Abstract
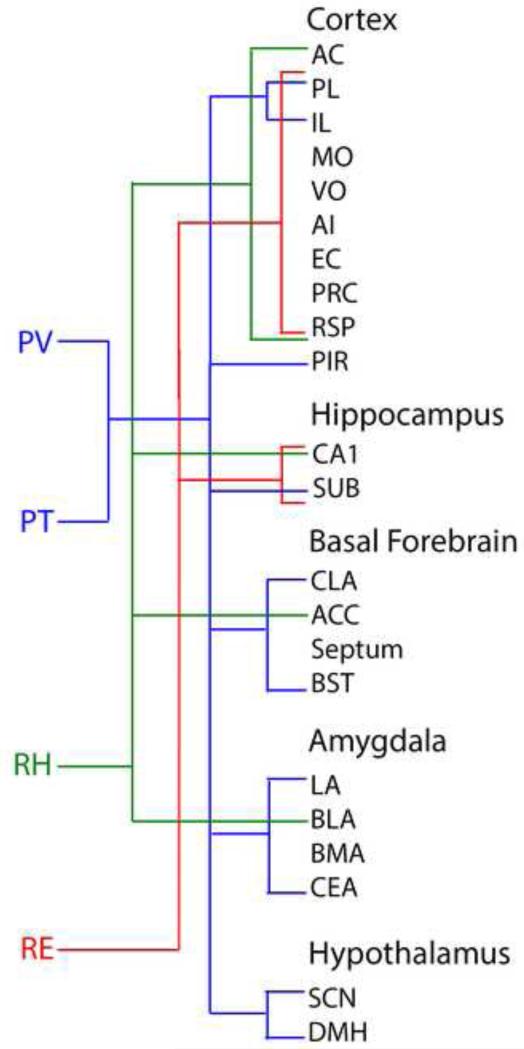
The thalamus was subdivided into three major groups: sensorimotor nuclei (or principal/relay nuclei), limbic nuclei and nuclei bridging these two domains. Limbic nuclei of thalamus (or ‘limbic thalamus’) consist of the anterior nuclei, midline nuclei, medial division of the mediodorsal nucleus (MDm) and central medial nucleus (CM) of the intralaminar complex. The midline nuclei include the paraventricular (PV) and paratenial (PT) nuclei, dorsally, and the reuniens (RE) and rhomboid (RH) nuclei, ventrally. The ‘limbic’ thalamic nuclei predominantly connect with limbic-related structures and serve a direct role in limbic–associated functions. Regarding the midline nuclei, RE/RH mainly target limbic cortical structures, particularly the hippocampus and the medial prefrontal cortex. Accordingly, RE/RH participate in functions involving interactions of the HF and mPFC. By contrast, PV/PT mainly project to limbic subcortical structures, particularly the amygdala and nucleus accumbens, and hence are critically involved in affective behaviors such as stress/anxiety, feeding behavior, and drug seeking activities. The anatomical/functional characteristics of MDm and CM are very similar to those of the midline nuclei and hence the collection of nuclei extending dorsoventrally along the midline/paramidline of the thalamus constitute the core of the ‘limbic thalamus’.
Keywords: nucleus reuniens, rhomboid nucleus, paratenial nucleus, paraventricular nucleus, mediodorsal nucleus, central medial nucleus, learning and memory, cognition, affect, stress/anxiety, feeding behavior, drug seeking activity
1. Introduction
Until recently, the role of the thalamus in complex, limbic-associated, functions has received relatively little attention. This stems from the rather entrenched view that the thalamus mainly serves to transfer sensorimotor information to the cortex – and the corollary that once information reaches the cortex the function of the thalamus is essentially complete. The cortex then uses this information for important processes that are almost entirely controlled by the cortex. In effect, a corticocentric view of brain function.
Despite the fact that corticothalamic projections significantly outnumber thalamocortical projections, the thalamus is generally seen as a gateway to the cortex, and at best, return corticothalamic projections are thought to modulate signals conveyed from the thalamus to the cortex. As will be described herein, this view of the thalamus is gradually changing with the demonstration that the thalamus does not merely route information to the cortex, but rather integrates signals reaching it from various sources. Depending on the nature of the information, the thalamus directs it to select subsets of cortical and subcortical structures for appropriate actions. Accordingly, discrete thalamic lesions alter a host of physiological, affective and cognitive functions (see below).
Classically, the thalamus has been described as consisting of specific and non-specific nuclei, with the specific nuclei mainly responsible for relaying modality-specific information to the cortex and the ‘non-specific’ nuclei exerting ‘modulatory’ effects on cortical targets. While this scheme has been revised over the years, it is tempting to speculate that it essentially evolved from an incomplete knowledge of the thalamus and its functions. While it was recognized early on that the thalamus was critical for the transfer of sensorimotor information to the cortex, comparatively little attention was paid to relatively vast ‘non-relay’ regions of the thalamus, which were characterized as ‘non- specific’. This would include the anterior nuclei (anterodorsal, anteroventral and anteromedial), lateral nuclei (lateral dorsal and lateral posterior), mediodorsal nucleus, submedial nucleus, intralaminar nuclei (central lateral, paracentral, central medial and parafascicular) and the midline nuclei (paratenial, paraventricular, rhomboid and reuniens). Although some of these cell groups were designated as ‘non-specific’ based on their (presumed) widespread projections to, and global effects on, the cortex, the designation of ‘non-specific’ probably more resulted from the fact that no specific function(s) could be attributed to them.
Presently, we will (1) describe an alternative classification for nuclei of the thalamus; (2) define the ‘limbic thalamus’ and its main anatomical/functional features; and (3) discuss, in depth, the circuitry and functions of the midline thalamus -- as an integral part of the limbic thalamus. The latter will not only include the midline nuclei, per se, but thalamic groups lying on or near the midline that share properties with the midline nuclei, primarily, the central medial nucleus and the medial part of the mediodorsal nucleus. The report generally deals with the rodent thalamus.
2. An alternative classification for nuclei of the thalamus
Aside from the general distinction between specific/non-specific nuclei of thalamus, traditionally, the thalamus has been divided into three anatomical/functional groups: the principal (or relay) nuclei, the association nuclei, and the midline and intralaminar nuclei (Price, 1995; Groenewegen and Witter, 2004). The principal ‘or relay’ nuclei receive specific sensory or motor information via ascending pathways and transmit it to discrete layers and regions of the cortex. The relay nuclei would include: the lateral geniculate complex (LGN), medial geniculate nucleus (MGN), ventral posteromedial (VPM) and posterolateral (VPL) nuclei, posterior nucleus (PO), ventral lateral nucleus (VL), ventral anterior nucleus (VA) and ventral medial nucleus (VM).
The “association” nuclei are a largely ill-defined group that differ from the principal nuclei in that they do not receive direct sensory (e.g., from the retina) or motor information and essentially do not project to primary sensorimotor cortices. The association nuclei receive major input from layer 5 pyramidal cells of the sensorimotor cortex and relay this information to associational areas of cortex – hence association nuclei of thalamus. The association thalamic nuclei include the mediodorsal nucleus (MD), the anterior nuclei, the submedial nucleus (SMT), and the lateral nuclei (Groenewegen and Witter, 2004).
The midline and intralaminar thalamic nuclei form a separate group primarily based on: (1) their distinct location along the midline and within the internal medullary lamina; (2) their rather prominent distribution to both subcortical and cortical structures; and (3) their involvement in processes of arousal and attention. The intralaminar nuclei consist of the CM, paracentral (PC), central lateral (CL), parafascicular (PF) and subparafascicular (SPF) nuclei, while the midline nuclei include the paratenial (PT), paraventricular (PV), rhomboid (RH) and reuniens (RE) nuclei – and in some classifications the intermediodorsal (IMD) nucleus (Groenewegen and Witter, 2004). The reticular nucleus of thalamus (RT) comprises a separate group in that it serves as an interface between thalamic nuclei and the cortex and lacks cortical projections.
A proposed alternative classification would group thalamic nuclei along a continuum from sensorimotor to limbic nuclei (Vertes et al., 2014). Similar to the classic system, this classification would consist of three sets of thalamic nuclei: sensorimotor nuclei of thalamus, ‘limbic’ nuclei of thalamus and thalamic nuclei bridging these two domains. While the connections and functions of these three groups would partly overlap (as with the classical divisions), the sensorimotor group would mainly consist of the principal thalamic nuclei; the ‘limbic group’ would include the anterior nuclei, MDm, CM and the midline nuclei (PT, PV, RH and RE), and the sensorimotor/limbic ‘bridging nuclei’ might include the submedial nucleus, PC, CL, and PF of the intralaminar complex, central and lateral parts of MD, and the laterodorsal (LD) and lateral posterior (LP) nuclei of the lateral thalamus. This organization will be further discussed below with an emphasis on the ‘limbic thalamus’.
3. The limbic thalamus
3.1 General considerations
As well documented, the collection of structures currently referred to as the ‘limbic system’ developed from the early description of Paul Broca of archi/paleo-cortical structures lying between diencephalon and the neocortex which he designated the limbic lobe. It mainly consisted of the cingulate gyrus, the parahippocampal gyrus and the hippocampal formation (HF). Even initially, the limbic lobe was thought to play a prominent role in affective behavior. The limbic lobe has steadily expanded to include several additional structures including the amygdala, septum, bed nucleus of the stria terminalis (BST), nucleus accumbens (ACC) and adjacent regions of the basal forebrain, the hypothalamus and parts of the brainstem, as well as the ‘limbic cortex’ and the ‘limbic thalamus’. As a whole, these structures constitute the generally accepted view of the “limbic system”. Although many of these (limbic) structures are interconnected by major fiber bundles (e.g., fornix, stria medullaris, stria terminalis, mammillothalamic tract), the feature which probably most unites them (into a system) is their presumed shared functional role(s) -- in affective and cognitive behaviors. If, as suggested, the limbic system is more a functional than anatomical entity, there are understandably differing views of the structures comprising the system, and importantly as we learn more about the brain, the limbic system will undoubtedly continue to expand. This might be particularly true for the limbic thalamus.
In the present re-classification of nuclei of the thalamus, nuclei were characterized as: (1) sensorimotor, (2) limbic or (3) a combination of both, thus bridging these two domains. As indicated, this classification represents a continuum (from sensorimotor to limbic): essentially what distinguishes sensorimotor from limbic regions of thalamus is relative degree of involvement in sensorimotor or limbic functions. Specifically, while it is undeniably the case that sensory and motor regions of the thalamus serve to relay signals associated sensations/movements to the cortex, this is by no means their sole role. Often overlooked is the fact that the principal nuclei of thalamus have significant ties to (strictly) non-sensorimotor regions of the brain and participate in a range of functions – only a subset of which are sensorimotor.
For example, the LGN of rats consists of three divisions, the dorsal (DLG) and ventral (VLG) lateral geniculate nuclei and the intergeniculate leaflet (IGL). Whereas the DLG relays visual information from the retina to the visual cortex, the DLG also receives ‘modulatory’ inputs from several extra-retinal sites including the visual cortex, RT, superior colliculus (SC), pretectal nuclei and brainstem cholinergic and monoaminergic nuclei (Groenewegen and Witter, 2004; Vertes et al., 2014). Along the same lines, but to a much greater degree, the VLG and IGL are extensively interconnected with non-visual structures, suggesting a role(s) well beyond an interface between the retina and visual cortex.
