Abstract
Fusarium infections are an important problem worldwide, commonly affecting immunocompromised individuals. We conducted a retrospective study in two Israeli tertiary medical centers of factors predisposing to infection by Fusarium spp. and their influence on the epidemiology and clinical outcome of this infection. Fusarium spp. were isolated from 89 patients with a median age of 57 years. Sixty-eight patients were considered immunocompetent. Seven patients had disseminated disease, 34 had locally invasive disease, and 48 had superficial infection. Most infections were limited and occurred mainly in lower limbs. Factors associated with in-hospital mortality were chronic renal failure, hematological malignancy, immunosuppression, disseminated infection, and positive blood culture. Multivariate analysis showed that chronic renal failure, hematological malignancy, burns, and disseminated infection were independently associated with mortality. A surge in the frequency of infections was noticed during the summer for patients from rural areas, involving mainly the eyes and lungs. At one of the hospitals (in a mountainous area), there was an increase in the isolation rate over time.
Invasive fungal infections are a major medical problem, particularly among immunocompromised hosts such as patients with hematological malignancies and those who have undergone stem cell or solid organ transplantation (3, 8, 20, 22, 23, 33). Some fungal species, including Fusarium spp., rarely cause disease but are considered emerging pathogens (8, 23). Since many of the data concerning such infections are gathered from small series or individual cases, it is difficult to evaluate the predisposing factors, natural history, clinical course, treatment, and prognosis of these patients.
Fusarium species are molds that have a worldwide distribution and are found mainly as saprophytic organisms in soil (26). In recent years, there have been an increasing number of reports of human infection due to Fusarium species, mostly involving immunocompromised hosts (3, 10, 16, 19). Some consider it the second most common mold pathogen in these patients (8), causing localized infection, deep-seated skin infections, and disseminated disease. Reports of infection in nonimmunocompromised hosts are infrequent and usually involve dialysis-related, burn wound, or ocular infections (1, 2, 5, 11, 12, 18, 26, 28, 31, 32, 34-37), although it has been suggested recently that, among these patients, the most frequent site of infection is the skin (27).
We reviewed all patients from whom Fusarium species were isolated in two major tertiary-care centers in Israel. Our purpose was to investigate the spectrum of fusarial infection among both immunocompromised hosts and other groups of patients and to determine factors which may influence the acquisition and outcome of infection in these diverse groups.
(Parts of this work were presented at the 15th Congress of the International Society for Human and Animal Mycology, San Antonio, Tex., in 2003.)
MATERIALS AND METHODS
We performed a retrospective study in the two main medical centers in which fungal diagnosis is performed in Israel. The two Hadassah University hospitals in Jerusalem together comprise a 1,000-bed tertiary medical center situated in a mountainous area at 800 m above sea level. Sheba Medical Center is a university hospital with 1,400 beds, situated on the coastal plain in the Tel Aviv metropolitan area. In both centers, all medical services are provided, including hemato-oncology and bone marrow transplant services. The computerized microbiology databases of Hadassah University Hospital (January 1987 to January 2002) and Sheba Medical Center (January 1992 to January 2002) were screened for reports of isolates of any Fusarium species. Hospital records of all patients with cultures positive for Fusarium species were reviewed, and data were extracted according to a predetermined protocol. Outpatients and nonresidents of Israel were excluded because the data available in the records of these patients were inadequate.
Immunocompromised patients were defined as those who had a hematological malignancy, were undergoing chemotherapy, or were receiving steroids at a dose higher than that equivalent to 10 mg of prednisone/day. Patients who did not meet these criteria were considered immunocompetent. Patients were regarded as having been treated for fusarial infection if they had received one of the following medications: amphotericin B (deoxycholate or lipid formulation), natamycin (ocular infections), or voriconazole (only one patient).
Only death occurring in the hospital was considered in the analyses involving mortality. Because of the retrospective nature of the study and the dearth of autopsies performed, attributable mortality was not evaluated.
Classification of infection was based on criteria proposed previously by several investigators (13, 16, 26) (Table 1).
