A 53-year-old woman presented to the hospital with near syncope, reporting that she crawled on the floor for the past 3 days to prevent passing out. An ECG performed in the emergency department showed complete heart block. A temporary transvenous pacemaker was emergently positioned. A transthoracic echocardiogram was suboptimal, but demonstrated a mass in the left atrium. Transesophageal echocardiography revealed that the large mass (3.2×2.7 cm) was much more extensive, with infiltration of both the interatrial and interventricular septae, both atria, the left ventricular outflow tract, and the ventricular side of the mitral valve anterior leaflet (Figure 1A through 1D, Movie IA through ID in the online-only Data Supplement). Differential diagnosis included a primary cardiac tumor, metastatic disease, sarcoidosis or other granulomatous process, or an infectious process. A computed tomography scan of the chest, abdomen, and pelvis demonstrated the intracardiac mass and no other abnormalities (Figure 2). A permanent pacemaker was placed. The patient declined surgical management of the intracardiac mass.
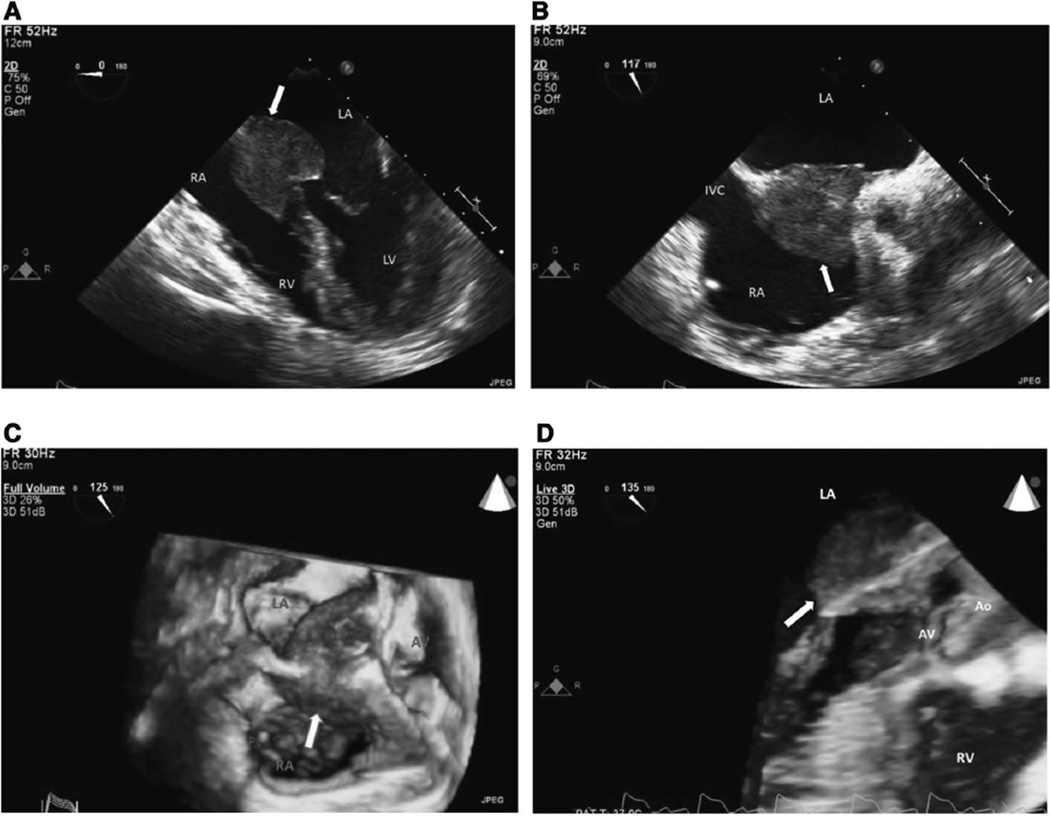
Figure 1.