The VLG is divided into a medial parvocellular and a lateral magnocellular part with differing inputs and outputs. The magnocellular VLG receives retinal input but the parvocellular division does not. Instead the parvocellular VLG receives a diverse array of afferents from visual structures (e.g., superior colliculus) as well as from several non-visual sites, mainly of the brainstem, including the periaqueductal gray (PAG), reticular formation (RF), parabrachial nucleus (PB), raphe nuclei and the locus coeruleus (LC) (Kolmac and Mitrofanis, 1997; Vertes et al. 2010). Unlike the DLG, the magnocellular and parvocellular divisions of VLG do not project to the visual cortex but rather distribute to other nuclei of the thalamus, to the hypothalamus, and to several brainstem structures (Vertes et al. 2014). In a comparable manner, the IGL receives retinal and non-retinal inputs, lacks visual cortical projections, and distributes widely throughout the diencephalon and brainstem and prominently to the suprachiasmatic nucleus (SCN).
In a sense, then, it may be a misnomer, even to characterize the lateral geniculate complex as a ‘relay’ nucleus for only the DLG carries visual information from the retina to the visual cortex. Seemingly, the VGL and IGL, which lack projections to the visual cortex, are not directly involved in the processing of visual information (visual discrimination), but rather mediate the effects of visual stimulation on a host of visual (e.g., visuomotor) and non-visual (e.g., circadian rhythms) processes. However, despite these caveats, it is certainly the case that the LGN is indispensable for the transfer of retinal signals to the visual cortex -- and hence is a thalamic ‘relay’ nucleus. In an analogous manner, but at the opposite end of the spectrum, a subset of thalamic nuclei predominantly serve ‘limbic-related’ functions and hence constitute the ‘limbic thalamus’.
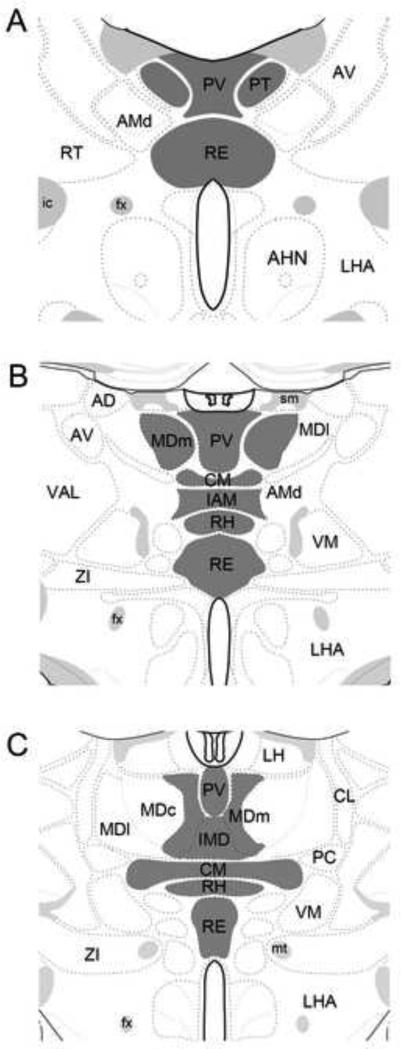
In the following, then, we discuss the anatomical and functional properties of midline nuclei of the thalamus as well as associated nuclei lying medially in the thalamus, as core components of the limbic thalamus (Fig. 1)
Figure 1.
Schematic representation of three rostrocaudally (A-C) aligned sections through the diencephalon depicting (in dark gray) the locations of dorsoventrally oriented nuclei along the midline/paramidline of the thalamus, which together with the anterior nuclei of thalamus, constitute the ‘limbic thalamus’. They include the paraventricular (PV), paratenial (PT), reuniens (RE) and rhomboid (RH) nuclei of the midline thalamus, the medial division of the mediodorsal nucleus (MDm), the central medial nucleus (CM) of the rostral intralaminar complex, the interanteromedial nucleus (IAM) and the intermediodorsal (IMD) nucleus. Sections modified from Swanson (2004). See list for abbreviations.
3.2 Nucleus reuniens
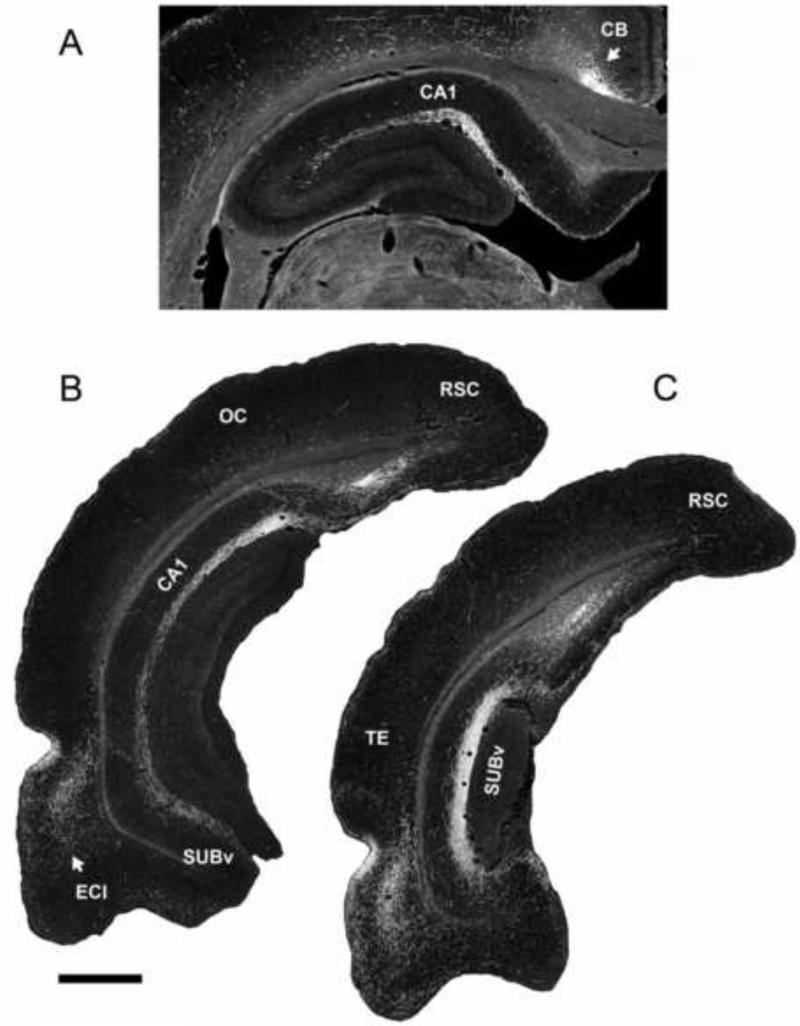
The nucleus reuniens (RE), the largest of the midline nuclei, is located in the anterior two-thirds of the thalamus. Rostrally, RE is divided into left and right halves, while further caudally the two sides fuse to become a mass of cells on the midline of the thalamus, lying immediately dorsal to the third ventricle. The RE is the most thoroughly investigated of the midline nuclei likely owing to the early demonstration of Herkenham (1978) that RE is the major source of thalamic input to the hippocampus. Confirming this, several subsequent studies have demonstrated that RE distributes massively to the hippocampus and in a highly organized manner (Ohtake and Yamada, 1989; Su and Bentivoglio, 1990; Wouterlood et al., 1990; Wouterlood, 1991; Dolleman-Van der Weel and Witter, 1996; Risold et al., 1997; Bokor et al., 2002; Van der Werf et al., 2002; Vertes, 2006; Vertes et al., 2006, 2007; Hoover and Vertes, 2007, 2012; Cavdar et al. 2008; Cassel et al., 2013; Varela et al., 2014). Specifically, RE fibers innervating the HF terminate selectively in the stratum lacunosum-moleculare (slm) of CA1 of the dorsal and ventral hippocampus as well as the molecular layer of the dorsal and ventral subiculum and the parasubiculum (Wouterlood et al., 1990; Vertes et al., 2006). This pattern of projection is illustrated in Figure 2. RE axons form asymmetric (excitatory) contacts predominantly on distal dendrites of pyramidal cells in slm of CA1 and the subiculum (Wouterlood et al., 1990). There is an absence of RE projections to CA2 and CA3 and to the dentate gyrus (DG) of the hippocampus.
Figure 2.
Low magnification darkfield photomicrographs showing patterns of labeling in the dorsal (A) and ventral hippocampus (B,C) produced by an injection of PHA-L in nucleus reuniens of the midline thalamus. Note the dense concentration of labeled fibers restricted to the stratum lacunosum moleculare of CA1 of the dorsal (A) and ventral (B) hippocampus and the molecular layer of the ventral subiculum (C). See list for abbreviations. Scale bar for A = 600 μm; for B,C = 1000 μm. Modified from Vertes et al. (2006).
