Abstract
Context:
The dermatophytoses constitute a group of superficial fungal infections of keratinized tissues, namely, the epidermis, hair, and nails. The distribution and frequency of dermatophytosis and their etiologic agents vary according to the geographic region studied, the socio-economic level of the population, the time of study, the climatic variations, the presence of domestic animals, and age.
Aims:
The present study was undertaken to assess the clinical profile of dermatophytic infections and to identify the causative fungal species in the various clinical presentations.
Settings and Design:
This was a hospital-based observational study.
Materials and Methods:
One hundred and fifty clinically suspected cases of dermatophytosis attending the outpatient department of a tertiary care hospital were included in the study. History was taken, general physical and cutaneous examination was done and details of skin lesions noted. Direct microscopy in 10% KOH (40% KOH for nail) and fungal culture on SDA with 0.05% chloramphenicol and 0.5% cycloheximide was done in every case.
Statistical Analysis Used:
Statistical analysis was done using SPSS 17.0 software. Chi-square test and contingency coefficient test were used as significant tests for analysis.
Results:
Out of 150 patients studied, majority belonged to the age group of 21–30 years (22.7%). Male-to-female ratio was 1.63:1. Tinea corporis (24.7%) was the most common clinical type observed. The overall positivity by culture was 40% and by direct microscopy was 59.3%. Trichophyton mentagrophytes was the predominant species isolated (48.3%).
Conclusions:
The present study reveals the changing trend in the prevalence of dermatophyte species in this part of Karnataka.
Keywords: Dermatophyte, dermatophytoses, tinea
INTRODUCTION
The dermatophytoses constitute a group of superficial fungal infections of keratinized tissues, namely, the epidermis, hair, and nails.[1] The causative fungi are moulds belonging to three asexual genera Microsporum, Trichophyton, and Epidermophyton.[2]
The distribution and frequency of dermatophytosis and their etiologic agents vary according to the geographic region studied, the socioeconomic level of the population, the time of study, the climatic variations, the presence of domestic animals, and age.[3]
The present study was undertaken to assess the clinical profile of dermatophytic infections and to identify the causative fungal species in the various clinical presentations.
MATERIALS AND METHODS
One hundred and fifty clinically suspected cases of dermatophytosis attending the outpatient department of a tertiary care hospital during a 1-year period from October 2007 to September 2008 were randomly selected for the study. Ethical clearance was obtained from the Institutional Ethical Committee prior to the start of the study.
Data was collected through a detailed history and clinical examination. Details of skin lesions were noted. Complete hemogram, blood sugar, urine routine, and renal function tests were done for all patients. Patients were referred to the ICTC for HIV testing whenever suspected.
All new cases of dermatophytosis of all age groups and both sexes, who gave consent for the required investigations were included in the study. Patients treated with antifungals or topical steroids in the recent past were excluded from the study. Depending on the presenting condition skin scales, crusts, nail clippings, or easily pluckable hair were collected in clean white paper packets. Specimen collected was subjected to potassium-hydroxide (KOH) wet preparation (10% KOH for skin and hair; 40% KOH for nail) for the presence of fungal elements. After direct microscopic examination, irrespective of demonstration of fungal elements, the specimen was inoculated into a test tube containing Sabouraud's dextrose agar with 0.05% chloramphenicol and 0.5% cycloheximide. This was incubated at 28°C for up to 4 weeks. If no growth was found after 4 weeks, it was taken as negative for growth of fungi. Fungal isolates were identified based on colony morphology, pigmentation, growth rate, microscopy (Lactophenol Cotton Blue mount), and slide culture. Special tests were done when necessary, namely, hair perforation test and urease test for species identification.
The statistical analysis was done using SPSS 17.0 software. Chi-square test and Contingency Coefficient test were used as significant tests for analysis.