TABLE 1.
Classification of infection
| Type of infection | Definition |
|---|---|
| Definitely disseminated | A culture positive for Fusarium from at least two noncontiguous organs or from one organ and from blood, or two positive blood cultures |
| Probably disseminated | One culture positive for Fusarium from blood or from one internal organ |
| Definitely locally invasive | Histological evidence of invasion confirmed by a positive culture from the same site |
| Probably locally invasive | Pertinent clinical symptoms without histological evidence of invasion associated with isolation of Fusarium on two different days from one nonsterile site or once from a sterile site |
| Superficial | Pertinent clinical symptoms associated with single Fusarium isolation from a nonsterile site |
Fungal isolation and identification.
Very similar procedures were used by both institutions for specimen handling, with only slight and insignificant variations. Fusarium isolates were recovered from clinical specimens following primary culture for 3 to 7 days at 30°C on Emmons' modified Sabouraud glucose agar supplemented with 50 mg of chloramphenicol and 5 mg of gentamicin per liter. A Cellotape flag preparation (15) for the rapid mounting of sporulating fungi was performed if the colonies produced abundant conidia. Microscopic observation of fusiform to sickle-shaped macroconidia after staining with lactophenol cotton blue were indicative of the presence of Fusarium spp. A pure culture obtained from a single conidium or hyphal tip inoculated on potato dextrose-sucrose agar (Difco Laboratories, Detroit, Mich.) was maintained at 30°C and examined for colony color and morphology.
Slide cultures with potato dextrose-sucrose agar were prepared from the pure culture. Conidial morphology and ontogeny were examined microscopically after 3 to 10 days of incubation at 30°C. Species determinations were made for 22 patient-unique isolates according to the overall micro- and macroscopic appearance, including the typical appearance and morphology of colonies (usually a loose cottony texture) and microscopic features and arrangement of macroconidia (usually hyaline, multiseptate, fusiform to sickle-shaped, mostly with an elongated apical cell and pedicellate basal cell), shape and mode of formation of microconidia, nature of the conidiogenous cell bearing microconidia, and presence or absence of chlamydoconidia (6, 14).
Data analysis was performed with SPSS statistical package release 11.01. Groups were compared with chi-square or Fisher's exact test for categorical variables and the Mann-Whitney U test for nonparametric continuous variables. In all cases, a two-sided P value was used. All parameters with a P value of <0.2 in the univariate analyses were included in a logistic multivariate analysis to predict mortality.
RESULTS
During the study period, cultures positive for Fusarium species were obtained from 89 patients (18 at Hadassah University Hospital and 71 at Sheba Medical Center).
Clinical manifestations.
Patient characteristics are described in Table 2. The majority of the patients (76%, 68 of 89) were not considered immunocompromised, although their characteristics (age, sex, and type of infection) were similar to those seen among immunocompromised patients (Table 2). The proportion with disseminated or locally invasive disease was almost identical in both groups. Although some organs or tissues, including eyes, gastrointestinal tract, and peritoneum, were more likely to be involved in the immunocompetent group, this did not achieve statistical difference. The respiratory tract (nasopharynx and lungs) was significantly more involved among immunocompromised patients. Interestingly, ischemic heart disease was a prominent feature of the immunocompetent patients (P = 0.034). As expected, burn patients were found exclusively among the nonimmunocompromised group.
TABLE 2.