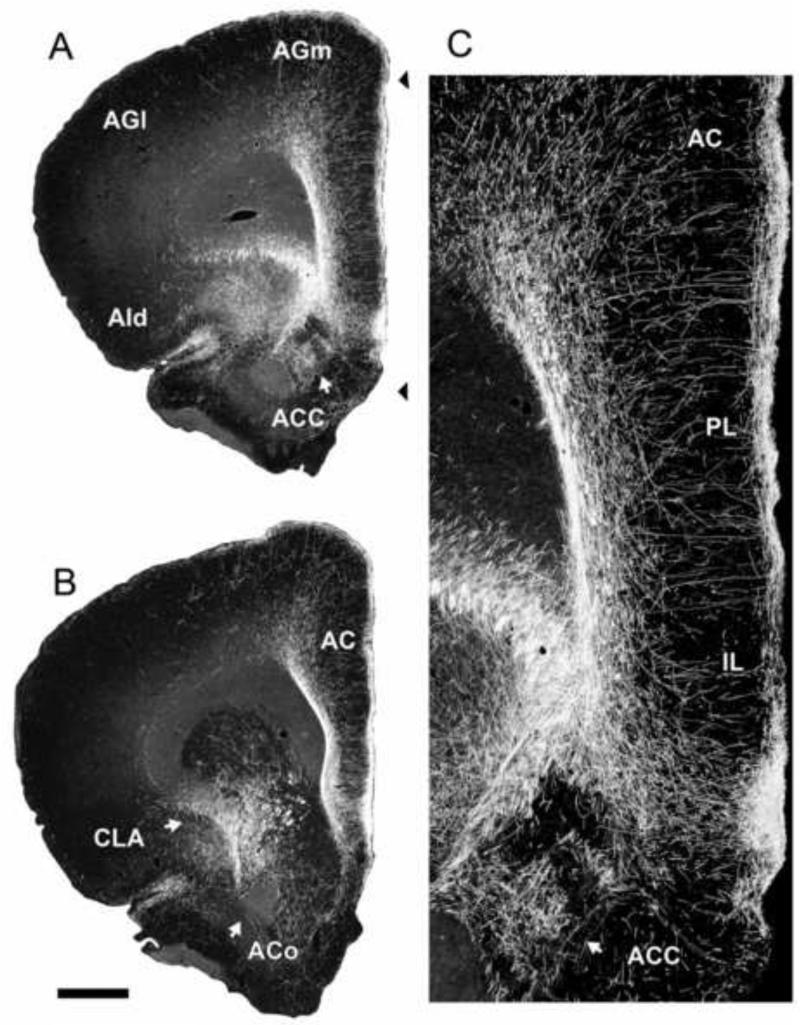
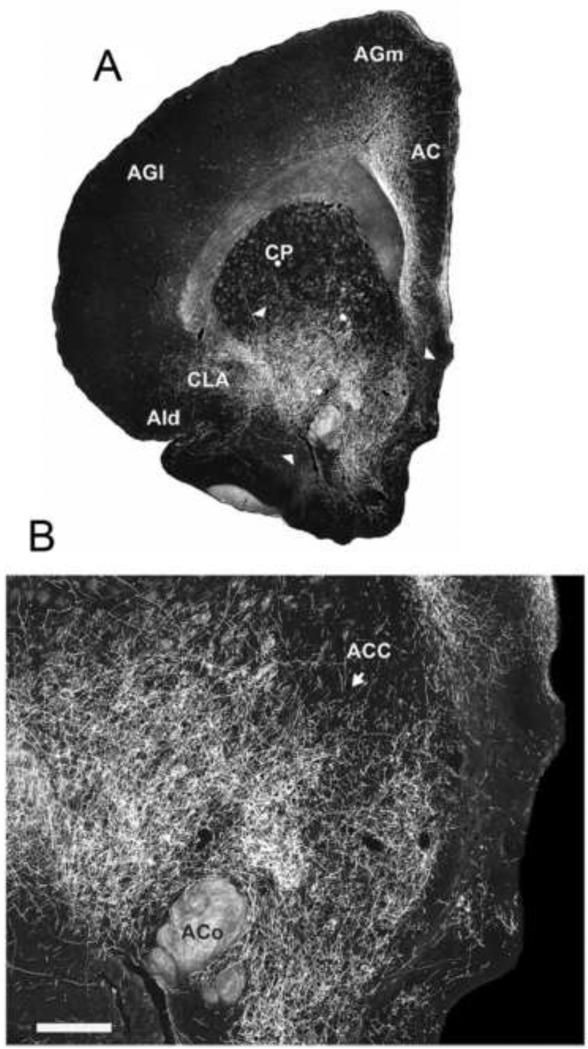
In addition to the hippocampus, RE is a rich source of projections to several cortical sites that collectively would be characterized as ‘limbic’ cortices (Berendse and Groenewegen, 1991; Wouterlood, 1991; Dolleman-Van der Weel and Witter, 1996; Van der Werf et al., 2002; Vertes et al., 2006, 2007; Hoover and Vertes, 2007, 2012; Cassel et al., 2013; Varela et al., 2014). They include the medial (MO) and ventral orbital (VO) cortices, the infralimbic (IL), prelimbic (PL) and anterior cingulate (AC) cortices of the medial prefrontal cortex (mPFC), the dorsal (AId) and ventral agranular insular cortices, rostral retrosplenial cortex, perirhinal cortex, and the medial and lateral entorhinal cortices. Figure 3 shows pronounced RE projections to the mPFC, most heavily concentrated in layers 1 and 5/6 of IL and PL of the ventral mPFC. The subcortical projections of RE are limited and mainly directed to the claustrum and to the anterior pole of ACC.
Figure 3.
A,B: low magnification darkfield photomicrographs of transverse sections through the rostral forebrain showing patterns of labeling within the medial prefrontal cortex produced by an injection of PHA-L in nucleus reuniens of the midline thalamus. Note the presence of pronounced numbers of labeled fibers in the infralimbic (IL), prelimbic (PL), anterior cingulate (AC) and medial (frontal) agranular (AGm) cortices, stronger in IL and PL than in AC and AGm Note additional labeling in the claustrum (CLA), the rostral pole of nucleus accumbens (ACC) and the dorsal agranular insular cortex (AId). C: high magnification darkfield photomicrograph from A (arrows) showing labeled fibers in all layers of IL, PL and AC, most densely concentrated in layers 1 and 5/6, and mediolaterally aligned fibers in the middle layers of these fields, oriented parallel to the cell layers. See list for abbreviations. Scale bar for A,B = 1000 μm; for C = 250 μm. Modified from Vertes et al. (2006).
Two very prominent targets of RE are the hippocampus and the mPFC (Vertes et al., 2006; Hoover and Vertes, 2012; Varela et al., 2014). Recent reports, using retrograde fluorescent techniques, have described collateral RE projections to these two structures (Hoover and Vertes, 2012; Varela et al., 2014). For instance, Hoover and Vertes (2012) demonstrated that, depending on specific sites of paired injections in the two sites, approximately 3-9% of RE cells projected, via collaterals, to the HF and mPFC. Although such cells (collateralizing) were rather dispersed throughout RE, they were most numerous in the lateral one-third of RE, just medial to the lateral wings of RE. It was further shown that, while intermingled, RE neurons projecting to one structure or the other (non-branching) were preferentially localized to distinct subregions of RE; that is, cells projecting to the mPFC were concentrated in the lateral wings of RE (or perireuniens nucleus), while those distributing to the hippocampus were most numerous in the rostral pole of RE. Finally, an approximately 10-fold greater number of RE cells projected to the ventral than to the dorsal hippocampus (Hoover and Vertes, 2012).
In accord with the foregoing, Varela et al. (2014) reported that on average 8% of RE cells, spanning its rostrocaudal axis, gave rise to collateral projections to the hippocampus and to the ventral mPFC. Interestingly, they further showed that only about 1% of hippocampal neurons (of the subiculum) projected dually (via collaterals) to RE and to the mPFC. It was suggested that RE cells with branching projections to the hippocampus and to the mPFC may play a critical role in systems consolidation of memory or in the synchronization of the theta rhythm during exploratory behaviors (Varela et al., 2014).
Whereas the output of RE is ‘relatively’ restricted to the hippocampus and limbic cortices (mainly the orbitomedial and parahippocampal cortices), reuniens receives a vast and diverse array of afferent projections from the cortex, hippocampus, basal forebrain, amygdala, hypothalamus and brainstem (Herkenham, 1978; Sesack et al., 1989; Witter et al., 1990; Wouterlood et al., 1990; Cullinan and Zaborszky, 1991; Hurley et al., 1991; Canteras and Swanson, 1992; Vertes, 1991, 1992; Risold et al., 1994, 1997; Canteras et al., 1995; Vertes et al., 1995; Naber and Witter, 1998; Canteras and Goto, 1999; Moore et al., 2000; Krout et al., 2002; Vertes, 2002, 2004; Jasmin et al., 2004; McKenna and Vertes, 2004; Hoover and Vertes, 2011).
Specifically, using retrograde tracers, McKenna and Vertes (2004) showed that RE receives widespread projections from subcortical and cortical sites. The main sources of afferents to RE were from the orbitomedial, insular, ectorhinal, perirhinal and retrosplenial cortices; the subiculum of hippocampus; the claustrum, lateral septum (LS), BST, and medial, lateral and magnocellular preoptic nuclei of the basal forebrain; the lateral habenula (LH), paraventricular and lateral geniculate nuclei of the thalamus; the zona incerta (ZI); the anterior, ventromedial, lateral, perifornical, posterior, supramammillary (SUM) and dorsal premammillary nuclei of the hypothalamus; and the ventral tegmental area (VTA), PAG, precommissural nucleus, parabrachial nuclei, laterodorsal tegmental nucleus (DR) (LDT) and the dorsal and median raphe (MR) nuclei of the brainstem.
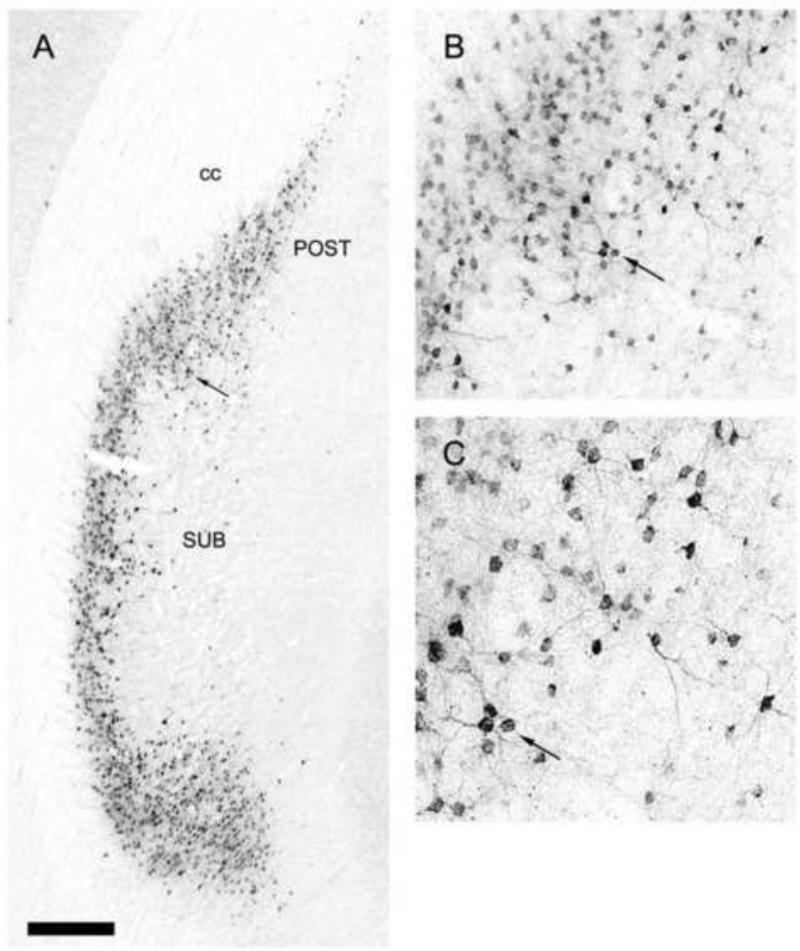
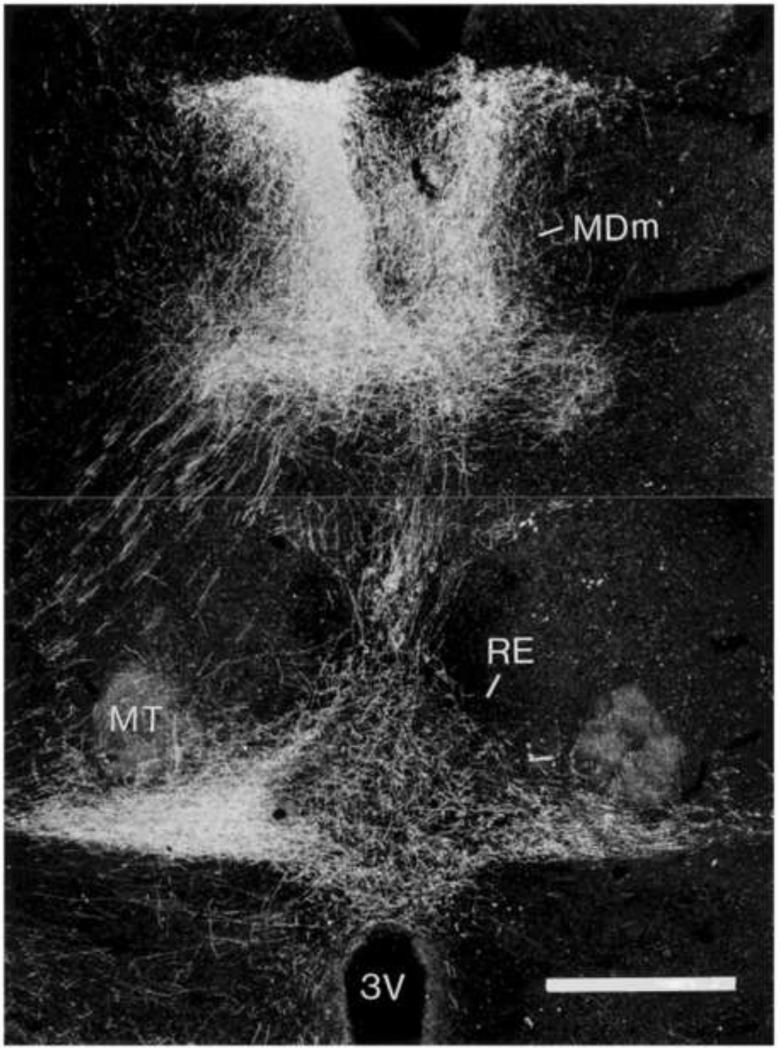
As described, RE prominently targets the hippocampus and mPFC and return projections are very pronounced indicating strong reciprocal RE connections with the HF and the mPFC. Regarding the hippocampus, retrograde tracer injections in RE were shown to produce marked cell labeling within the ventral subiculum of the hippocampus (McKenna and Vertes, 2004). As depicted in Figure 4, a Fluorogold injection in RE gave rise to a dense band of labeled cells dorsoventrally throughout the ventral subiculum of hippocampus. With respect to the mPFC, anterograde injections in the ventral mPFC produced substantial terminal labeling throughout the extent of the midline thalamus, with an absence of labeling in non-midline, or lateral, regions of the thalamus (Vertes, 2002). As shown in Figure 5, labeling was particularly strong rostrocaudally throughout the medial division of the mediodorsal nucleus (MDm) and within nucleus reuniens.
