Abstract
Macrophage colony-stimulating factor (CSF-1 or M-CSF) is important for kidney repair after acute kidney injury (AKI). CSF-1 is upregulated in tubule epithelial cells in response to kidney injury stimuli and binds to its sole receptor, CSF1R, in an autocrine and paracrine manner. Wang and colleagues used a genetic approach to constitutively delete Csf1 in proximal tubules to establish that proximal tubule production of CSF-1 is important for polarizing and skewing macrophages toward an M2 phenotype, and for recovery from AKI.
Following an acute insult, kidney function may either recover or progressively decline. During kidney recovery, renal and extrarenal cells participate in the wound-healing response and can initiate fibrosis. Immune cells of the mononuclear phagocyte system, including macrophages and dendritic cells, have emerged as important cells in the recovery of kidney function, as well as in the development of fibrosis and can dictate the balance between wound healing and progressive fibrosis. The intrinsic plasticity of monocytes/macrophages and dendritic cells, as well as attempts to relate in vitro studies to in vivo findings makes the functional definition and phenotype of this myeloid population in kidney pathophysiology complex.1–4 The distinct roles of macrophage-derived mediators of tubule repair and/or fibrosis in vivo have not been well established. In vitro studies have led to two well-defined mononuclear phagocytes. Classically activated macrophages (M1 mononuclear phagocytes, including macrophages and dendritic cells) are produced by exposure to lipopolysaccharide or interferon-γ and are widely thought to be proinflammatory and contribute to initial kidney injury. Alternatively activated macrophages (M2 mononuclear phagocytes) are produced by interleukin-4 and interleukin-10, appear later after acute kidney injury (AKI), and have a genetic signature associated with wound healing and/or fibrosis.5 The in vitro-defined relative polarization status of macrophages/dendritic cells may not reflect their true in vivo phenotype. These mononuclear phagocyte phenotypes depend on the complex local tissue microenvironment, which may induce phenotype switching.
Previously, macrophage colony-stimulating factor (CSF-1 or M-CSF) has been shown to be important for renal macrophage proliferation and polarization during kidney repair after AKI.1 Wang et al.6 (this issue) now establish proximal tubule production of CSF-1 as important for polarization of renal macrophages and recovery from AKI (Figure 1). CSF-1 is upregulated in tubule epithelial cells in response to kidney injury stimuli and binds to its sole receptor, CSF1R, in an autocrine and paracrine manner.7 CSF-1 is also well known for its role in the bone marrow to facilitate the production of blood monocytes, the precursors for infiltrating tissue macrophages. Tissue production of CSF-1 can induce monocyte recruitment from the blood and proliferation and survival of tissue-resident macrophages, as well as skew macrophages toward an M2 phenotype.8
Figure 1. Colony-stimulating factor-1 (CSF-1 or M-CSF) produced by the proximal tubule polarizes renal macrophages and recovery from acute kidney injury (AKI).
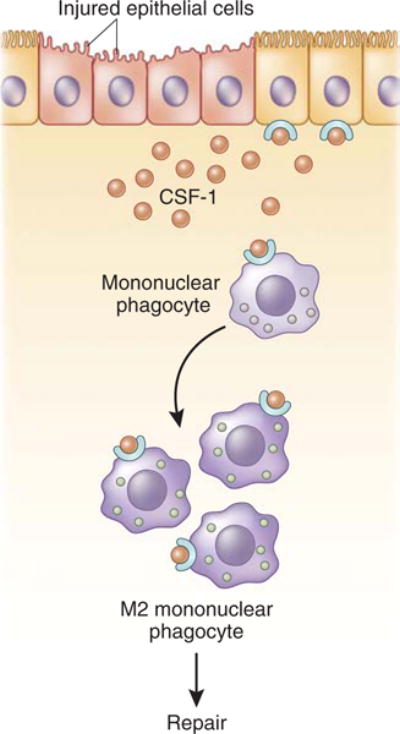
Macrophage CSF-1 is upregulated in tubule epithelial cells in response to kidney injury stimuli and binds to its sole receptor, CSF-1R, in an autocrine and paracrine manner. Tissue production of CSF-1 can induce monocyte recruitment from the blood and proliferation and survival of tissue-resident macrophages, as well as skew macrophages toward an M2 phenotype.