RESULTS
Out of 150 cases, 93 (62%) were males and 57 (38%) were females. Male-to-female ratio was 1.63:1. The youngest patient was 1.5 years old and the oldest was 70 years old. Majority of the patients belonged to the age group of 21–30 years (22.7%), followed by 0–10 years (21.3%) and 11–20 years (19.3%) [Figure 1]. Out of 150 patients studied, 92 (61.3%) were poor, 26 (17.3%) belonged to the lower middle class, 26 (17.3%) were below poverty line, and 6 (4%) belonged to the upper middle class [Modified Updated B.G. Prasad classification (Per capita per month) July 2008].
Figure 1.
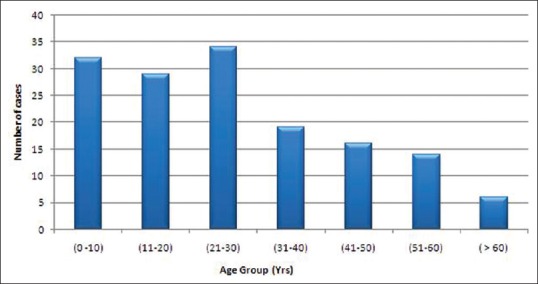
Distribution of cases according to age
Family history of dermatophytosis was present in 30 cases (20%). Tinea corporis [Figure 2] was the most common clinical type with 37 cases (24.7%), followed by tinea capitis [Figures 3 and 4] with 35 cases (23.3%) and tinea unguium [Figure 5] with 16 cases (10.7%) [Table 1]. Out of 150 cases, 51 cases (34%) had mixed infection with multiple site involvement [Table 2] [Figure 6]. In this study, tinea corporis was more common in the age group of 21–30 years with 10 cases (27%) and in females with 20 cases (54.1%) compared with males with 17 cases (45.9%). Tinea capitis was more common in the age group of 0–10 years with 23 cases (65.7%). It was more common in males with 26 cases (74.3%) [Figure 7]. Tinea unguium was more common in the age group of 31–40 years with 7 cases (43.8%) and in females with 9 cases (56.2%). Tinea faciei was more common in the age groups of 0–10 years and 11–20 years with 2 cases each (33.3%). It was more common in males with 4 cases (66.7%). Tinea pedis was more common in the age group of 11–20 years with 2 cases (50%) and in females with 3 cases (75%). Mixed clinical types were more common in the age group of 21–30 years with 16 cases (31.4%) and in males with 38 cases (74.5%). In this study the correlation between age, gender, and clinical types was statistically significant.
Figure 2.
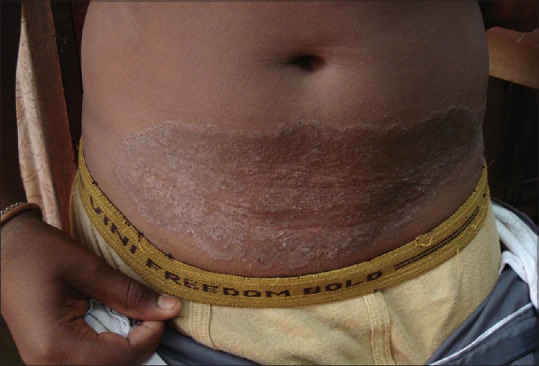
Tinea corporis over lower abdomen
Figure 3.
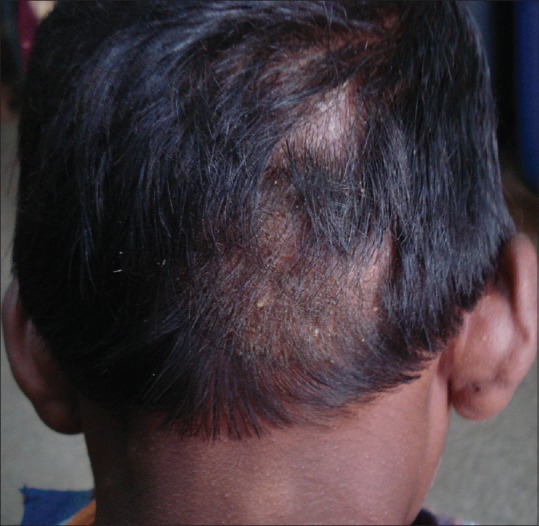
Tinea capitis: grey patch-type showing patchy alopecia with lustreless hair and scaling in the occipital region
Figure 4.