Figure 4.
Low (A) and high (B,C) magnification light-field photomicrographs depicting retrogradely labeled cells in the ventral subicular complex of the hippocampus produced by a Fluorogold injection in nucleus reuniens of the midline thalamus. As shown, pronounced numbers of labeled cells extended dorsal-ventrally throughout the subiculum (SUB) within the ventral subiculum and the postsubiculum (POST). B,C: Clusters of labeled cells dorsally in the ventral subiculum (arrow in A) at higher levels of magnification. See list for abbreviations. Scale bar for A = 325 μm; for B,C = 65 μm. Modified from McKenna and Vertes (2004).
Figure 5.
Darkfield photomicrograph of a transverse section through the diencephalon showing patterns of labeling within nuclei of the midline thalamus produced by a PHA-L injection in the infralimbic cortex. Note dense labeling virtually confined to the paraventricular and medial division of the mediodorsal nucleus (MDm), dorsally, and the nucleus reuniens (RE), ventrally. See list for abbreviations. Scale bar = 450 μm. Modified from Vertes (2002).
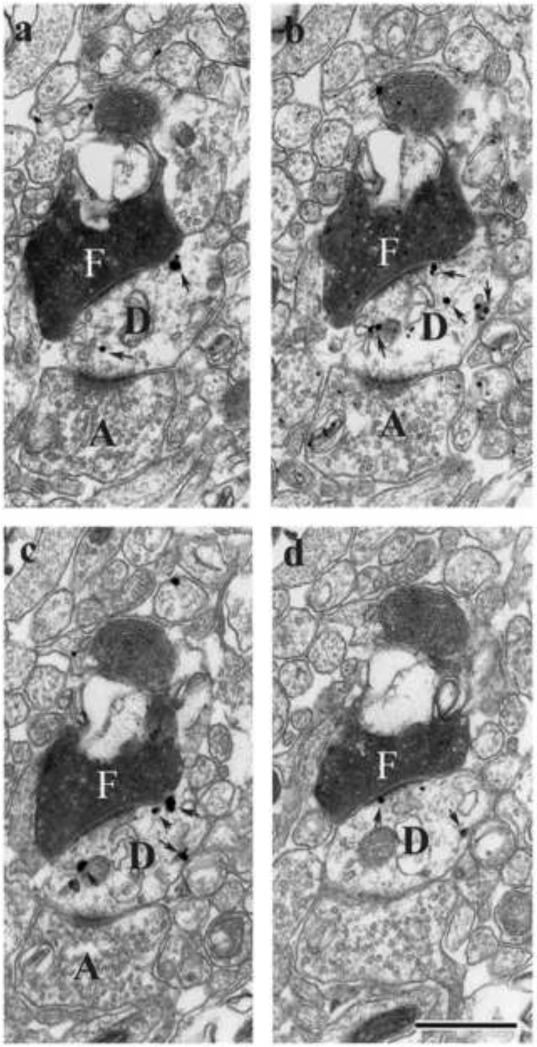
While it is well recognized that the hippocampus heavily targets the mPFC (Swanson, 1981, Ferino et al., 1987; Jay and Witter, 1991; Carr and Sesack, 1996; Hoover and Vertes, 2007), interestingly there are no return projections from the mPFC to the hippocampus (Laroche et al., 2000; Vertes, 2004). The demonstration, however, of strong projections from the mPFC to RE, and, in turn, from RE to the hippocampus (Vertes, 2002; Vertes et al., 2006) suggests that RE may be the principal route for the transfer of information from the mPFC to the hippocampus -- thus completing an important functional loop between these structures. Supporting this, at the ultrastructural level, mPFC fibers distributing to the RE have been shown to form asymmetric (excitatory) contacts on proximal dendrites of RE cells projecting to the hippocampus (Fig. 6) (Vertes et al., 2007).
Figure 6.
Series of electron micrographic sections through the nucleus reuniens of the midline thalamus (a-d) showing asymmetric contacts of a single PHA-L labeled (F) fibers from the medial prefrontal cortex onto a labeled dendritic shaft, identified by the presence of numerous silver intensified gold deposits (arrows in D) of a nucleus reuniens cell retrogradely from a Fluorogold injection in the ventral hippocampus. Note also the presence of asymmetric contacts of an unlabeled fiber (A) on the same labeled dendrite segment (D). Scale bar, 1 μm. Modified from Vertes et al. (2007).
Consistent with pronounced RE projections to HF and mPFC, nucleus reuniens has been shown to exert strong excitatory actions at CA1 of the hippocampus and at the mPFC (Dolleman-Van der Weel et al., 1997; Bertram and Zhang 1999; Viana Di Prisco and Vertes, 2006). Dolleman-Van der Weel et al. (1997) demonstrated that RE stimulation produced large negative-going field potentials (sink) at slm of CA1 as well as paired pulse facilitation at CA1. Bertram and Zhang (1999) compared the effects of RE and CA3 stimulation on population responses (field EPSPs and spikes) at CA1 and reported that RE actions at CA1 were equivalent to, and in some cases considerably greater than, those of CA3 at CA1. They concluded that the RE projection to the hippocampus “allows for the direct and powerful excitation of the CA1 region”. This thalamo-hippocampal connection bypasses the trisynaptic/commissural pathway that has been thought to be the exclusive excitatory drive to CA1” (Bertram and Zhang, 1999). Viana Di Prisco and Vertes (2006) confirmed the excitatory effects of RE on the hippocampus, and further showed that RE stimulation produced large monosynaptically-elicited evoked responses dorsoventrally throughout the mPFC, with most pronounced actions (latency and amplitude) at the ventral mPFC -- or at PL and IL.
3.3 Rhomboid nucleus
The rhomboid nucleus (RH) is a rather small cell group that lies just dorsal to nucleus reuniens and overlaps with approximately the caudal two-thirds of RE. It is quite recognizable by its rhomboid-like shape, hence its name.
By comparison with RE, relatively few reports have examined the anatomical connections of RH (Ohtake and Yamada, 1989; Berendse and Groenewegen, 1990, 1991; Van der Werf et al., 2002; Vertes et al., 2006, Hoover and Vertes, 2007). With notable exceptions (see below), the inputs and outputs of RH are similar to those of RE. Whereas RE projections are essentially confined to limbic cortices, RH, however, distributes more widely over the cortex to limbic and non-limbic regions. The primary cortical targets of RH are the medial orbital, the mPFC (AGm, AC, PL, IL), posterior agranular insular, primary and secondary somatosensory, retrosplenial, posterior parietal, perirhinal, occipital, and temporal cortices, and the hippocampus. Although present, RH projections to the hippocampus are much less pronounced than those from RE.
The subcortical projections of RH are also considerably more widespread than those of RE. Subcortically, RH fibers distribute fairly widely throughout the anterior forebrain to the claustrum, the dorsal striatum (caudate putamen, C-P), LS, the core and shell of ACC, the olfactory tubercle (OT), and to the basal nuclei of the amygdala. RH projections to the striatum are restricted to medial aspects of the caudate-putamen, and are considerably less dense than those to C-P from the dorsally adjacent central medial nucleus (see below). Two prominent subcortical targets of RH are the nucleus accumbens (Fig. 7) and the basolateral nucleus (BLA) of the amygdala.
Figure 7.
Low (A) and high (B) magnification darkfield photomicrographs of a transverse section through the anterior forebrain showing patterns of labeling in the dorsal and ventral striatum produced by a PHA-L injection in the rhomboid nucleus of the midline thalamus. B taken from A (arrowheads). Note dense labeling in the nucleus accumbens (ACC) and adjacent ventromedial parts of the dorsal striatum. See list for abbreviations. Scale bar: for A = 750 μm; for B = 400 μm. Modified from Vertes et al. (2006).
Although data is limited, it appears that RH receives a diverse and widespread set of afferent projections from cortical and subcortical sites (Sesack et al., 1989; Hurley et al., 1991; Krout et al., 2002; Vertes, 2002, 2004; McKenna and Vertes, 2004; Owens, 2005). With some overlap, afferents to RH are distinct from those to RE -- with a major difference being that unlike RE, the rhomboid nucleus receives significant input from non-limbic (sensory/motor) structures.
Cortical afferents to RH derive from limbic and non-limbic regions of the cortex. While fibers throughout the medial wall of the PFC (or mPFC) distribute to RH, they arise most strongly from the dorsally located AGm and taper ventrally in the mPFC. In contrast to other midline nuclei, RH receives marked input from the primary motor cortex and from the primary and secondary somatosensory cortices. Aside from input from parts of the insular and medial PFC, RH receives limited projections from ‘limbic’ cortices, and notably few projections from parahippocampal cortices and the hippocampus (Van der Werf et al., 2002; McKenna and Vertes, 2004; Cassel et al., 2013; Vertes et al., 2014).