Characteristics of patients
| Characteristic | All patients (n = 89) | Immunocompetent (n = 68) | Immunocompromised (n = 21)a | P (immunocompetent vs. immunocompromised) |
|---|---|---|---|---|
| Age, yr (median; range) | 57; 0-92 | 61; 0-92 | 48; 1-87 | 0.095 |
| No. male:no. female | 47:42 | 39:29 | 8:13 | NSb |
| Hospital (HUH:SMC)c | 9:59 | 9:12 | 0.003 | |
| Residence | ||||
| Rural:urban | 17:72 | 15:53 | 2:19 | NS |
| Mountain:plain | 10:78 | 5:63 | 6:15 | 0.01 |
| Type of infection (no. of patients) | ||||
| Disseminated and probably disseminated | 7 | 5 | 2 | NS |
| Locally invasive and probably locally invasive | 34 | 24 | 10 | NS |
| Superficial | 48 | 39 | 9 | NS |
| Species (no. of patients) | ||||
| F. oxysporum | 10 | 7 | 3 | NS |
| F. solani | 8 | 6 | 2 | NS |
| F. dimerum | 4 | 3 | 1 | NS |
| Not determined | 67 | 52 | 15 | NS |
| Site of infection (no. of patients) | ||||
| Lower limb | 48 | 40 | 8 | 0.096 |
| Eye | 12 | 11 | 1 | NS |
| Lungs and pleural space | 6 | 2 | 4 | 0.01 |
| Genitourinary tract | 5 | 3 | 2 | NS |
| Blood | 3 | 1 | 2 | NS |
| Nasopharynx | 3 | 0 | 3 | 0.002 |
| Gastrointestinal tract and/or peritoneum | 4 | 4 | 0 | NS |
| Underlying disease (no. of patients) | ||||
| Hematological/stem cell transplantation | 14 | 0 | 14 | <0.001 |
| Chronic renal failure | 22 | 17 | 5 | NS |
| Dialysis (hemodialysis:peritoneal dialysis) | 5:3 | 3:3 | 2:0 | NS |
| Peripheral vascular disease | 15 | 12 | 3 | NS |
| Diabetes mellitus | 27 | 23 | 4 | NS |
| Ischemic heart disease | 14 | 14 | 0 | 0.034 |
| Burn | 8 | 8 | 0 | NS |
| Outcome (no. of patients) | ||||
| Organ removed surgically | 13 | 9 | 4 | NS |
| Patient died during same hospitalization (/of available patients) | 19 (/86) | 11 (/65) | 8 (/21) | 0.042 |
One patient, admitted twice 2 years apart, was regarded as having had two infections.
NS, not significant.
HUH, Hadassah University Hospital; SMC, Sheba Medical Center.
One of the interesting features of fusarial infection was the involvement of extremities, especially the legs, in more than 50% of patients. The next most common organ involved was the eye (13%).
Invasiveness of infection.
Most of the infections were superficial (54%, 48 of 89 patients) (Table 2). The next most common type of infection was locally invasive disease (38%, 34 patients), and disseminated disease was observed in seven patients (8%). These types of infection did not differ between the hospitals or according to the immune status of the patients. Peripheral vascular disease was a risk factor for superficial infection with Fusarium spp. (13 of 48, 27%) versus 2 of 41 (5%) patients with locally invasive and disseminated infections (P = 0.005).
Treatment.
Data regarding treatment with systemic fungal drugs were available for 82 patients. Treatment was administered to 20% (9 of 45) of patients with superficial infection, 33.3% (10 of 30) of those with locally invasive disease, and 71.4% (5 of 7) of those with disseminated disease.
Outcome.
Data on outcome during hospitalization were available for 86 patients. Nineteen (11%) died during the hospital admission during which a Fusarium species was isolated. The variables associated with in-hospital mortality by univariate analysis are presented in Table 3. Hematological malignancies and immunosuppression were associated with a higher mortality rate, as was chronic renal failure (P = 0.001). As expected, disseminated infection and its surrogate marker (fungemia) were also associated with a higher mortality rate. In multiple logistic regression analysis (Table 4), chronic renal failure, hematological malignancy, burns, and disseminated infection were independently associated with poor outcome.
TABLE 3.
Univariate analysis of factors associated with mortality
| Factor | No. of patients
|
P | |
|---|---|---|---|
| Deceased (n = 19) | Survived (n = 67) | ||
| Disseminated infection | 5 | 2 | 0.005 |
| Positive blood culture | 3 | 0 | 0.009 |
| Immunosuppressive therapy | 8 | 13 | 0.042 |
| Hematological malignancies | 7 | 7 | 0.006 |
| Chronic renal failure | 10 | 11 | 0.001 |
| Burn | 4 | 4 | 0.068 |
TABLE 4.