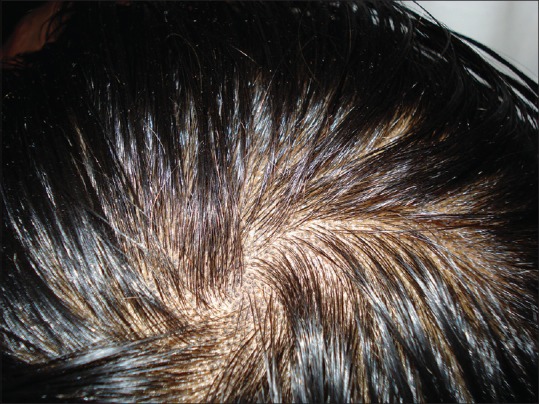
Tinea capitis: black dot type
Figure 5.
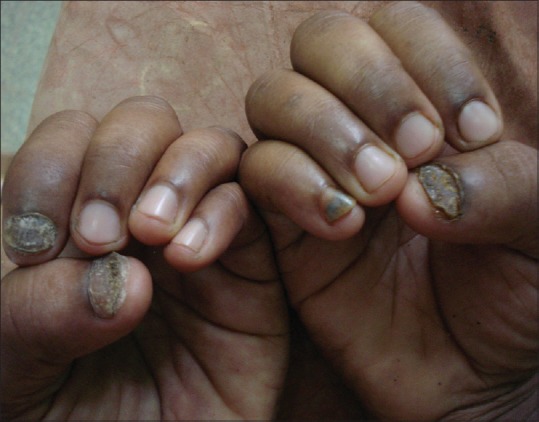
Tinea unguium: fingernails showing distal subungual and total dystrophic onychomycosis
Table 1.
Age and sex wise distribution with relation to clinical types
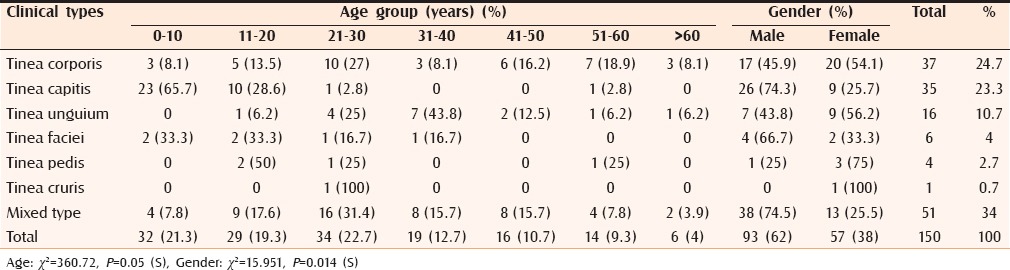
Table 2.
Mixed clinical types
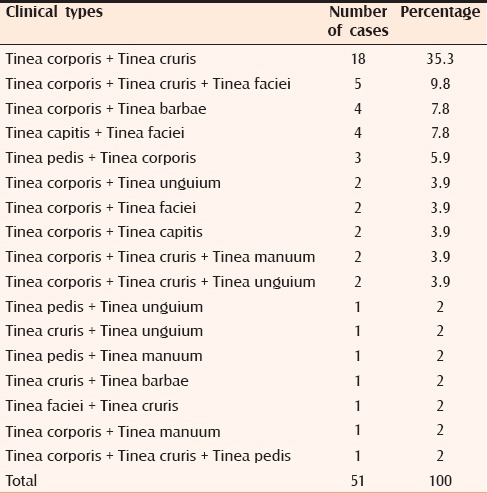
Figure 6.