In general, subcortical forebrain inputs to RH are not extensive, arising most heavily from the claustrum, substantia innominata, and ZI, and to a lesser degree from the posterior and lateral nuclei of the hypothalamus and parts of the amygdala. As with other midline and intralaminar nuclei (Krout et al., 2002; Van der Werf et al., 2002; Groenewegen and Witter, 2004; McKenna and Vertes, 2004; Cassel et al., 2013; Vertes et al., 2014), the brainstem is a major source of afferents to RH. Fibers originate from ‘limbic-related’ brainstem sites such as the VTA, PAG, pedunculopontine tegmental nucleus (PPT), LDT and the parabrachial complex, but also from ’motor’ nuclei including the substantia nigra-pars reticulata, mesencephalic RF, SC, and the anterior pretectal nucleus
3.4 Reuniens and rhomboid nuclei: Functional Considerations
A number of recent reports have described the effects of lesions/inactivation of RE on behavior, with many studies including RH with RE due to their close proximity and difficulty in separately evaluating their effects on behavior (Dolleman-van der Weel et al., 2009; Davoodi et al., 2009, 2011; Eleore et al., 2011; Hembrook and Mair, 2011; Hembrook et al., 2012; Loureiro et al., 2012; Cholvin et al., 2013; Hallock et al., 2013; Prasad et al., 2013). While a consensus has not been reached, it appears that RE (or RE/RH) is critically involved in behaviors associated with the mPFC or those that depend on interactions between the hippocampus and mPFC.
In an initial study, Dolleman-van der Weel et al. (2009) reported that rats with excitotoxic lesions of RE did not significantly differ from controls in the acquisition and retention of a water maze reference memory task. For instance, on the probe test following acquisition, RE lesioned rats swam directly to the correct quadrant of the pool. However, interestingly, upon not finding the platform, these rats immediately adopted a strategy of searching the entire pool for the platform. The deficit was described as non-mnemonic -- or a reduced ability to shift strategies. Accordingly, it was viewed as a medial PFC rather than a hippocampal dysfunction. Subsequent studies have similarly shown that inactivation of RE/RH does not alter acquisition or retrieval on a standard water maze task (Loureiro et al., 2012; Cholvin et al., 2013). In accord with a RE involvement mPFC-associated functions (Dolleman-van der Weel et al., 2009), Prasad et al. (2013) showed that rats with RE lesions were unable to inhibit premature responses on a five choice reaction time task -- an impulsive behavioral pattern characteristic of damage to the prefrontal cortex (Chudasama et al. 2003).
While the foregoing suggests that RE lesions may affect mPFC- but not hippocampal-dependent tasks, the developing notion is that RE is most directly involved in tasks requiring the interactions of the HF and mPFC. For instance, Hembrook and Mair (2011) initially showed that RE/RH lesions significantly altered performance on a delayed non-match to sample radial arm maze task which is sensitive to damage to the hippocampus or to the mPFC (McDonald and White, 1993; Porter and Mair, 1997; Mair et al., 1998), but were without effect on a visuospatial reaction time task sensitive to alterations of the striatum or the motor cortex. Hembrook et al. (2012) subsequently examined the effects of reversible inactivation of RE/RH: (1) on a delayed non-match to position task in an operant chamber sensitive to lesions of the HF or the mPFC; and (2) on a variable choice radial maze delayed non-matching task responsive to hippocampal but not to mPFC lesions (Porter et al., 2000). RE/RH inactivation significantly disrupted performance on the delayed non-match to position but not on the radial maze task. The authors concluded that “RE and RH affect measures of spatial working memory that depend on interactions of between the hippocampus and mPFC, but not measures that depend on the hippocampus alone” (Hembrook et al., 2012).
Using a different set of tasks, Cassel and colleagues similarly concluded that RE/RH selectively participate in functions requiring the cooperative actions of the hippocampus and the mPFC (Loureiro et al., 2012; Cholvin et al., 2013; Cassel et al., 2013). Specifically, lesions of RE/RH had no effect on either the acquisition or short term retention (5 days post-acquisition) of a water maze task, but disrupted long-term retention (25 days) on the task (Loureiro et al., 2012). As was pointed out, recent memory (5 days) involves the hippocampus, whereas remote memory (25 days) enlists both the hippocampus and the mPFC (Clark et al., 2005; Broadbent et al., 2006; Teixeira et al., 2006; Lopez et al., 2012). In a follow-up report, Cholvin et al. (2013) compared the effects of selective inactivation of the hippocampus, the mPFC or the RE/RH on a standard water maze task and on a ‘double-H’ water maze task that places demands on both the hippocampus (place identification) and the mPFC (strategy-shifting) for successful completion. Only hippocampal inactivation impaired performance on the standard water maze task, whereas inactivation of the hippocampus, mPFC, or the RE/RH disrupted performance, and to a similar degree, on the double-H water maze task. According to the authors, the hippocampus serves a recognized role in spatial memory, the mPFC in set shifting, and RE/RH may act “as the coordinator of this processing” (Cholvin et al., 2013).
Finally, Hallock et al. (2013) compared the effects of RE/RH suppression on two versions of a conditional discrimination T-maze task: one involving a working memory component and the other not. RE/RH inactivation severely disrupted performance on the working memory version but not on the conditional discrimination version of the task, leading the authors to conclude that RE/RH is a necessary component of working memory performance which is “thought to depend on the hippocampal-prefrontal circuit.”
3.5 Paraventricular nucleus
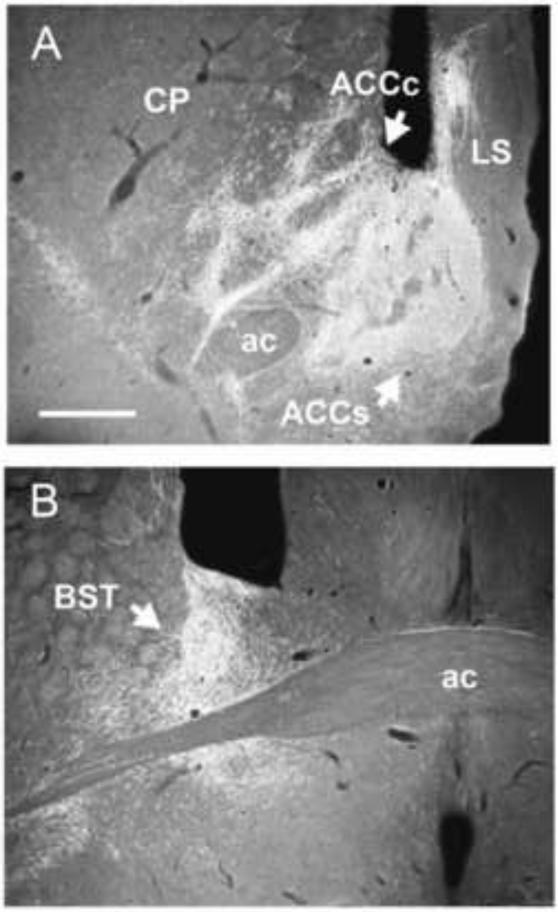
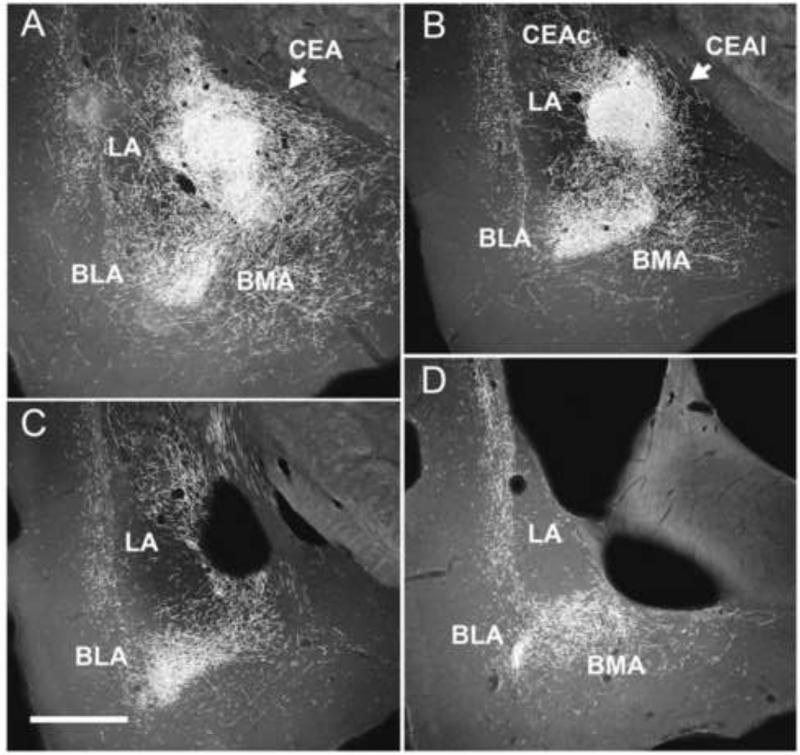
The paraventricular nucleus (PV) is located medially below the third ventricle and dorsomedial to the mediodorsal nucleus, and essentially spans the rostro-caudal length of the thalamus. PV distributes fairly widely throughout the forebrain to cortical and subcortical structures (Berendse and Groenewegen, 1990, 1991; Meredith and Wouterlood, 1990; Su and Bentivoglio, 1990; Turner and Herkenham, 1991; Brog et al., 1993; Conde et al., 1995; Moga et al. 1995; Bubser and Deutch, 1998; Otake and Nakamura, 1998; Pinto et al., 2003; Jasmin et al., 2004; Peng and Bentivoglio, 2004; Parsons et al., 2006, 2007; Hoover and Vertes, 2007; Shin et al., 2008; Li and Kirouac, 2008; Vertes and Hoover, 2008). The principal targets of PV are IL and PL of the mPFC, dorsal agranular insular cortex, ventral subiculum of hippocampus, claustrum, lateral septum, core and shell of ACC, OT, BST, medial, central, cortical and basal nuclei of amygdala, and the suprachiasmatic, arcuate, and dorsomedial nuclei of the hypothalamus (Li and Kirouac, 2008; Vertes and Hoover, 2008). In addition, the caudal PV distributes to dorsal striatum. Figure 8 shows pronounced projections from the rostral PV to ACC and BST, while Figure 9 depicts massive caudal PV projections to the central and basal nuclei of the amygdala at four rostro-caudal levels of the amygdala.
Figure 8.
Low magnification darkfield photomicrographs of transverse sections through anterior forebrain showing patterns of labeling in the nucleus accumbens (ACC) (A) and the bed nucleus of the stria terminalis (BST) (B) produced by a PHA-L injection in the anterior paraventricular nucleus of the midline thalamus. Note the pronounced labeling in both the shell and core of ACC, with additional labeling in the adjacent lateral septum (LS) as well as in BST above and below the anterior commissure. See list for abbreviations. Scale bar = 500 μm. Modified from Vertes and Hoover (2008).
Figure 9.