A, 2D TEE at midesophageal 4-chamber view at 0° shows a large cardiac mass (arrow) at interatrial septum and A-V node area that extends into both atria at end diastole. B, 2D TEE at lower midesophageal view at 117° shows large cardiac mass (arrow) at mid and basal interatrial septal area. C, 3D TEE at upper-esophageal short-axis view at 125° shows large mass (arrow) involving mid and basal interatrial septum and extending into midatrioventricular area. D, 3D TEE at upper-esophageal view at 135° shows large intracardiac mass (arrow) extending into left atrium, atrioventricular conjunction, and left ventricular outflow tract. Ao indicates aorta; AV, aortic valve; A-V, atrioventricular; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; MV, mitral valve; PL, pacemaker lead; RA, right atrium; RV, right ventricle; 2D, 2-dimensional; 3D, 3-dimensional; and TEE, transesophageal echocardiography.
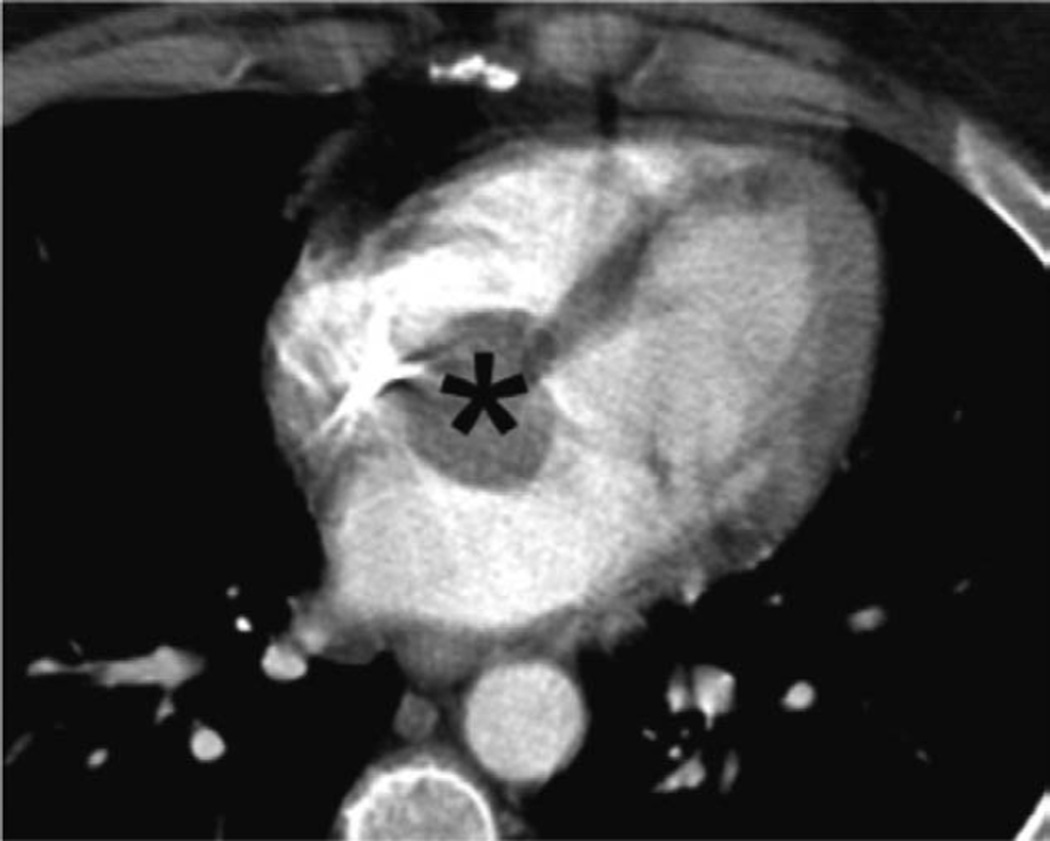
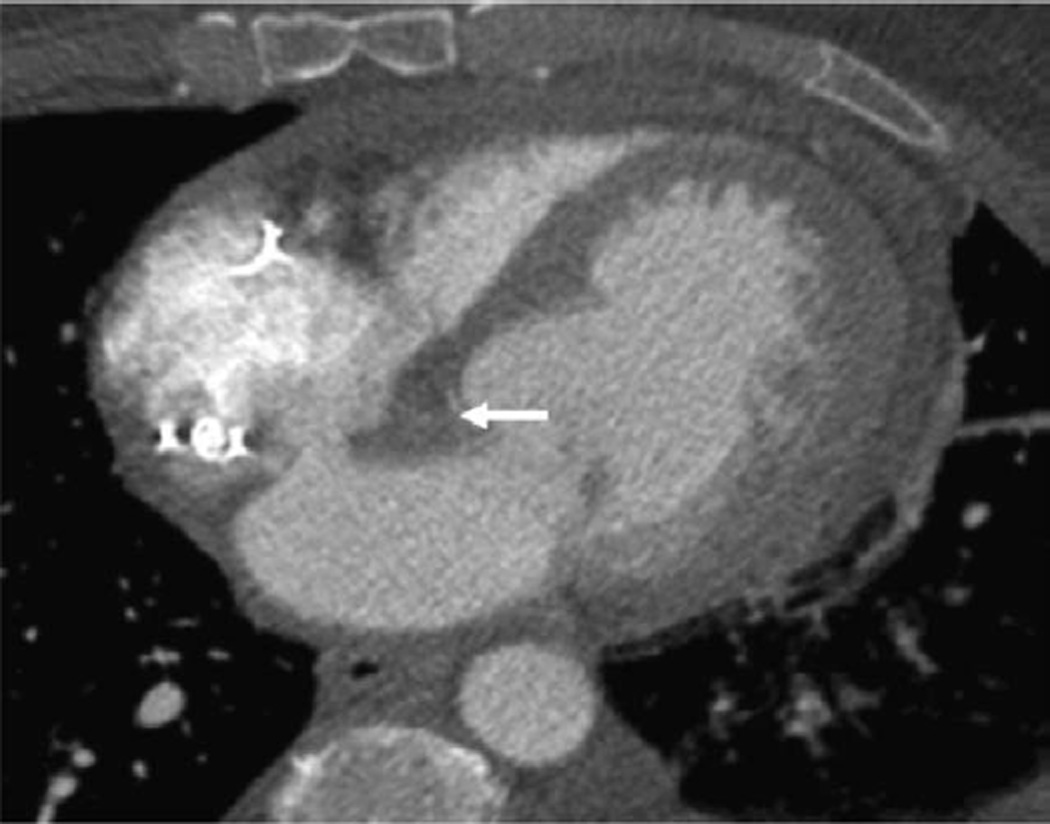
Figure 2.
Chest CT scan, axial long-axis image. Initial CT scan shows large cardiac mass (asterisk) at interatrial septum and A-V node area that extends to both atria. A-V indicates atrioventricular; and CT, computed tomography.
The cardiac biopsy was done percutaneously by using intracardiac echocardiographic guidance. The biopsy of the mass excluded neoplasm and suggested either an infectious or a chronic inflammatory process. Histological evaluation of the biopsy material showed: mixed lymphoplasmacytic and neutrophilic inflammation; and poorly formed granulomas with negative special stains for bacteria, fungus, mycobacteria, and spirochetes (Figure 3). Blood cultures grew no organisms. The white blood cell count was 17.600 /µL with a normal differential, C-reactive protein was 200 mg/L, and sedimentation rate was 115 mm/h. Autoimmune serologies were notable for positive anti-myeloperoxidase antibodies at 7.5 and negative antinuclear antibodies, classic antineutrophilic cytoplasmic antibodies, and anti-proteinase 3 antibodies. Subsequent classic antineutrophilic cytoplasmic antibodies was positive at 1:64, and anti-myeloperoxidase antibodies were 7.5. At that time, in the final diagnostic comments, granulomatosis with polyangiitis (GPA, formerly known as Wegener granulomatosis) was suggested, and the patient was treated with rapidly tapering doses of prednisone.