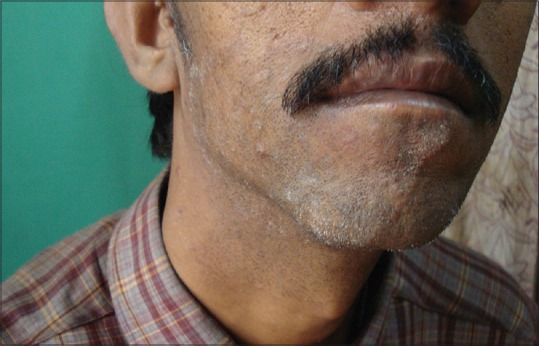
Tinea barbae
Figure 7.
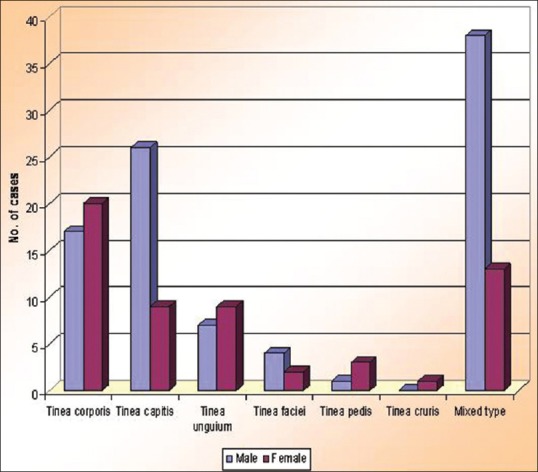
Sex distribution with respect to clinical types
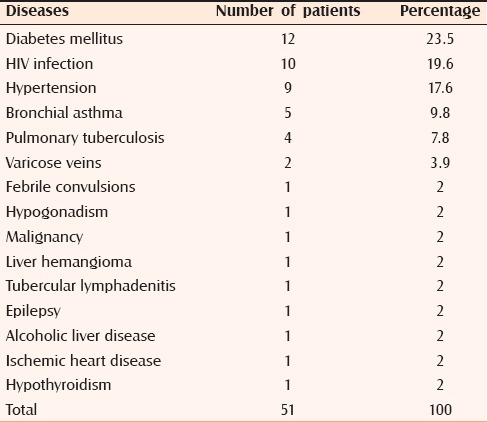
In the present study, diabetes mellitus was the most common systemic disorder associated with dermatophytosis with 12 cases (23.5%) followed by HIV infection with 10 cases (19.6%) [Table 3].
Table 3.
Associated systemic disorders
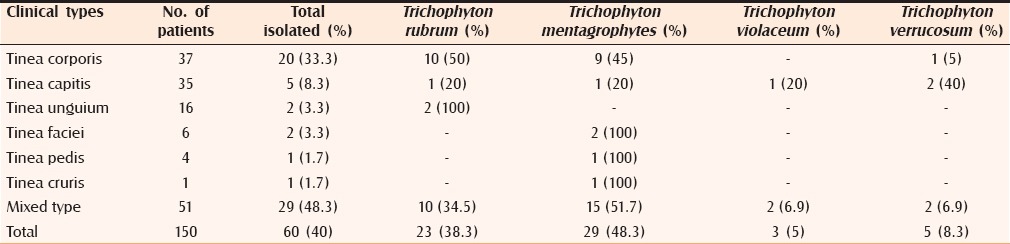
Out of the 150 clinically suspected cases of dermatophytosis, fungi were demonstrated in 90 cases (60%) by direct microscopy and/or by culture [Table 4]. Contingency coefficient test revealed that there is significant association between KOH and culture. The overall positivity by culture was 40% and by direct microscopy was 59.3%. Out of 150 cases, 60 cases (40%) were culture positive. Among these 60 cases, the most common isolate was Trichophyton mentagrophytes with 29 cases (48.3%) [Figures 8 and 9], followed by Trichophyton rubrum with 23 cases (38.3%) [Figures 10 and 11], Trichophyton verrucosum with 5 cases (8.3%) [Figures 12a, b and 13] and Trichophyton violaceum with 3 cases (5%) [Figures 14 and 15 and Table 5]. No species of Epidermophyton or Microsporum were isolated.