A-D: Series of low magnification darkfield photomicrographs of transverse sections rostrocaudally (A-D) through the forebrain depicting patterns of labeling within the amygdala produced by a PHA-L injection in the posterior paraventricular nucleus of the midline thalamus. A,B: Note dense labeling in the central (CEA), basomedial (BMA) and basolateral (BLA) nuclei of amygdala, and marked but less pronounced labeling in parts of the medial, lateral (LA) and anterior cortical nuclei of the amygdala. C,D: Note prominent labeling caudally within the amygdala mainly confined to BMA and BLA. See list for abbreviations. Scale bar = 750 μm. Modified from Vertes and Hoover (2008).
The PV receives a relatively diverse array of afferents from the forebrain and the brainstem (Cornwall and Phillipson, 1988; Sesack et al., 1989; Chen and Su, 1990; Hurley et al., 1991; Vertes, 1991, 1992, 2002; Otake and Nakamura, 1995; Otake et al., 1995; Vertes et al., 1995, 1999; Ruggiero et al., 1998; Novak et al., 2000; Krout et al., 2002; Goto and Swanson, 2004; Peng and Bentivoglio, 2004; Kirouac et al., 2005, 2006; Otake, 2005; Hoover and Vertes, 2011; Li and Kirouac, 2012).
A major source of input to PV is from the cortex, or specifically from the mPFC and insular cortices (Groenewegen, 1988; Sesack et al., 1989; Chen and Su, 1990; Hurley et al., 1991; Vertes, 2002, 2004; Jasmin et al., 2004; Hoover and Vertes, 2011; Li and Kirouac, 2012). Whereas fibers throughout the mPFC project to PV, there is a dorsal to ventral gradient in intensity such that AGm and AC distribute moderately, and PL and IL massively, to PV (Vertes, 2002, 2004; Li and Kirouac, 2012). Some cortical inputs differentially favor anterior or posterior parts of PV (Li and Kirouac, 2012); that is, the ventral subiculum projects more heavily to the anterior than to the posterior PV, whereas IL, PL and the posterior agranular insular cortex preferentially distribute to the posterior PV.
The main subcortical afferents to PV arise from structures of the hypothalamus and brainstem, with additional but more limited input from BST and parts of the amygdala. PV receives projections from several nuclei of the hypothalamus including the tuberomammillary, SUM, dorsomedial, posterior, lateral and parasubthalamic nuclei -- as well as from the medial preoptic area and diagonal band nuclei (Vertes, 1992; Vertes et al., 1995; Goto and Swanson, 2004; Kirouac et al., 2005, 2006; Menzie et al., 2010; Li and Kirouac, 2012). In addition, PV is essentially unique among the midline nuclei in that it receives afferents from the suprachiasmatic nucleus and the intergeniculate leaflet (Moore et al., 2000; Kawano et al., 2001). While varying in density, brainstem projections to PV derive from VTA, the pontomesencephalic RF, nucleus cuneiformis, nucleus of Darkschewitsch, the dorsal and median raphe nuclei, regions of the PAG, parabrachial nucleus, LDT and PPT, LC, and the solitary nucleus (Chen and Su, 1990; Takada et al., 1990; Bester et al., 1999; Krout and Loewy, 2000a, 2000b; Krout et al., 2002; Li and Kirouac, 2012
3.6 Paratenial nucleus
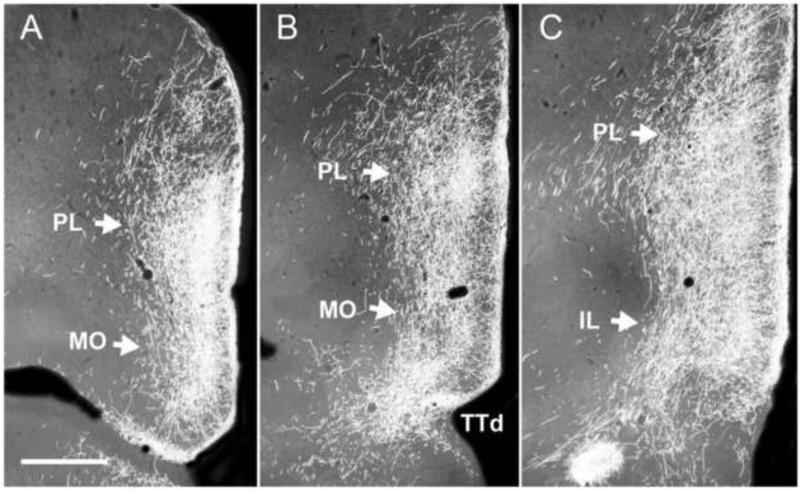
The paratenial nucleus (PT) is a slender, elongated nucleus located in the anterior thalamus. It borders the paraventricular nucleus laterally and overlaps with approximately the rostral one-third of PV. Compared to PV, less attention has been paid to the efferent projections of PT, probably owing to its small size (Berendse and Groenewegen, 1990, 1991; Turner and Herkenham, 1991; Brog et al., 1993; Conde et al., 1995; Van der Werf et al., 2002; Jasmin et al., 2004; Hoover and Vertes, 2007; Vertes and Hoover, 2008). Nonetheless, the output of PT is substantial and largely parallels that of PV (Vertes and Hoover, 2008). The main cortical targets of PT are the medial frontal polar, AC, PL, IL, medial orbital, dorsal agranular insular, piriform and entorhinal cortices, and the ventral subiculum of the hippocampus. Figure 10 shows dense PT projections to the medial orbital, PL and IL cortices at three rostrocaudal levels of the mPFC.
Figure 10.
A-C: Series of rostrocaudally (A-C) aligned low magnification darkfield photomicrographs of transverse sections through the anterior forebrain depicting patterns of labeling within the medial prefrontal cortex (mPFC) produced by a PHA-L injection into the paratenial nucleus of the midline thalamus. Note intense labeling along the ventral medial wall of the mPFC mainly confined to the prelimbic (PL) (A-C), medial orbital (MO) (A,B) and infralimbic (C) (IL) cortices. As depicted, labeling was particularly dense in layers 1 and 3 of these prefrontal cortical fields. See list for abbreviations. Scale bar = 750 μm. Modified from Vertes and Hoover (2008).
Principal subcortical sites of projection of PT are the claustrum, the core and shell of ACC, the medial sector of C-P, BST, and caudal parts of the central and basal nuclei of the amygdala. PT also distributes lightly/moderately to the ventral orbital and perirhinal cortices, the dorsal subiculum, lateral septum, medial and cortical nuclei of amygdala, and the lateralhypothalamus (Vertes and Hoover, 2008).
As with PT projections, much less is known of inputs to PT than for other midline thalamic nuclei including PV. Nonetheless, an understanding of major sources of afferents to PT has emerged from anterograde studies that traced projections to PT (Cornwall and Phillipson, 1988; Groenewegen, 1988; Sesack et al., 1989; Chen and Su, 1990; Hurley et al., 1991; Krout et al., 2002; Vertes, 2002, 2004; Jasmin et al., 2004; Hoover and Vertes, 2011). With some notable exceptions, PT and PV receive similar sets of inputs. PT receives substantial input from rostral regions of the ‘limbic’ cortex -- or from the medial, orbital, and insular PFC (Sesack et al., 1989; Hurley et al., 1991; Vertes, 2002, 2004; Jasmin et al., 2004; Hoover and Vertes, 2011). Specifically, PT receives prominent projections from the ventral mPFC (IL and PL), from the (rostral) agranular insular cortex and from medial and ventral orbital cortices (Jasmin et al., 2004; Hoover and Vertes, 2011). Forebrain subcortical structures distributing to PT mainly include the ventral subiculum and the claustrum, and to a lesser degree, LS, diagonal band nuclei, BST, medial nuclei of amygdala, reticular thalamic nucleus, ZI, and parts of the hypothalamus (Chen and Su, 1990). Like PV, brainstem afferents to PT originate from DR, MR, PAG, LC, parabrachial nucleus, LDT, and the solitary nucleus
3.7 Differential projections of the paraventricular and paratenial nuclei
While largely overlapping, there are some notable differences in the projections of PT and PV. In brief, the output of PT is more strongly weighted to (limbic) cortical than to subcortical structures, whereas the opposite is true for PV: limbic subcortical greater than cortical. In particular, PT distributes more widely throughout the cortex, and more intensely than PV to the medial orbital cortex, the ventral mPFC, the lateral entorhinal cortex, the ventral subiculum, and the dorsal striatum. On the other hand, PV fibers terminate more heavily in BST, the central and basal nuclei of the amygdala and most of the hypothalamus. Both PT and PV, however, distribute massively to the ACC (Vertes and Hoover, 2008).
3.8 Paraventricular nucleus: Functional considerations
By comparison with RE and RH which target the hippocampus and limbic cortical structures involved in cognitive functions, the paraventricular nucleus of the dorsal midline thalamus preferentially distributes to limbic subcortical sites such as the septum, BST, ACC, and the amygdala. Accordingly, PV has been directly implicated in affective functions such as stress and anxiety, feeding behavior, and drug seeking activities (for review, Hsu et al., 2014; Matzeu et al., 2014). Little is known regarding the functions of PT.
The paraventricular nucleus is activated by wide range of stressors such as fear/anxiety, immobilization, and footshock-induced stress, and thus appears responsive to stress, per se, independent of its specific source (Chastrette et al., 1991; Bubser and Deutch, 1999). PV has been shown to be instrumental in the adaptation to acute stressors following periods of chronic stress. For example, rats exposed to a repeated (chronic) stressor become habituated to that stressor, so when the stressor is re-introduced it produces less of a response than to its initial exposure. However, if a different stressor is introduced after habituation, the response to it is magnified, possibly signaling the presence of novel, potentially threatening, stimuli. PV appears to be involved in both processes: that is, adaptation to the original stressor and the heightened response to a novel stressor (Bhatnagar and Dallman, 1998; Bhatnagar et al., 2002; Heydendael et al., 2011). Specifically, posterior PV lesions block habituation to repeated restraint stress (Bhatnagar et al., 2002) and c-fos expression in PV is significantly elevated when novel stressors are applied after a chronic stressor (Bhatnagar and Dallman, 1998).
Although more typically responsive to “negative” stimuli, PV cells are also activated by rewarding conditions and thus may generally encode emotionally salient events (Igelstrom et al., 2010; Hsu et al., 2014; Matzeu et al., 2014). Specifically, PV has been shown to serve a role in feeding behavior -- which may be a model system for appetitive functions. For instance, c-fos levels in PV are enhanced in anticipation of feeding in food-deprived rats, and PV lesions attenuate anticipatory locomotor activity associated with feeding (Nakahara et al. 2004; Angeles-Castellanos et al. 2007). More recently, Choi et al. (2012) demonstrated that direct injections of orexin-A in PV produced a marked increase in dopamine release in ACC and elicited hedonic feeding -- an overconsumption of palatable foods. Similar to the reward circuit for food (Choi et al., 2010, 2012), the core circuitry for drug seeking behavior reportedly involves orexinergic projections from the lateral hypothalamus to PV and in turn PV projections to the nucleus accumbens (Martin-Fardon and Boutrel, 2012; Matzeu et al., 2014). Regarding a role for PV in drug-seeking behavior, lesions or inactivation of PV suppress drug-seeking activities (Hamlin et al., 2009; James et al. 2010; Marchant et al., 2010), while cue-induced reinstatement of cocaine seeking behavior strongly correlates with c-fos activation in PV (James et al., 2011). In addition, injections of orexin-A in PV reinstated extinguished cocaine and sweetened condensed milk seeking behaviors (Martin-Fardon et al., 2011). Finally, there may be a link between a PV involvement in stress/anxiety and in overeating and drug seeking behavior in that stress tends to exacerbate the latter behaviors (Martin-Fardon and Boutrel, 2012; James and Dayas, 2013).
