Sir,
Pityriasis versicolor, also known as tinea versicolor is a superficial chronically recurring fungal infection of the stratum corneum, characterized by scaly, dyspigmented irregular macules most often occurring on the trunk and extremities.[1] The organism can easily be diagnosed by treating skin scraping with 10% KOH,[2] and shows short, thick hyphae with a large number of variously sized spores (spaghetti and meat-ball appearance). Many systemic and topical antifungals in various forms are used in the treatment of pityriasis versicolor with an overall positive result.
Our study was carried out over three months among 86 consecutive patients of pityriasis versicolor attending the dermatology outpatient department. A thorough clinical examination was done to determine the characteristics and distribution of lesions, and any other associated dermatological or systemic diseases. Skin scrapings were collected from the active (symptomatic) lesion and treated with 10% KOH. Mycological confirmation was done under the microscope. After informed consent, patients were randomly allocated to two groups by coin flipping method. The first group was advised to apply ketoconazole 2% cream twice daily and second group were asked to apply topical luliconazole 1% cream twice daily. On the 14th and 28th day, the therapeutic response was evaluated and KOH preparation of skin scrapings was repeated. There were a total six dropouts (two in first follow-up and four in the second follow-up), whose data was excluded during the final calculation.
Among the 80 patients with pityriasis versicolor, 55 (68.75%) patients were male and 25 (31.25%) patients were female, with a male preponderance (M: F = 2.2:1). Forty-two patients (52.5%) were in the age group of 21–40 years. The disease was rare above 60 years of age (4.55%). At baseline, KOH mount was positive in 80% case in the ketoconazole group and 85% in the luliconazole group. At first follow up (day 14), KOH mount negativity among the ketoconazole group was 67.50% and in the luliconazole group it was 80.00%. At the second follow up (day 28), KOH mount was negative in 72.50% in the ketoconazole group and 92.50% in the luliconazole group.
Individual improvement within both groups was statistically significant at the follow ups (P < 0.0001). Comparison between two groups showed that there was no statistically significant difference between two groups at first follow up (P = 0.2178). However, at the second follow up the improvement in luliconazole-treated group was better than in the ketoconazole-treated group (P = 0.0367), which is statistically significant [Table 1 and Figure 1].
Table 1.
Number of patients with negative KOH mount in each group, at baseline, after 14 days and after 28 days
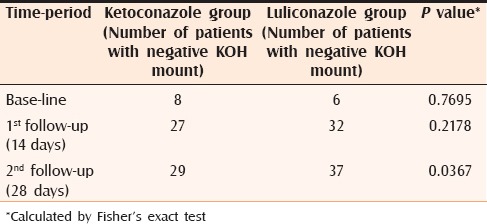
Figure 1.
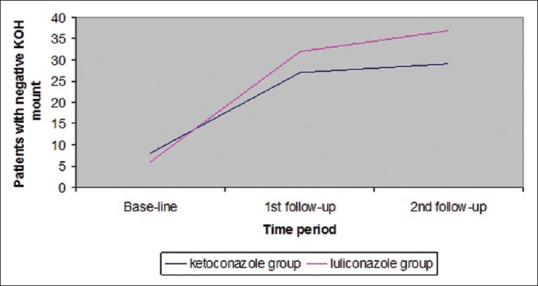
Graphical representation of KOH mount negativity in each group, as they increases over time
Different modalities of treatment are available for pityriasis versicolor including topical and systemic azoles, allylamines and also selenium sulphide. However, a recent evidence based review concluded most treatment options were similarly effective in the treatment of pityriasis versicolor but randomized controlled trials are needed to compare their relative efficacies.[3]