Multiple-regression analysis for factors predicting mortality
| Factor | Odds ratio | 95% Confidence interval | P |
|---|---|---|---|
| Chronic renal failure | 16.4 | 3.1-86.0 | 0.001 |
| Hematological malignancy | 15.2 | 2.5-91.0 | 0.003 |
| Burn | 15.1 | 1.8-125.3 | 0.012 |
| Disseminated infection | 8.7 | 1.2-64.5 | 0.033 |
Mycology.
Of the 22 patient-unique isolates identified to species level, 10 were Fusarium oxysporum, 8 were Fusarium solani, and 4 were Fusarium dimerum (all F. dimerum were from Sheba Medical Center). Among the other 67 patients, identification of the species was either not available on file or had not been performed, so detailed statistical analysis of the different species could not be carried out. The most common reason for not undertaking species identification was that the fungal culture was thought to represent noninvasive infection by the laboratory or treating physician.
Seasonal and annual variations.
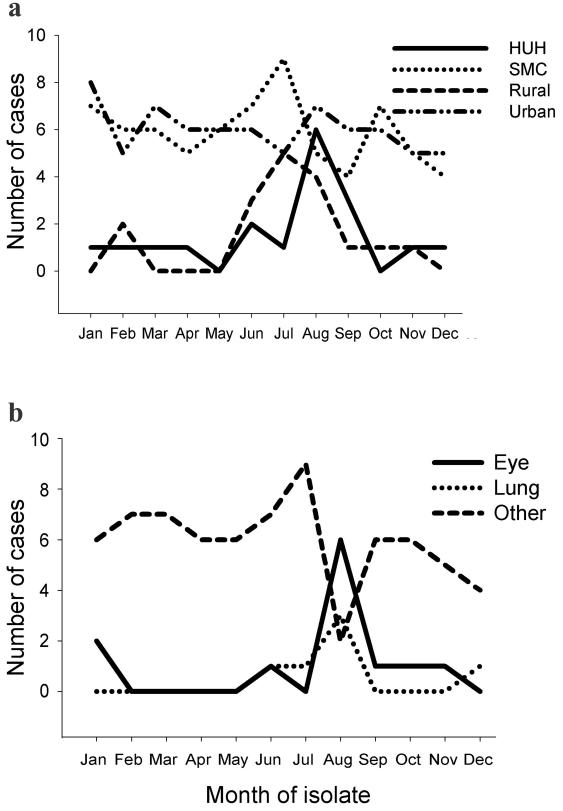
Among patients living in rural areas, most infections occurred between June and August (12 of 17), compared to 18 of 54 among residents of urban communities (P = 0.001) (Fig. 1a).
FIG. 1.
Fusarium isolation by month. (a) Patients are grouped according to area of residence or hospital. HUH, Hadassah University Hospital; SMC, Sheba Medical Center; Rural, patients living in a rural setting; Urban, urban residents. (b) Patients grouped according to organ involved. Other, all organs except eye and lung.
Ocular and pulmonary infections peaked during August, with 50% of infections in eyes (6 of 12) and lungs (3 of 6) compared to only 13% (10 of 77) and 10% (8 of 83), respectively, of all isolates from other anatomical sites (P < 0.001 and P = 0.047, respectively). A summer peak was observed in patients from Hadassah University Hospital (Fig. 1), while at the Sheba Medical Center, no significant seasonal variation was observed.