3.9 Mediodorsal nucleus, medial division
The mediodorsal nucleus (MD) is a large nucleus situated dorsally and medially in the thalamus. The two halves of MD are separated by PV and by the intermediodorsal nucleus (IMD). MD is bordered dorsally by the habenular complex, rostrally by PT, and caudally by the parafascicular nucleus of the intralaminar thalamus. Hence MD is virtually ‘encircled’ by the midline and intralaminar nuclei of thalamus. The MD in rats is divided into three segments: medial (MDm), central (MDc) and lateral (MDl) divisions, and generally a fourth part, lateral to MDl, termed the paralamellar MD (MDpl) -- as it abuts the internal medullary lamina (Leonard, 1969, 1972; Krettek and Price, 1977; Groenewegen, 1988).
The different segments of MD are characterized by distinct input-output relationships and distinct sets of functions. On the whole, MD is strongly reciprocally connected with the medial, orbital and insular prefrontal cortices, but interestingly is the source of relatively minor projections to other parts of the cortex (Krettek and Price, 1977; Reep and Winans, 1982; Groenewegen, 1988; Conde et al., 1990; Ray and Price, 1992; Reep and Corwin, 1999; Vertes, 2002, 2004; Jasmin et al., 2004; Gabbott et al., 2005; Rotaru et al., 2005; Hoover and Vertes, 2007, 2011). In fact, the prefrontal cortex of rodents was initially designated as a “MD projection cortex” (Leonard, 1969; Uylings and van Eden, 1990). As with other regions of the PFC, mediodorsal connections with the medial prefrontal cortex are highly topographically organized, such that medial to lateral segments of MD are mapped onto ventral to dorsal regions of the mPFC. Specifically, MDm reciprocally connects with IL, the lateral part of MDm and MDc with PL and the ventral AC, the MDl with the dorsal AC, and MDpl with AGm (Groenewegen, 1988; Ray and Price, 1992; Vertes, 2002; Hoover and Vertes, 2007). Figure 5 shows massive projections from the infralimbic cortex to MDm.
In a similar manner, specific segments of MD connect with distinct regions of the orbital cortex. MDm is reciprocally linked to the medial orbital (MO) cortex, MDc to the lateral orbital cortex, MDl to the ventral (VO) and ventrolateral (VLO) orbital cortices, and ventral aspects of MDc and MDl to the dorsolateral orbital (DLO) cortex (Groenewegen, 1988; Ray and Price, 1992; Hoover and Vertes, 2011). insular cortex, MDm interconnects with the dorsal (AId) and posterior Regarding the agranular insular cortices, and MDc with the ventral agranular insular cortex (Groenewegen, 1988; Ray and Price, 1992; Jasmin et al., 2004). With some overlap, the foregoing indicates that MDm, in contrast to lateral parts of MD, primarily communicates with limbic regions of the PFC. This is consistent with the functional properties of MDm (see below).
Similar to the interconnections of MD with the PFC, most subcortical structures projecting to MD target specific segments of MD, but some (mainly of the brainstem) distribute throughout MD (Young et al., 1984; Groenewegen, 1988; Kuroda and Price, 1991a, 1991b; Ray and Price, 1992; Vertes, 1991, 1992; Zahm et al., 1996; Morin and Meyer-Berstein, 1999; Vertes et al., 1999; Krout et al., 2002; Tripathi et al., 2012). MD is typically described as composed of three functional units: motor (MDl/MDpl), olfactory (MDc) and limbic (MDm), and with some overlap, subcortical inputs to MD reflect these functional divisions. For instance, afferents to lateral segments of MD (MDl/MDpl) mainly derive from structures of the basal ganglia and the brainstem. These include the internal segment of the globus pallidus (GPi) and substantia nigra-pars reticulata of the basal ganglia, and the SC, pretectum, and the acetylcholine-containing LDT of the brainstem. Inputs to MDc predominately originate from olfactory structures including piriform cortex, endopiriform nucleus, OT, horizontal limb of diagonal band nucleus and the lateral preoptic area. Afferents to MDm arise from a greater number and more diverse set of structures than to the other divisions likely reflecting its involvement in limbic-associated functions. They include: ACC/ventral pallidum, diagonal band nuclei, the anterior cortical, BLA and basomedial nuclei of the amygdala, VTA, the substantia nigra-pars compacta (SNc), the supramammillary nucleus, and the lateral entorhinal cortex (Groenewegen, 1988; Ray and Price, 1992; Vertes, 1992). All divisions of MD receive common inputs from several sites including the reticular nucleus of thalamus, the mesopontine reticular formation, PPT, LDT, LC and the dorsal and median raphe nuclei of the brainstem (Groenewegen, 1988; Vertes, 1991; Vertes et al., 1999; Krout et al., 2002).
In summary, the foregoing indicates that MDm, largely in contrast to other divisions of MD, is predominantly interconnected with limbic subcortical and cortical structures, and in this respect, MDm is much more aligned with the midline nuclei (RE, RH, PV, PT) than with lateral regions of MD.
3.10 Mediodorsal nucleus, medial division: Functional considerations
As discussed, each division of MD displays a unique set of connections which in general constitute a continuum from limbic to motor proceeding medially to laterally in MD. The functional properties of MD generally parallel this anatomical organization (Groenewegen et al., 1990; Deniau et al., 1994; Zahm, 2000; Block et al., 2007; Aggleton et al., 2011; Mailly et al., 2013; Mitchell and Chakraborty, 2013). With regard to MDm, it is strongly reciprocally linked with the prefrontal cortex and as such its functions significantly overlap with those of the PFC. A function commonly associated with MD (or MDm) is behavioral flexibility -- or the capacity to alter behavior to meet changing contingencies (McAlonan et al., 1993; Hunt and Aggleton, 1998; Block et al., 2007; Dolleman-van der Weel et al., 2009; Mitchell and Chakraborty, 2013). Rats with MDm lesions (or inactivation) are unable to readily switch from one response to another in the face of changing task demands. Although several factors are likely involved, the deficit is generally attributed to perseverative responding, or maintaining a previously rewarded response strategy despite its being incorrect or unrewarded upon changed conditions. For example, Block et al. (2007) demonstrated that rats with bilateral MD lesions, or disconnecting MD from the mPFC, were unable to successfully switch from a “response” strategy (e.g., all right turns) to a visually cued strategy (or vice versa) on a four arm cross-maze. They postulated that MD “directs” the mPFC to switch strategies and the mPFC, in turn, suppresses incorrect responses in favor of correct ones to the new paradigm.
Perhaps a more striking example of failures in set shifting with MD (or MDm) lesions involved the use of a relatively undemanding reference memory task in the water maze (Dolleman-van der Weel et al., 2009). MD lesioned rats were slow to acquire a water maze task, which reportedly was the result of “perseverance” of edge swimming (thigmotaxis) carried over from pretraining. The rats were also impaired in the change of task conditions in the probe test from a hidden platform to a visible one. In effect, even though the platform was visible, MD lesioned rats continued to search for the hidden platform -- a clearly perseverative response. The deficits in switching strategies described with MD lesions are characteristic (or a hallmark) of dysfunctions of the prefrontal cortex (Ragozzino et al., 1999; Birrell and Brown, 2000; Dias and Aggleton, 2000; Stefani et al., 2003; Floresco et al., 2006; Rich and Shapiro, 2009). The foregoing would suggest that disruption of communication between these structures leads to perseverative behaviors -- or otherwise stated, the integrative actions of MD and the mPFC are critical for flexible choice behavior.
In addition to a role in behavioral flexibility, the mPFC is an important component of an extended circuitry subserving recognition memory; that is, a circuitry which also includes the perirhinal cortex, hippocampus and MD (Ennaceur et al., 1996; Norman and Eacott, 2004; Barker et al., 2007; Barker and Warbuton, 2011; Cross et al., 2012). Recognition memory involves the ability to identify stimuli based on previous experience with them, and judgments can be made using various types of information such as an object's characteristics (familiarity) or where (spatial location) or when (temporal order) it was encountered. Barker et al. (2007) examined the contribution of the mPFC to the different components of recognition memory showing that mPFC lesions spared single item recognition (novel object preference) as well as object location (spatial displacement), but significantly disrupted associative object recognition (object in place task) and recency judgments (temporal order). With respect to MD, Cross et al. (2012) subsequently demonstrated that MD lesions (or disconnecting MD from the mPFC) produced the same types of recognition memory deficits as seen with mPFC lesions (Barker et al., 2007); that is, intact single item recognition but marked impairments on associative recognition memory and recency discrimination. These findings suggest the conjoint action of MD and the mPFC in recognition memory.
3.11 Central medial nucleus
The intralaminar nuclei (ILt) of thalamus form a conspicuous collection of nuclei in the medial and dorsal part of the thalamus. The intralaminar thalamic nuclei are located lateral to the mediodorsal nucleus and embedded within the internal medullary lamina. The intralaminar nuclei are made up of a rostral group, consisting of the central medial, paracentral (PC), and central lateral (CL), and a caudal group composed of the parafascicular (PF) and subparafascicular nuclei (SPF) in rats (Swanson, 2004; Paxinos and Watson, 2014). The central medial nucleus can be clearly recognized as a centrally located group of large, deeply staining, flattened cells distinct from midline nuclei lying dorsal and ventral to it. Laterally, CM is continuous with the paracentral nucleus on both sides.
On the whole, the principal targets of the intralaminar nuclei are regions of the cortex and the dorsal striatum, with additional projections to the ventral striatum and amygdala. In general, the lateral (PC, CL) and caudal (PF, SPF) intralaminar nuclei innervate sensorimotor regions of the cortex and the dorsal striatum, whereas CM distributes over a much wider region of the forebrain to both limbic and non-limbic sites (Berendse and Groenewegen, 1990, 1991; Conde et al., 1990, 1995; Su and Bentivoglio, 1990; Hicks and Huerta, 1991; Turner and Herkenham, 1991; Brog et al., 1993; Reep and Corwin, 1999; Erro et al., 2002; Van der Werf et al., 2002; Jasmin et al., 2004; Wang and Shyu, 2004; Hoover and Vertes, 2007; Vertes et al., 2012, 2104). In this regard, the projections of CM closely resemble those of the midline nuclei and the MDm (see above) than projections of the other intralaminar nuclei.