Wang et al.6 used a genetic approach to constitutively delete Csf1 in proximal tubules to determine whether proximal tubule production of CSF-1 was necessary for kidney recovery from AKI. They used two murine models of reversible kidney injury: ischemia/reperfusion injury and the diphtheria toxin receptor (DTR) mouse, in which administration of diphtheria toxin (DT) to transgenic mice expressing the human DTR in proximal tubule cells results in cellular apoptosis. As anticipated, in mice lacking proximal tubule expression of Csf1, activation of CSF1R was diminished in tubules and macrophages. Proximal tubule production of CSF-1 was not necessary for kidney injury at day 4 after DT. However, kidneys from mice with proximal tubule-deficient CSF-1 had reduced recovery of kidney function by day 6 and kidney function similar to that of control mice by day 10. Reduced kidney function on day 6 was associated with cellular and biochemical indicators of kidney damage such as increased kidney neutrophil numbers, Kim1 levels, and tubular oxidative stress and secondary necrosis. CSF-1 has been shown to induce tubule proliferation, thereby mediating kidney repair, and macrophages may be only partially responsible for CSF-1-dependent renal repair.7 Therefore, the relative contribution of CSF1R activation in tubules versus macrophages in response to tubule production of CSF-1 in kidney recovery has yet to be determined. Notably, previous studies by this group reported that mice globally deficient in Csf1 were unable to recover kidney function by day 10 in the DT model, suggesting that non-proximal tubule production of CSF-1 may also be important for kidney recovery from DT-mediated AKI. Alternatively, as CSF-1 is important in myeloid-cell development in the bone marrow,8 global deletion of Csf1 may have a greater impact on kidney recovery owing to extrarenal effects of CSF-1.
The study by Wang et al.6 also sought to determine whether there was a role for proximal tubule production of CSF-1 in renal macrophage/dendritic-cell number and polarization during recovery from DT-mediated AKI. Indeed, there were fewer total renal macrophages/dendritic cells at day 6 after DT in kidneys of mice lacking proximal tubule production of CSF-1, which may be owing to reduced proliferation of resident macrophages/dendritic cells or macrophages/dendritic cells derived from infiltrating monocytes, and/or reduced monocyte recruitment to the kidney. In addition, macrophages/dendritic cells purified from kidneys of mice lacking proximal tubule production of CSF-1 at day 6 after DT were less M2 polarized relative to control mice, and there was no difference in M1 markers. However, macrophage depletion by liposome clodronate at the time of DT administration in the absence of proximal tubule production of CSF-1 increased initial kidney injury and reduced recovery, suggesting that macrophage proliferation and polarization during kidney recovery is dependent on other factors in addition to CSF-1.
Consistent with a role for M2 macrophages in promoting renal repair and prevention of fibrosis, kidneys of mice lacking proximal tubule production of CSF-1 at 4 weeks after DT had increased fibrosis, fibrotic markers, and oxidative stress relative to control mice. Interestingly, although kidney function was not measured in mice lacking Csf1 in proximal tubules at this time point, it was probably similar to that of controls, as kidney function was not different at day 10. These data suggest that there is dissociation between fibrosis and kidney function, and the functional consequence of increased fibrosis is not evident at this time point; longer follow-up may be necessary.
Last, this study also convincingly demonstrates that proximal tubule production of CSF-1 is necessary for recovery from AKI mediated by ischemia/reperfusion injury. Similar to the findings in the DT model, there was no difference in initial injury in mice with the proximal tubule-specific deletion of Csf1 compared with control mice. However, these mice had reduced recovery of kidney function by 72 h of reperfusion. At day 5 of reperfusion, kidneys from mice with proximal tubule-specific Csf1 deletion also contained more neutrophils and fewer macrophages/dendritic cells. In addition, proximal tubule deletion of Csf1 resulted in a decrease in M2 markers relative to control mice in purified kidney macrophages/dendritic cells. Four weeks after ischemia/reperfusion injury, there was increased fibrosis in kidneys of mice with proximal tubule-specific deletion of Csf1 compared with control mice. Altogether, these findings support that modulation of macrophage polarization and renal repair by proximal tubule production of CSF-1 is common to both ischemia/reperfusion and direct tubule apoptosis-mediated AKI.
This study advances our understanding of the importance of the kidney interstitial microenvironment in recovery from AKI. It identifies proximal tubule production of CSF-1 as an important factor in macrophage polarization and recovery of kidney injury. Further studies are needed to elucidate the role of local tissue production of CSF-1 in proliferation and polarization of resident macrophages/dendritic cells versus recruitment of monocytes. In addition, as M2 macrophages have also been shown to be associated with fibrosis,5 future studies to clarify the functional differences within this heterogeneous population may be determined through selective, inducible deletion of key factors in specific subsets of macrophages and dendritic cells in a time-dependent manner.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
References
- 1.Zhang MZ, Yao B, Yang S, et al. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest. 2012;122:4519–4532. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huen SC, Huynh L, Marlier A, et al. GM-CSF promotes macrophage alternative activation after renal ischemia/reperfusion injury. J Am Soc Nephrol. 2015;26:1334–1345. doi: 10.1681/ASN.2014060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clements M, Gershenovich M, Chaber C, et al. Differential Ly6C expression after renal ischemia-reperfusion identifies unique macrophage populations. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014111138. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin SL, Castano AP, Nowlin BT, et al. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 5.Cao Q, Harris DC, Wang Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology. 2015;30:183–194. doi: 10.1152/physiol.00046.2014. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Chang J, Yao B, et al. Proximal tubule-derived colony stimulating factor-1 mediates polarization of renal macrophages and dendritic cells, and recovery in acute kidney injury. Kidney Int. 2015;88:1274–1282. doi: 10.1038/ki.2015.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menke J, Iwata Y, Rabacal WA, et al. CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J Clin Invest. 2009;119:2330–2342. doi: 10.1172/JCI39087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]