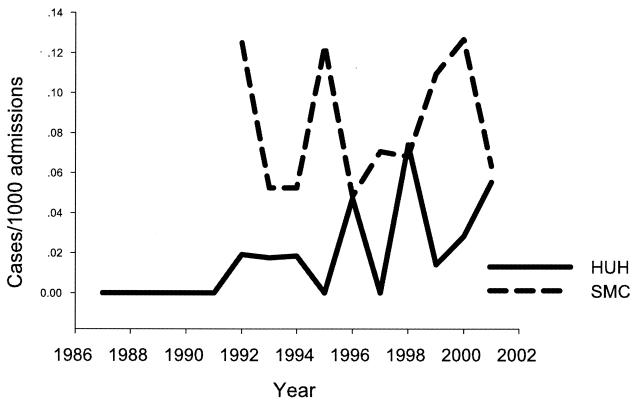
Although the number of patients from whom Fusarium spp. were isolated ranged from 5 to 13 per year, a statistically significant increasing trend in the rate of cases per 1,000 admissions was noted at the Hadassah University Hospital (P = 0.008) (Fig. 2). At the Sheba Medical Center, there was no such trend, although the incidence at the Sheba Medical Center was higher than at the Hadassah University Hospital over virtually the entire study period.
FIG. 2.
Incidence of patients with Fusarium infections per 1,000 admissions. HUH, Hadassah University Hospital; SMC, Sheba Medical Center.
DISCUSSION
Fusarium spp. have become one of the important causes of mold infections in humans, especially in immunocompromised hosts, in whom it is second only to Aspergillus spp. (3, 8, 10, 13, 16, 20, 23, 25-27, 30, 33). Reports of fusarial infection in immunocompetent patients are sparse. These include mostly infection of the eyes, skin and nails, peritoneum, or lungs (1, 2, 5, 11, 12, 18, 21, 26-29, 31, 32, 34-37). Contrary to other studies, the majority of patients (76%) in our series were immunocompetent. These patients tended to be older than immunocompromised patients and consequently had a greater prevalence of underlying diseases such as ischemic heart disease. Diabetes mellitus, peripheral vascular disease, and chronic renal failure were also more frequent but not significantly so. Major sites of infection in the immunocompetent patients included the eyes and the lower limbs, where the common presentation was skin ulceration. Although most infections were superficial, locally invasive disease was observed in 38% (34 patients), and in 8% (seven patients) disseminated infection was documented, including one patient with bloodstream infection, two with burns, and one with multiple abscesses of both lower limbs. Only two of the latter group survived (one with burns and one with multiple leg abscesses), emphasizing the severity of this infection, when disseminated, among immunocompetent patients.
Most of our patients were not treated for fungal infection. We were unable to judge which patients for whom this was appropriate from the data available. The administration of treatment may merely reflect the presence of clinically more aggressive disease. Clinicians generally consult with the laboratory or infectious diseases service when an unusual finding is reported. In addition to possible delays emanating from late reporting due to the time required for growth and identification, isolation of molds from superficial specimens is poorly understood by clinicians. On the other hand, when the patient has a special problem, e.g., neutropenia or burns, contact with the infectious diseases service or laboratory is often initiated before isolation of any organism. In such cases, communication clearly works, and the significance of the fungal isolate is understood.
We found several risk factors for higher in-hospital mortality among patients with fusarial infections; chronic renal failure, hematological malignancy, and burns were associated with increased odds (by multiple logistic regression analysis) for death during hospitalization (odds ratio, ≥15.0), as was disseminated infection (odds ratio, 8.7). Fleming et al. (8) and Boutati and Anaissie (3) have also commented on dissemination being associated with higher mortality.
It has been suggested previously (3, 13, 26) that F. solani is more frequently associated with disseminated disease than other Fusarium species. We noted a similar tendency, which may reflect the greater pathogenic potential in a murine model (24). However, since the species of most of our isolates were not determined, no firm conclusion can be drawn.
Fusarial infection has a tendency for seasonal variation. The infection is most prevalent in autumn in France (16) and in summer in Texas and Italy (3, 10, 30). In Israel, the highest incidence of Fusarium species isolation was in the summer, particularly among patients from rural areas, and was associated with ocular and respiratory tract infections (6, 30). This may reflect sporulation of Fusarium spp. during this season. Interestingly, in Israeli agricultural practice, Fusarium spp. are usually considered pathogens of various field and vegetable crops during the summer (17). However, there are two agriculturally significant F. oxysporum forms that are considered winter pathogens (9, 17). Moreover, in Israel, fusarial infections are unrelated to rain and wind, in contrast to previous reports from other countries (3, 10, 30), since Israeli summers are rainless and hot (around 30°C). It is conceivable that the humidity, which is high in the coastal plain in the summer (55 to 70%) and low in the mountains (40 to 55%) (4), may partly explain the differences between these regions, as presented by more cases per 1,000 admissions in Sheba Medical Center, which is located in the coastal plain, than in the Hadassah University Hospital, located in the mountains of Jerusalem.