Ketoconazole is a water-soluble imidazole derivative. It is a synthetic antimycotic with a broad spectrum of activity against dermatophytes and yeasts.[4,5] Ketoconazole exhibits a wide spectrum of activity against dermatophytes, Candida, Malassezia in vitro.[6] In this study we have used 2% ketoconazole cream, which has previously shown 80%–90% cure rate in the treatment of pityriasis versicolor.[7,8,9,10]
Luliconazole is a novel, optically active imidazole antifungal.[11] The compound has a unique chemical structure, which is augmented by introduction of a ketene dithioacetate structure in the imidazole moiety. It has high potency inhibitory action against filamentous fungi, including dermatophytes. Preliminary studies suggested that luliconazole could also be effective against Malassezia species.[12]
In this study we have found both luliconazole and ketoconazole to be effective in the treatment of pityriasis versicolor. However, over a 4-week period, luliconazole was found to be more effective than ketoconazole.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hay RJ, Ashbee HR. Mycology. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Wiley-Blackwell; 2010. pp. 36.1–36.93. [Google Scholar]
- 2.Gupta AK, Batra R, Bluhm R, Faergemann J. Pityriasis versicolor. Dermatol Clin. 2003;21:413. doi: 10.1016/s0733-8635(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 3.Hu SW, Bigby M. Pityriasis versicolor: A systematic review of interventions. Arch Dermatol. 2010;146:1132–40. doi: 10.1001/archdermatol.2010.259. [DOI] [PubMed] [Google Scholar]
- 4.Odds FC, Milne LJ, Gentles JC, Ball EH. The activity in vitro and in vivo of a new imidazole antifungal Ketoconazole. J Antimicrobe Chemother. 1980;6:97–104. doi: 10.1093/jac/6.1.97. [DOI] [PubMed] [Google Scholar]
- 5.Thienpont D, Van Cutsem J, Van Gerven F, Heeres J, Janssen PA. Ketoconazole - a new broad spectrum orally active antimycotic. Experientia. 1979;35:606–7. doi: 10.1007/BF01960348. [DOI] [PubMed] [Google Scholar]
- 6.Heeres J, Backx LJ, Mostmans JH, Van Cutsem J. Antimycotic imidazoles. Part 4. Synthesis and antifungal activity of ketoconazole, a new potent orally active broad-spectrum antifungal agent. J Med Chem. 1979;22:1003–5. doi: 10.1021/jm00194a023. [DOI] [PubMed] [Google Scholar]
- 7.Savin RC, Horwitz SN. Double-blind comparison of 2% ketoconazole cream and placebo in the treatment of pityriasis versicolor. J Am Acad Dermatol. 1986;15:500–3. doi: 10.1016/s0190-9622(86)70200-4. [DOI] [PubMed] [Google Scholar]
- 8.Balwada RP, Jain VK, Dayal S. A doubleblind comparison of 2% ketoconazole and 1% clotrimazole in the treatment of pityriasis versicolor. Indian J Dermatol Venereol Leprol. 1996;62:298–300. [PubMed] [Google Scholar]
- 9.Chopra V, Jain VK. Comparative study of topical terbinafine and topical ketoconazole in pityriasis versicolor. Indian J Dermatol Venereol Leprol. 2000;66:299–300. [PubMed] [Google Scholar]
- 10.Nagpal VB, Jain VK, Aggarwal K. Comparative study of oral and topical ketoconazole therapy in pityriasis versicolor. Indian J Dermatol Venereol Leprol. 2003;69:287–8. [PubMed] [Google Scholar]
- 11.Niwano Y, Kuzuhara N, Kodama H, Yoshida M, Miyazaki T, Yamaguchi H. In vitro and in vivo antidermatophyte activities of NND-502, a novel optically active imidazole antimycotic agent. Antimicrob Agents Chemother. 1998;42:967–70. doi: 10.1128/aac.42.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchida K, Nishiyama Y, Tanaka T, Yamaguchi H. In vitro activity of novel imidazole antifungal agent NND-502 against Malassezia species. Int J Antimicrob Agents. 2003;21:234–8. doi: 10.1016/s0924-8579(02)00362-x. [DOI] [PubMed] [Google Scholar]