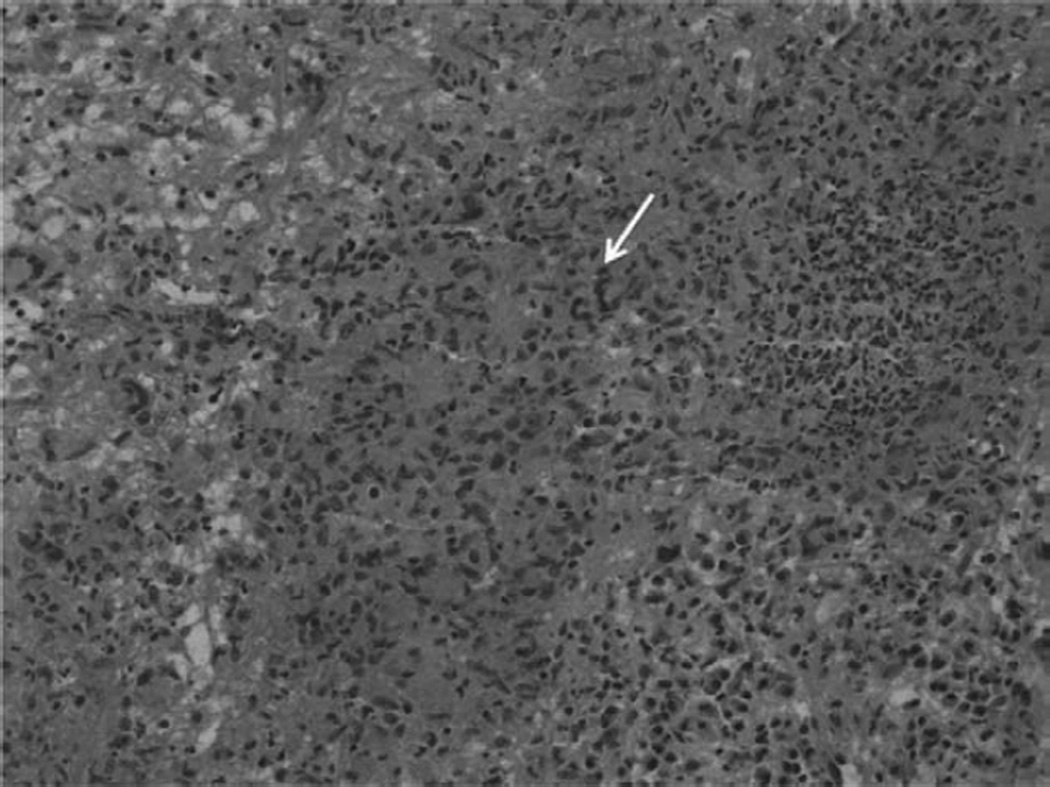
Figure 3.
Right atrial biopsy showing mixed lymphoplasmacytic and neutrophilic inflammation with poorly formed granulomas and associated necrosis and microabscesses. Special stains for bacteria, fungus, mycobacteria, and spirochetes were negative. Arrow points to a multinucleated giant cell.