In our series, as in others (14, 27), the skin of the lower limbs was the main anatomical site involved (48 of 89 patients, including the eight patients with burns). Other major organs with relatively frequent involvement included the eye (n = 11), respiratory tract (n = 9), and gastrointestinal tract and genitourinary tract (n = 9). Since our series is based on hospitalized patients, it is likely that the true incidence of superficial infections of the limbs or the eyes is much higher than estimated here.
The frequency of isolation of Fusarium species varies between different countries (3, 10, 16). Such variation was also observed between two different geographical regions within Israel. The major differences between the hospitals that we studied are their location and elevation: Sheba Medical Center is situated in the coastal plain, and Hadassah University Hospital is located in the mountains of Jerusalem. While the frequency of isolation at Sheba Medical Center during the 10 years of the study remained constant, there was a steady increase in the number of isolations at the Hadassah University Hospital (Fig. 2). Indeed, after a single case of disseminated fusarial infection was diagnosed histologically in 1987 at Hadassah University Hospital (7), there were no further isolations until 1992. Even at Sheba Medical Center, there may have been some increase in the number of isolations. In an earlier report from Sheba Medical Center (11), the number of isolations from 1982 to 1991 was 40, compared to 71 in 1992 to 2001 (our study), but this trend is not statistically significant. Therefore, we consider that Fusarium species are emerging pathogens in the mountainous area of Israel and possibly to a lesser extent in the coastal plain as well.
REFERENCES
- 1.Becker, W. K., W. G. Cioffi, Jr., A. T. McManus, S. H. Kim, W. F. McManus, A. D. Mason, and B. A. Pruitt, Jr. 1991. Fungal burn wound infection. A 10-year experience. Arch. Surg. 126:44-48. [DOI] [PubMed] [Google Scholar]
- 2.Boonpasart, S., N. Kasetsuwan, V. Puangsricharern, L. Pariyakanok, and T. Jittpoonkusol. 2002. Infectious keratitis at King Chulalongkorn Memorial Hospital: a 12-year retrospective study of 391 cases. J. Med. Assoc. Thai. 85(Suppl. 1):S217-S230. [PubMed] [Google Scholar]
- 3.Boutati, E. I., and E. J. Anaissie. 1997. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer center and implications for management. Blood 90:999-1008. [PubMed] [Google Scholar]
- 4.Central Bureau of Statistics. 2002. Statistical abstracts of Israel 2002, no. 53. Central Bureau of Statistics, Tel Aviv, Israel.
- 5.Chiaradia, V., D. Schinella, L. Pascoli, F. Tesio, and G. F. Santini. 1990. Fusarium peritonitis in peritoneal dialysis: report of two cases. Microbiologica 13:77-78. [PubMed] [Google Scholar]
- 6.de Matos, A. P., N. F. Sanches, and J. L. D. Costa. 1997. Patterns of diurnal and seasonal airborne spore concentrations of Fusarium subglutinans in a pineapple orchard in Brazil. Acta Hortic. 425:515-524.