Table 4.
KOH and culture findings
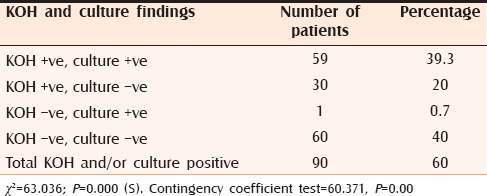
Figure 8.

Obverse view of macroscopic colony morphology of T. mentagrophytes
Figure 9.
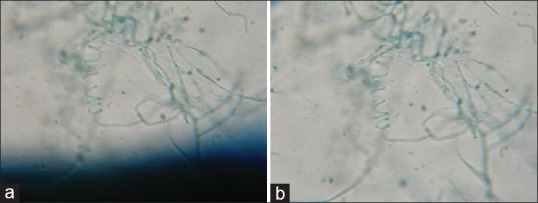
(a and b) LPCB mount of Trichophyton mentagrophytes showing spiral hyphae
Figure 10.

Obverse view of macroscopic colony morphology of Trichophyton rubrum
Figure 11.
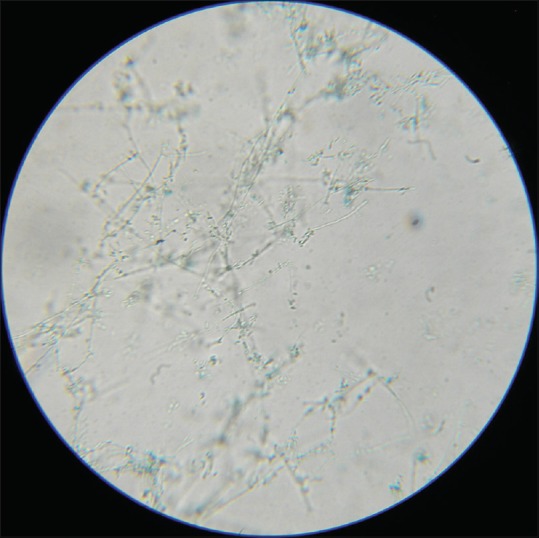
LPCB mount of Trichophyton rubrum showing abundant microconidia
Figure 12.

Trichophyton verrucosum: Growth on Sabouraud's dextrose agar. (a) Obverse view. (b) Reverse view
Figure 13.
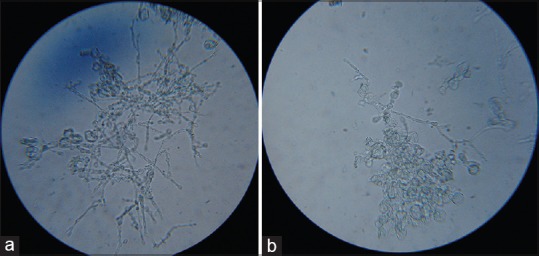
(a and b) LPCB mount of Trichophyton verrucosum showing chains of chlamydoconidia
Figure 14.

Obverse view of macroscopic colony morphology of Trichophyton violaceum
Figure 15.
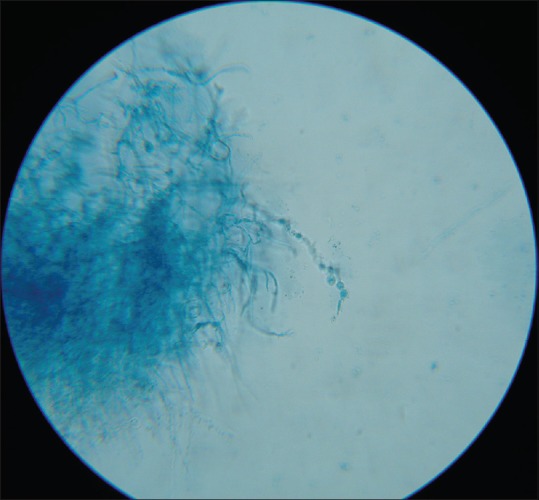
LPCB mount of Trichophyton violaceum showing irregular hyphae with intercalary chlamydoconidia
Table 5.