Eight months later, the patient developed acute respiratory distress and kidney failure. After successful resuscitation, the chest computed tomography revealed lung nodules and bilateral pleural effusions. A renal biopsy showed crescentic and necrotizing glomerulonephritis. Involving all sampled glomeruli and IgG, linear staining of the glomerular capillary walls was suggestive of an antiglomerular basement membrane and antineutrophilic cytoplasmic antibody–related glomerulonephritis. The patient was treated with pulse steroid, cytoxan, and plasmapheresis and remained dialysis-dependent. An interim 2-dimensional–3-dimensional transthoracic echocardiogram showed decrease in the size of the intracardiac mass. A 2-dimensional–3-dimensional transesophageal echocardiography performed 2 years after the initial transesophageal echocardiography showed a significant decrease in mass size to 1.5 × 1.6 cm (Figure 4A through 4D, Movie IIA through IID in the online-only Data Supplement). A chest computed tomography also confirmed that finding (Figure 5). Inflammatory serology measurements also improved with erythrocyte sedimentation rate, C-reactive protein, and white blood cell count having nadirs 44, 23, and 6.19, respectively. The antimyeloperoxidase nadir was 3.1.
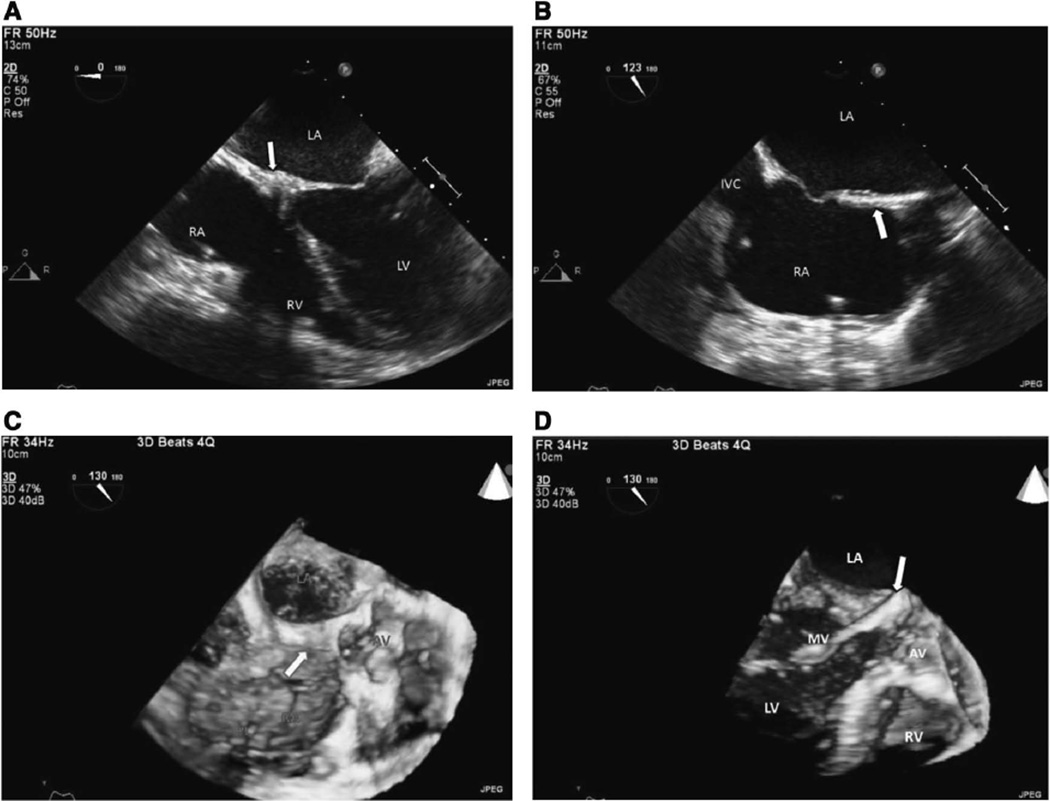
Figure 4.