- 7.Engelhard, D., A. Eldor, I. Polacheck, I. Hardan, D. Ben-Yehuda, S. Amselem, I. F. Salkin, G. Lopez-Berestein, T. Sacks, E. A. Rachmilewitz, et al. 1993. Disseminated visceral fusariosis treated with amphotericin B-phospholipid complex. Leukemia Lymphoma 9:385-392. [DOI] [PubMed] [Google Scholar]
- 8.Fleming, R. V., T. J. Walsh, and E. J. Anaissie. 2002. Emerging and less common fungal pathogens. Infect. Dis. Clin. N. Am. 16:915-933, vi-vii. [DOI] [PubMed] [Google Scholar]
- 9.Gamliel, A., T. Katan, H. Yunis, and J. Katan. 1996. Fusarium wilt of crown rot of sweet basil: involvement of soilborne and airborne inoculum. Phytopathology 86:56-62. [Google Scholar]
- 10.Girmenia, C., L. Pagano, L. Corvatta, L. Mele, A. del Favero, and P. Martino. 2000. The epidemiology of fusariosis in patients with haematological diseases. Gimema Infection Programme. Br. J. Haematol. 111:272-276. [DOI] [PubMed] [Google Scholar]
- 11.Goldschmied-Reouven, A., J. Friedman, and C. S. Block. 1993. Fusarium species isolated from non-ocular sites: a 10 year experience at an Israeli general hospital. J. Mycol. Med. 3:99-102. [Google Scholar]
- 12.Gopinathan, U., P. Garg, M. Fernandes, S. Sharma, S. Athmanathan, and G. N. Rao. 2002. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea 21:555-559. [DOI] [PubMed] [Google Scholar]
- 13.Guarro, J., and J. Gene. 1995. Opportunistic fusarial infections in humans. Eur. J. Clin. Microbiol. Infect. Dis. 14:741-754. [DOI] [PubMed] [Google Scholar]
- 14.Gupta, A. K., R. Baran, and R. C. Summerbell. 2000. Fusarium infections of the skin. Curr. Opin. Infect. Dis. 13:121-128. [DOI] [PubMed] [Google Scholar]
- 15.Harris, J. L. 2000. Safe, low-distortion tape touch method for fungal slide mounts. J. Clin. Microbiol. 38:4683-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennequin, C., V. Lavarde, J. L. Poirot, M. Rabodonirina, A. Datry, S. Aractingi, J. Dupouy-Camet, D. Caillot, F. Grange, L. Kures, O. Morin, B. Lebeau, S. Bretagne, C. Guigen, D. Basset, and R. Grillot. 1997. Invasive Fusarium infections: a retrospective survey of 31 cases. The French ‘Groupe d'Etudes des Mycoses Opportunistes' GEMO. J. Med. Vet. Mycol. 35:107-114. [PubMed] [Google Scholar]
- 17.Katan, T., E. Shelvin, and J. Katan. 1997. Sporulation of Fusarium oxisporum f. sp. lycopersici on stem surfaces of tomato plants and aerial dissemination of inoculum. Phytopathology 87:712-719. [DOI] [PubMed] [Google Scholar]
- 18.Kerr, C. M., J. R. Perfect, P. C. Craven, J. H. Jorgensen, D. J. Drutz, J. D. Shelburne, H. A. Gallis, and R. A. Gutman. 1983. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis. Ann. Intern. Med. 99:334-336. [DOI] [PubMed] [Google Scholar]
- 19.Krcmery, V., Jr., Z. Jesenska, S. Spanik, J. Gyarfas, J. Nogova, R. Botek, J. Mardiak, J. Sufliarsky, J. Sisolakova, M. Vanickova, A. Kunova, M. Studena, and J. Trupl. 1997. Fungaemia due to Fusarium spp. in cancer patients. J. Hosp. Infect. 36:223-228. [DOI] [PubMed] [Google Scholar]
- 20.Krcmery, V., Jr., E. Kunova, Z. Jesenska, J. Trupl, S. Spanik, J. Mardiak, M. Studena, and E. Kukuckova. 1996. Invasive mold infections in cancer patients: 5 years' experience with Aspergillus, Mucor, Fusarium and Acremonium infections. Supp. Care Cancer 4:39-45. [DOI] [PubMed] [Google Scholar]