Dermatophytes isolated in relation to clinical types
DISCUSSION
In the present study, dermatophytosis was more common in the age group of 21–30 years (22.7%). Studies conducted by Verenkar et al.[4] and Sumana et al.[5] also showed a higher prevalence in the same age group. The increased incidence of dermatophytosis in this age group may be due to the fact that this population group takes part in maximum outdoor activities such as agriculture and manual labor, which predisposes them to acquire infection from environmental exposure. However Bindu et al.[6] observed a higher prevalence in the age group of 11–20 years.
In the present study, males (62%) were more commonly affected than females (38%). Male-to-female ratio was 1.63:1, which was similar to studies by Amin et al.[7] and Singh et al.[8] but differed from the study by Belurkar et al.[9] The increased prevalence in males may be due to the occupational hazards related to their nature of work and increased risk of exposure to infections. Furthermore, lower incidence in females may also be due to the nonreporting of female patients to hospitals due to the prevailing social stigma in the rural population of India.
In the present study, dermatophytosis was most common in the poor class (61.3%), followed by lower middle class and those below poverty line (17.3% each). Four percent belonged to the upper middle class. These findings are similar to the observations of Ranganathan et al. (1995)[10] (N = 462), who reported that 69.2% of infected people were from low income group, followed by middle income group (23.3%) and moderately rich group (1.8%). Overcrowding, poor hygienic conditions, sharing of clothes, lack of proper nutrition, and lack of proper education about sanitation seen among the patients of low socioeconomic groups may be the factors that promote the growth of dermatophytes.
In the present study, family history of dermatophytosis was present in 30 cases (20%). In a study by Bindu et al.,[6] history of contact with infected family members was seen in 16.6%. Overcrowding and sharing of clothes and towels are important factors in the household transmission of dermatophytes.
In the present study, tinea corporis was the most common clinical type seen in 24.7% of patients, which is comparable with studies by Verenkar et al.,[4] Bindu et al.,[6] Singh et al.,[8] and Surendran et al.[11] However in a study by Lyngdoh et al.,[12] tinea pedis (26.6%) was found to be the most common clinical type.
Tinea capitis was the second most common clinical type in the present study, seen in 23.3% of patients. In a study by Karmakar et al.,[13] tinea capitis accounted for 16.8% of cases. Other studies have reported relatively lower incidence. Of the 35 cases of tinea capitis in the present study, 10 cases (28.6%) gave positive family history of dermatophytosis and some of our patients were siblings. Overcrowding and sharing of combs and caps may be responsible for higher incidence of tinea capitis in the present study.
In the present study, tinea unguium was seen in 10.7% of cases, which is comparable with the study by Bindu et al. (13.3%). Lower incidence has been noted in studies by Karmakar et al. (2.8%) and Singh et al. (1.9%). This variation is dependent on the predominant age group and associated systemic diseases present in the study population such as diabetes mellitus and HIV/AIDS.
In the present study, tinea pedis was seen in 2.7% of cases, which is comparable with studies by Karmakar et al. (2%) and Bindu et al. (3.3%). Majority of patients in the present study belonged to lower socioeconomic groups and wore open shoes.
In the present study, 34% of patients showed multiple site involvement. This is comparable with the study by Siddappa et al.[14] (23%). Among those with multiple site involvement, tinea corporis with tinea cruris was the commonest type with 18 cases (35.3%), followed by tinea corporis with tinea cruris with tinea faciei with 5 cases (9.8%). The increased prevalence of multiple site involvement observed in our study may be due to associated systemic diseases such as diabetes mellitus and HIV/AIDS along with poor hygiene and delay in seeking treatment.