A, 2D TEE midesophageal 4-chamber view at 0° shows regression of cardiac mass (arrow) at the base of the interatrial septum and A-V node area at end diastole. B, 2D TEE at lower midesophageal view at 123° shows that residual small mass (arrow) has almost completely disappeared at mid and basal interatrial septal area. C, 3D TEE at upper-esophageal short-axis view at 130° shows residual small mass (arrow) involving mid and basal interatrial septum and extending midatrioventricular area. D, 3D TEE at lower-esophageal view at 130° shows that residual small mass (arrow) has almost completely disappeared. Ao indicates aorta; AV, aortic valve; A-V, atrioventricular; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; MV, mitral valve; PL, pacemaker lead; RA, right atrium; RV, right ventricle; 2D, 2-dimensional; 3D, 3-dimensional; and TEE, transesophageal echocardiography.
Figure 5.
Chest CT scan, axial long-axis image. After 16 months from the initial CT scan, the mass (arrow) has markedly regressed. CT indicates computed tomography.
The patient’s clinical picture deteriorated despite stable cardiac function and optimal medical management over 4 years of follow-up. Her terminal hospitalization was for septic shock; emergent laparotomy identified ischemic necrosis of the distal ileum and the right and transverse colon. Autopsy did not identify any active vasculitis in the mesenteric vessels. Cardiac autopsy showed transmural fibrosis and chronic inflammation of interatrial septum, postinflammatory changes of the mitral and aortic valves, pericardial adhesion, and chronic pericarditis.
Granulomatosis with polyangiitis (GPA) affects the heart at the time of diagnosis in 8% to 16% of cases, and some time during the disease course in 4% to 25%.1 The most frequent cardiac complications of GPA are pericarditis, coronary vasculitis, myocarditis, and valvulitis.1 Cardiac conduction system abnormalities and intracardiac mass are extraordinarily rare complications of GPA. We present the first case described in the literature with GPA associated with the combination of complete heart block and a large intracardiac mass. The mass was located in very close proximity to the atrioventricular node (A-V) node, if not directly infiltrating the A-V node; that relationship was clearly demonstrated by 3D transesophageal echocardiography and cardiac computed tomography.
Intracardiac masses of unknown cause or with complications are usually surgically removed for definitive diagnosis and treatment.2 We identified only 1 other report in the literature in which regression of an intracardiac mass occurred after immunosuppressive therapy of GPA.3 This suggests that inflammatory granulomatous cardiac masses without complication should be considered for immunosuppressive therapy before or instead of surgical resection.
Complete A-V block in GPA is a rare complication and is usually associated with early active systemic disease. The A-V block may resolve after the induction therapy of GPA.4 A patient with granulomatous changes or a mass involving the A-V conduction systems will need a permanent pacemaker. In this case, despite regression of the intracardiac mass, the complete A-V block did not resolve and the patient was pacemaker dependent. GPA with multiorgan involvement, particularly cardiac involvement, is associated with poor clinical prognosis despite optimal medical management.1
Supplementary Material
Footnotes
The patient gave verbal and written consent for this presentation.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.115.016851/-/DC1.
Disclosures
None
References
- 1.Oliveira GH, Seward JB, Tsang TS, Specks U. Echocardiographic findings in patients with Wegener granulomatosis. Mayo Clin Proc. 2005;80:1435–1440. doi: 10.4065/80.11.1435. [DOI] [PubMed] [Google Scholar]
- 2.Herbst A, Padilla MT, Prasad AR, Morales MC, Copeland JG. Cardiac Wegener’s granulomatosis masquerading as left atrial myxoma. Ann Thorac Surg. 2003;75:1321–1323. doi: 10.1016/s0003-4975(02)04662-3. [DOI] [PubMed] [Google Scholar]
- 3.Mortazavi M, Nasri H. Granulomatosis with polyangiitis (Wegener’s) presenting as the right ventricular masses: a case report and review of the literature. J Nephropathol. 2012;1:49–56. doi: 10.5812/jnp.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin CH, Tsai SH, Chen HC, Chen SJ. Heart blockage in a patient with cavitary lung lesions. Am J Emerg Med. 2012;30:1663.e1–1663.e3. doi: 10.1016/j.ajem.2011.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.