- 21.Leck, A. K., P. A. Thomas, M. Hagan, J. Kaliamurthy, E. Ackuaku, M. John, M. J. Newman, F. S. Codjoe, J. A. Opintan, C. M. Kalavathy, V. Essuman, C. A. Jesudasan, and G. J. Johnson. 2002. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br. J. Ophthalmol. 86:1211-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marr, K. A., and R. A. Bowden. 1999. Fungal infections in patients undergoing blood and marrow transplantation. Transplant. Infect. Dis. 1:237-246. [DOI] [PubMed] [Google Scholar]
- 23.Marr, K. A., R. A. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 24.Mayayo, E., I. Pujol, and J. Guarro. 1999. Experimental pathogenicity of four opportunist Fusarium species in a murine model. J. Med. Microbiol. 48:363-366. [DOI] [PubMed] [Google Scholar]
- 25.Musa, M. O., A. Al Eisa, M. Halim, E. Sahovic, M. Gyger, N. Chaudhri, F. Al Mohareb, P. Seth, M. Aslam, and M. Aljurf. 2000. The spectrum of Fusarium infection in immunocompromised patients with haematological malignancies and in non-immunocompromised patients: a single institution experience over 10 years. Br. J. Haematol. 108:544-548. [DOI] [PubMed] [Google Scholar]
- 26.Nelson, P. E., M. C. Dignani, and E. J. Anaissie. 1994. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 7:479-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nucci, M., and E. Anaissie. 2002. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin. Infect. Dis. 35:909-920. [DOI] [PubMed] [Google Scholar]
- 28.O'Day, D. M., W. S. Head, P. Akrabawi, and J. Ives. 1979. Fusarium oxy-sporum endophthalmitis. Arch. Ophthalmol. 97:1545. [DOI] [PubMed] [Google Scholar]
- 29.Pushker, N., M. Chra, M. S. Bajaj, S. Ghose, N. Naik, S. Kashyap, and G. Satpathy. 2002. Necrotizing periorbital Fusarium infection-an emerging pathogen in immunocompetent individuals. J. Infect. 44:236-239. [DOI] [PubMed] [Google Scholar]
- 30.Raad, I., J. Tarrand, H. Hanna, M. Albitar, E. Janssen, M. Boktour, G. Bodey, M. Mardani, R. Hachem, D. Kontoyiannis, E. Whimbey, and K. Rolston. 2002. Epidemiology, molecular mycology, and environmental sources of Fusarium infection in patients with cancer. Infect. Control Hosp. Epidemiol. 23:532-537. [DOI] [PubMed] [Google Scholar]
- 31.Rippon, J. W., R. A. Larson, D. M. Rosenthal, and J. Clayman. 1988. Disseminated cutaneous and peritoneal hyalohyphomycosis caused by Fusarium species: three cases and review of the literature. Mycopathologia 101:105-111. [DOI] [PubMed] [Google Scholar]
- 32.Rowsey, J. J., T. E. Acers, D. L. Smith, J. A. Mohr, D. L. Newsom, and J. Rodriguez. 1979. Fusarium oxysporum endophthalmitis. Arch. Ophthalmol. 97:103-105. [DOI] [PubMed] [Google Scholar]
- 33.Sampathkumar, P., and C. V. Paya. 2001. Fusarium infection after solid-organ transplantation. Clin. Infect. Dis. 32:1237-1240. [DOI] [PubMed] [Google Scholar]
- 34.Verma, S., and S. J. Tuft. 2002. Fusarium solani keratitis following LASIK for myopia. Br. J. Ophthalmol. 86:1190-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vismer, H. F., W. F. Marasas, J. P. Rheeder, and J. J. Joubert. 2002. Fusarium dimerum as a cause of human eye infections. Med. Mycol. 40:399-406. [DOI] [PubMed] [Google Scholar]
- 36.Wheeler, M. S., M. R. McGinnis, W. A. Schell, and D. H. Walker. 1981. Fusarium infection in burned patients. Am. J. Clin. Pathol. 75:304-311. [DOI] [PubMed] [Google Scholar]
- 37.Young, J. B., I. H. Ahmed-Jushuf, A. M. Brownjohn, F. M. Parsons, S. J. Foulkes, and E. G. Evans. 1984. Opportunistic peritonitis in continuous ambulatory peritoneal dialysis. Clin. Nephrol. 22:268-269. [PubMed] [Google Scholar]