In the present study, tinea capitis was common in the age group of 0–10 years (65.7%) and among males (74.3%), which is also observed in other studies by Siddappa et al. and Kalla et al.[15] This is because of the lack of fungistatic action of sebum in the scalp of prepubertal children. The lower frequency in females could be due to the custom of regular application of vegetable oil over the scalp, which has fungistatic properties.[15] The higher frequency in males may be because they frequently visit saloons for their hair dressing compared with females, where they use contaminated razors and hence spreading infection from one child to another.
Tinea unguium was more common among the age group of 31–40 years (43.8%), and further females (56.2%) were more commonly affected compared with males (43.8%). This differs from studies by Kaur et al.[16] and Veer et al.[17] where males were more commonly affected. The higher occurrence of tinea unguium among females in the present study may be because females perform domestic chores and hands and feet remain wet for most of the day.
In the present study, 8% of patients had associated diabetes mellitus, 6.7% of patients had HIV infection and 3.3% of patients had asthma. In the study by Bindu et al., diabetes mellitus was seen in 10.6%, atopic diathesis in 10%, and HIV infection in 2% of patients. This variation can be due to regional difference in the incidence of various diseases.
In the present study, out of 150 clinically diagnosed cases total culture positivity was 40% (60 isolates). This was comparable with the studies by Karmakar et al., Bindu et al., and Singh et al.
In the present study, the most common culture isolate was T. mentagrophytes (48.3%), followed by T. rubrum (38.3%), T. verrucosum (8.3%), and T. violaceum (5%). This is in contrast to other studies by Siddappa et al. and Singh et al. where T. rubrum was the most common culture isolate. In a study done by Agarwal et al.,[18] T. mentagrophytes was the most common isolate.
In the present study, isolation rate of T. verrucosum, a zoophilic species was higher compared with other studies. Out of 150 patients studied, 51 (34%) gave a history of contact with domestic animals, which explains this higher isolation rate. Domestic animals constitute an important reservoir of human ringworm epidemics.
In tinea corporis out of 20 culture isolates, T. rubrum was the most common isolate with 10 cases (50%) followed by T. mentagrophytes with 9 cases (45%) and T. verrucosum with 1 case (5%).
In the present study, the predominant isolate in tinea capitis was T. verrucosum with 2 cases (40%), followed by T. rubrum, T. mentagrophytes, and T. violaceum with 1 case each (20%). This differs from the study by Kalla et al. where T. violaceum was the predominant isolate. Belurkar et al. found T. tonsurans as the predominant isolate (57.1%) from tinea capitis. These differences may be due to regional variation in the prevalence of various dermatophyte species and affinity of certain species for particular anatomical sites.
In the present study, among mixed clinical types T. mentagrophytes (51.7%) was the predominant isolate followed by T. rubrum (34.5%), T. violaceum, and T. verrucosum (6.9% each). This differs from the study by Bindu et al.[6] where T. rubrum was the predominant isolate (66.7%) from mixed clinical types, followed by T. mentagrophytes (26.7%) and T. tonsurans (6.7%). The higher isolation rate of T. mentagrophytes from mixed clinical types observed in this study may be due to changing trends in the prevalence of dermatophyte species in this part of Karnataka.
CONCLUSION
In our study, fungi were demonstrated by direct microscopy and/or by culture in 90 cases (60%) out of 150 cases. Hence direct microscopy with or without culture is an important diagnostic tool in dermatophytosis.
