/radiol.2492080269. [DOI] [PubMed] [Google Scholar]
- 23.Zisman DA, Karlamangla AS, Ross DJ, et al. High-resolution chest CT findings do not predict the presence of pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2007;132(3):773–779. doi: 10.1378/chest.07-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raymond TE, Khabbaza JE, Yadav R, Tonelli AR. Significance of main pulmonary artery dilation on imaging studies. Ann Am Thorac Soc. 2014;11(10):1623–1632. doi: 10.1513/AnnalsATS.201406-253PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swigris JJ, Olson AL, Shlobin OA, Ahmad S, Brown KK, Nathan SD. Heart rate recovery after six-minute walk test predicts pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Respirology. 2011;16(3):439–445. doi: 10.1111/j.1440-1843.2010.01877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zisman DA, Karlamangla AS, Kawut SM, et al. Validation of a method to screen for pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2008;133(3):640–645. doi: 10.1378/chest.07-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zisman DA, Ross DJ, Belperio JA, et al. Prediction of pulmonary hypertension in idiopathic pulmonary fibrosis. Respiratory medicine. 2007;101(10):2153–2159. doi: 10.1016/j.rmed.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American journal of respiratory and critical care medicine. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 Pt 2):1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 30.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786-688. [DOI] [PubMed] [Google Scholar]
- 31.Minai OA, Gudavalli R, Mummadi S, Liu X, McCarthy K, Dweik RA. Heart rate recovery predicts clinical worsening in patients with pulmonary arterial hypertension. American journal of respiratory and critical care medicine. 2012;185(4):400–408. doi: 10.1164/rccm.201105-0848OC. [DOI] [PubMed] [Google Scholar]
- 32.HC B. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 33.Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346(8982):1085–1087. doi: 10.1016/s0140-6736(95)91748-9. [DOI] [PubMed] [Google Scholar]
- 34.Authors/Task Force M. Galie N, Humbert M, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2015 doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 35.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 36.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd. New York, NY: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 37.Breiman L, Friedman J, Stone CJ, Olshen RA. Classification and regression trees. CRC press; 1984. [Google Scholar]
- 38.Burman P. A comparative study of ordinary cross-validation, v-fold cross-validation and the repeated learning-testing methods. Biometrika. 1989;76(3):503–514. [Google Scholar]
- 39.Breiman L. Random forests. Machine learning. 2001;45(1):5–32. [Google Scholar]
- 40.Boerrigter B, Mauritz GJ, Marcus JT, et al. Progressive dilatation of the main pulmonary artery is a characteristic of pulmonary arterial hypertension and is not related to changes in pressure. Chest. 2010;138(6):1395–1401. doi: 10.1378/chest.10-0363. [DOI] [PubMed] [Google Scholar]
- 41.D'Andrea A, Stanziola A, Di Palma E, et al. Right Ventricular Structure and Function in Idiopathic Pulmonary Fibrosis with or without Pulmonary Hypertension. Echocardiography. 2015 doi: 10.1111/echo.12992. [DOI] [PubMed] [Google Scholar]
- 42.Kanemoto N. Electrocardiogram in primary pulmonary hypertension--with special reference to prognosis. The Tokai journal of experimental and clinical medicine. 1987;12(3):173–179. [PubMed] [Google Scholar]
- 43.Bossone E, Paciocco G, Iarussi D, et al. The prognostic role of the ECG in primary pulmonary hypertension. Chest. 2002;121(2):513–518. doi: 10.1378/chest.121.2.513. [DOI] [PubMed] [Google Scholar]
- 44.Mahammedi A, Oshmyansky A, Hassoun PM, Thiemann DR, Siegelman SS. Pulmonary artery measurements in pulmonary hypertension: the role of computed tomography. Journal of thoracic imaging. 2013;28(2):96–103. doi: 10.1097/RTI.0b013e318271c2eb. [DOI] [PubMed] [Google Scholar]
- 45.Ng CS, Wells AU, Padley SP. A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. Journal of thoracic imaging. 1999;14(4):270–278. doi: 10.1097/00005382-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Haimovici JB, Trotman-Dickenson B, Halpern EF, et al. Relationship between pulmonary artery diameter at computed tomography and pulmonary artery pressures at right-sided heart catheterization. Massachusetts General Hospital Lung Transplantation Program. Academic radiology. 1997;4(5):327–334. doi: 10.1016/s1076-6332(97)80111-0. [DOI] [PubMed] [Google Scholar]
- 47.McCall RK, Ravenel JG, Nietert PJ, Granath A, Silver RM. Relationship of main pulmonary artery diameter to pulmonary arterial pressure in scleroderma patients with and without interstitial fibrosis. Journal of computer assisted tomography. 2014;38(2):163–168. doi: 10.1097/RCT.0b013e3182aa7fc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest. 2007;132(3):998–1006. doi: 10.1378/chest.06-3087. [DOI] [PubMed] [Google Scholar]
- 49.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Annals of internal medicine. 1987;107(2):216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 50.Shetler K, Marcus R, Froelicher VF, et al. Heart rate recovery: validation and methodologic issues. Journal of the American College of Cardiology. 2001;38(7):1980–1987. doi: 10.1016/s0735-1097(01)01652-7. [DOI] [PubMed] [Google Scholar]
- 51.Swigris JJ, Swick J, Wamboldt FS, et al. Heart rate recovery after 6-min walk test predicts survival in patients with idiopathic pulmonary fibrosis. Chest. 2009;136(3):841–848. doi: 10.1378/chest.09-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corte TJ, Wort SJ, Gatzoulis MA, Macdonald P, Hansell DM, Wells AU. Pulmonary vascular resistance predicts early mortality in patients with diffuse fibrotic lung disease and suspected pulmonary hypertension. Thorax. 2009;64(10):883–888. doi: 10.1136/thx.2008.112847. [DOI] [PubMed] [Google Scholar]
- 53.Rivera-Lebron BN, Forfia PR, Kreider M, Lee JC, Holmes JH, Kawut SM. Echocardiographic and hemodynamic predictors of mortality in idiopathic pulmonary fibrosis. Chest. 2013;144(2):564–570. doi: 10.1378/chest.12-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]