The overall positivity by culture was 40%. T. mentagrophytes was the most common isolate obtained (48.3%) followed by T. rubrum (38.3%). Most Indian studies have reported T. rubrum as the predominant isolate from dermatophytosis. The present study reveals the changing trends in the prevalence of various dermatophyte species in this part of Karnataka.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Emmons CW. In: Dermatophytoses. Medical Mycology. 3rd ed. Emmons CW, Binford CH, Utz JP, Kwon-Chung KJ, editors. Philadelphia: Lea and Febiger; 1977. pp. 117–64. [Google Scholar]
- 2.Hay RJ, Moore MK. In: Mycology. Rook's Textbook of Dermatology. 7th ed. Burns T, Breathnach S, Cox N, Griffiths C, editors. Oxford: Blackwell Science; 2004. pp. 31.5–31.60. [Google Scholar]
- 3.Chinelli PA, Sofiatti Ade A, Nunes RS, Martins JE. Dermatophyte agents in the city of Sao Paulo, from 1992 to 2002. Rev Inst Med Trop Sao Paulo. 2003;45:259–63. doi: 10.1590/s0036-46652003000500004. [DOI] [PubMed] [Google Scholar]
- 4.Verenkar MP, Pinto MJ, Rodrigues S, Roque WP, Singh I. Clinico-microbiological study of dermatophytoses. Indian J Pathol Microbiol. 1991;34:186–92. [PubMed] [Google Scholar]
- 5.Sumana V, Singaracharya MA. Dermatophytosis in Khammam (Khammam district, Andhra Pradesh, India) Indian J Pathol Microbiol. 2004;47:287–9. [PubMed] [Google Scholar]
- 6.Bindu V, Pavithran K. Clinico-mycological study of dermatophytosis in Calicut. Indian J Dermatol Venereol Leprol. 2002;68:259–61. [PubMed] [Google Scholar]
- 7.Amin AG, Shah HS. Dermatophytosis. Indian J Dermatol. 1973;19:22–7. [PubMed] [Google Scholar]
- 8.Singh S, Beena PM. Profile of dermatophyte infections in Baroda. Indian J Dermatol Venereol Leprol. 2003;69:281–3. [PubMed] [Google Scholar]
- 9.Belurkar DD, Bharmal RN, Kartikeyan S, Vadhavkar RS. A mycological study of Dermatophytoses in Thane. Bombay Hospital Journal 2004. [Last accessed on 2008 Dec 08]. Available from: http://www.bhj.org/journal/2004 4602 april/index.htm .
- 10.Ranganathan S, Menon T, Selvi SG, Kamalam A. Effect of socio-economic status on the prevalence of dermatophytosis in Madras. Indian J Dermatol Venereol Leprol. 1995;61:16–8. [PubMed] [Google Scholar]
- 11.Surendran K, Bhat RM, Boloor R, Nandakishore B, Sukumar D. A clinical and mycological study of dermatophytic infections. Indian J Dermatol. 2014;59:262–7. doi: 10.4103/0019-5154.131391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyngdoh CJ, Lyngdoh WV, Choudhury B, Sangma KA, Bora I, Khyriem AB. Clinico-mycological profile of dermatophytosis in Meghalaya. Int J Med Public Health. 2013;3:254–6. [Google Scholar]
- 13.Karmakar S, Kalla G, Joshi KR, Karmakar S. Dermatophytoses in a desert district of Western Rajasthan. Indian J Dermatol Venereol Leprol. 1995;61:280–3. [PubMed] [Google Scholar]
- 14.Siddappa K, Mahipal OA. Dermatophytoses in Davangere. Indian J Dermatol Venereol Leprol. 1982;48:254–9. [PubMed] [Google Scholar]
- 15.Kalla G, Begra B, Solanki A, Goyal A, Batra A. Clinicomycological study of tinea capitis in desert district of Rajasthan. Indian J Dermatol Venereol Leprol. 1995;61:342–5. [PubMed] [Google Scholar]
- 16.Kaur R, Kashyap B, Bhalla P. A five-year survey of onychomycosis in New Delhi, India: Epidemiological and Laboratory aspects. Indian J Dermatol. 2007;52:39–42. [Google Scholar]
- 17.Veer P, Patwardhan NS, Damle AS. Study of onychomycosis: Prevailing fungi and pattern of infection. Indian J Med Microbiol. 2007;25:53–6. doi: 10.4103/0255-0857.31063. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal US, Saran J, Agarwal P. Clinico-mycological study of dermatophytes in a tertiary care centre in northwest India. Indian J Dermatol Venereol Leprol. 2014;80:194. doi: 10.4103/0378-6323.129434. [DOI] [PubMed] [Google Scholar]