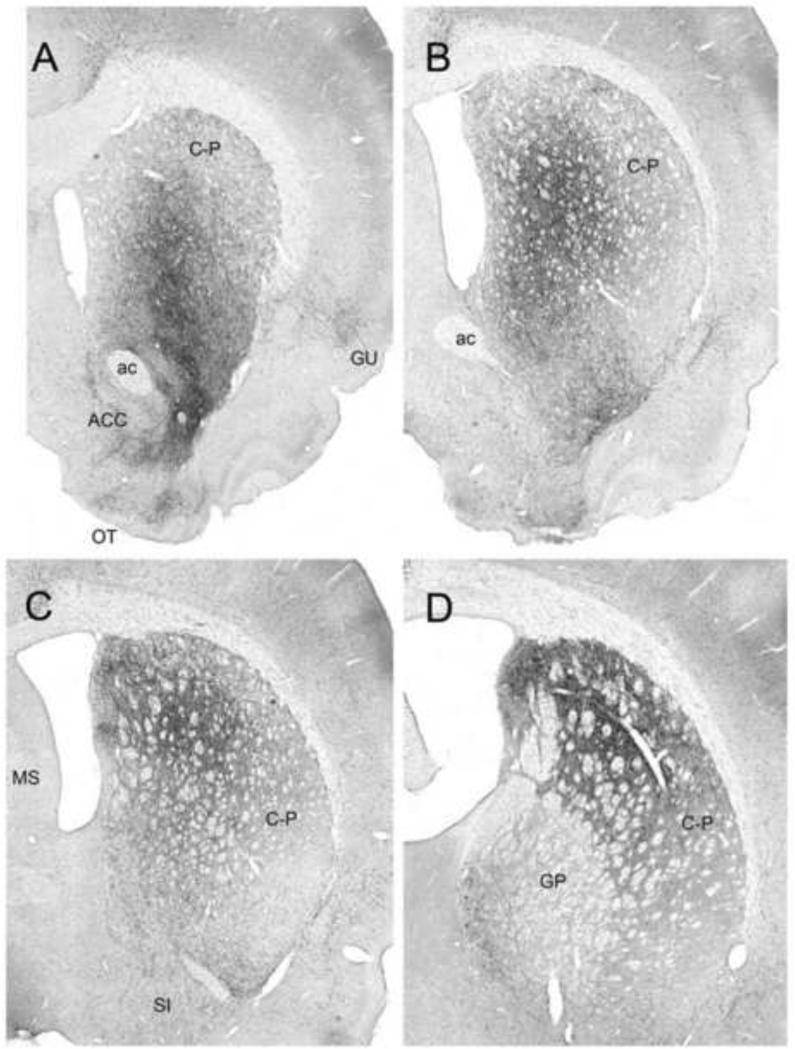
The principal CM targets are anterior and posterior regions of cortex, the claustrum, dorsal striatum, ACC, OT, and the amygdala. There are distinct differences in projections of the rostral and caudal CM (Vertes et al., 2012, 2014). The cortical projections of rostral CM (CMr) are virtually restricted to anterior regions of cortex, with fibers distributing to AGm, AC, PL, the dorsolateral orbital and dorsal agranular insular cortices. By contrast, the caudal CM primarily targets lateral and caudal structures of the cortex including the lateral and dorsolateral orbital cortices, dorsal and posterior agranular insular cortices, the gustatory/visceral cortex, the primary somatosensory and motor cortices, and the perirhinal cortex. Subcortically, rostral CM distributes rostrocaudally throughout C-P, to the anterior core and shell of ACC, and throughout the BLA of amygdala. Figure 11 shows pronounced projections from rostral CM to the dorsal striatum, distributing virtually throughout mid-central and dorsolateral regions of the anterior C-P.
Figure 11.
Low power lightfield photomicrographs of four rostrocaudally aligned (A-D) transverse sections through the rostral forebrain showing patterns of labeling in the dorsal striatum (C-P) produced by a PHA-L injection rostrally in the central medial nucleus of the thalamus. Note dense labeling spread throughout C-P, concentrated rostrally (A,B), dorsoventrally within the mid-central C-P, and caudally (C,D) within the medial/dorsomedial half of C-P. See list for abbreviations. Scale bar for A = 975 μm; for B,C = 1000 μm; for D = 950 μm. Modified from Vertes et al. (2012).
Caudal CM fibers, on the other hand, innervate lateral/ventrolateral regions of C-P and several nuclei of the amygdala including the anterior, lateral, central, medial, cortical, and basal nuclei. With the exception of nucleus accumbens, which essentially only receives input from the rostral CM, rostral and caudal CM target separate parts of the same structures. When outputs are combined, however, CM distributes very widely over the cortex, to the entire dorsal striatum, to the anterior ACC, and to most subnuclei of the amygdala (Vertes et al., 2012, 2014).
CM lies between the dorsal (PV, PT) and ventral (RE, RH) nuclei of the midline thalamus and shares connections with each group -- with additional projections to mainly sensorimotor structures. Specifically, comparable to the ventral midline nuclei, CM distributes widely throughout limbic cortices, substantially targeting the lateral and dorsolateral orbital, dorsal and posterior agranular insular, PL, AC, gustatory/visceral, ectorhinal and perirhinal cortices. Unlike RE/RH, however, CM does not project to the hippocampus and only minimally to the entorhinal cortex. On the other hand, comparable to the dorsal midline nuclei, CM strongly targets the ACC, OT and virtually the entire amygdala -- with pronounced rostral CM projections to the basolateral nucleus of amygdala.
By contrast with the midline nuclei, however, CM distributes massively to the dorsal striatum (Fig. 11), essentially blanketing the C-P. In addition, CM is the source of prominent projections to the primary and secondary motor cortices, and significant but lesser projections to the somatosensory cortex. Accordingly, CM shares “limbic” subcortical and cortical projections with the midline nuclei of thalamus (and with MDm), and sensorimotor projections with the laterally adjacent rostral intralaminar nuclei: PC and CL. In this respect, CM appears to bridge the limbic and sensorimotor domains.
Afferent projections to CM, much like those to PT (see above) have not been examined in detail; no report has systematically described the totality of projections to CM. Loewy and colleagues, however, using retrograde tracers, described brainstem afferents to CM (Krout and Loewy, 2000a, 2000b; Krout et al., 2001, 2002) and some reports, as part of larger investigations of inputs to the thalamus, have traced fibers to CM from various subcortical and cortical structures.
Evidence gained from descriptions of cortical projections to the thalamus (see above) shows that the PFC (or the mPFC) strongly targets CM (Reep et al., 1987; Sesack et al., 1989; Jasmin et al., 2004; Vertes, 2002, 2004; Hoover and Vertes, 2011). Regarding the mPFC, ventral (or limbic) regions of the mPFC (i.e., IL, PL and AC) distribute densely to CM, whereas the dorsally located AGm is the source of weak projections to CM (Vertes, 2002, 2004). By comparison, the orbital and insular cortices distribute moderately to CM with heaviest input to CM arising from the medial orbital cortex (Shi and Cassell, 1998; Jasmin et al., 2004; Hoover and Vertes, 2011).
CM receives a diverse set of afferents from subcortical structures, particularly those of the brainstem. The following subcortical structures have been found to project to CM: the mesencephalic, pontine, and medullary reticular formation, the serotonergic dorsal raphe, median raphe and raphe magnus, the cholinergic PPT and LDT nuclei, the nucleus prepositus hypoglossi, the spinal trigeminal nucleus, the medial and lateral vestibular nuclei, the parabrachial complex, the LC, nucleus incertus, distinct regions of the PAG, deep layers of the SC, the nucleus of Darkschewitsch, and the pars reticulata and pars compacta of the substantia nigra (Peschanski and Besson, 1984; Jones and Yang, 1985; Vertes et al., 1986, 1999, 2010; Yamasaki et al., 1986; Hallanger et al., 1987; Vertes and Martin, 1988; Vertes, 1991; Villanueva et al., 1998; Bester et al., 1999; Groenewegen et al., 1999; Shiroyama et al., 1999; Goto et al., 2001; Krout and Loewy, 2000a, 2000b; Krout et al., 2001, 2002; Olucha-Bordonau et al., 2003). In addition, CM receives input from deep cerebellar nuclei, the supramammillary nucleus, zona incerta, and the reticular thalamic nucleus (Haroian et al., 1981; Vertes, 1992; Kolmac and Mitrofanis, 1997; Power et al., 1999; Power and Mitrofanis, 2001).
3.12 Central medial nucleus: Functional considerations
As indicated, although partially overlapping, the projections of CM differ from those of the other intralaminar nuclei, notably CM is a source of rather prominent projections to limbic sites (Van der Werf et al., 2002; Vertes et al., 2012). Accordingly, the functions of CM would be expected to differ from those of other intralaminar (ILt) groups and might be more aligned with functions attributed to the midline nuclei. Unfortunately, the functions of CM have not for the most part been examined separately from those of the adjacent paracentral and central lateral nuclei. Most reports examining intralaminar functions have, in fact, focused on PC and CL, largely to the exclusion of CM. This is likely due to the greater accessibility of PC/CL, than CM, for behavioral manipulations.
The functions of the rostral intralaminar (ILt) complex in large measure parallel those of its main targets, namely, the prefrontal cortex and the dorsal striatum -- and accordingly are generally described with reference to these targets. In early studies, Mair and colleagues (Burk and Mair, 1998; Bailey and Mair, 2005) demonstrated that rostral ILt lesions produced significant deficits on a delayed match to position task which is sensitive to PFC damage (Mair et al., 1998). In a subsequent report, using the same task, Mair and Hembrook (2008) showed that the reversible suppression of the rostral ILt with muscimol impaired performance on the delayed match to position task. Conversely, procedures that either reduced GABA tone (with FG-7142) to the intralaminar nuclei and or activated (with orexin A) them improved performance on the task. It was further demonstrated electrical stimulation of the intralaminar thalamus at low currents similarly enhanced performance on this task, when applied at the start of the delay period or just prior to the selection of appropriate responses. They concluded that ILt neurons “carry response-related information across brief memory delays and mediate memory-guided responding” (Mair and Hembrook, 2008). Consistent with this, intralaminar neurons in the monkey have been shown to respond selectively during the delay period of delayed-response tasks signaling the location of visual targets (Wyder et al., 2003, 2004).
It has further been demonstrated CM/intralaminar thalamus plays a role in striatal-dependent activities. For instance, Mitchell and Dalrymple-Alford (2006) described a double dissociation between the effects of ILt or anterior thalamic (ATh) lesions on striatal- or hippocampal-dependent tasks; namely, on an (egocentric) working memory response-related task or on an (allocentric) radial arm maze task. Specifically, only ILt lesions impaired performance on the non-hippocampal-dependent working memory task, whereas ATh lesions produced deficits solely on the radial arm maze task. Hembrook and Mair (2011) similarly found no association between ILt impairments and spatial learning. ILt lesions significantly disrupted performance and on a visuospatial reaction time task which is sensitive to striatal damage (Mair et al., 2002), but not on a delayed non-match to sample radial arm maze task responsive to hippocampal alterations (McDonald and White, 1993; Mair et al., 1998).
In contrast to the foregoing, Wolff et al. (2008) found that ILt lesions did not disrupt performance on an egocentric reference memory task in the water maze -- despite the fact that dorsal caudate lesions produced severe impairments on a land-based version of this task (Mitchell and Hall, 1988). Further, Moreau et al. (2013) reported that rats with ILt lesions exhibited no deficits on a visual discrimination task in the water maze, which is sensitive to striatal disruption (Packard and McGaugh, 1992). Although several factors could account for the differing results, including subtle changes in task demands, a most likely explanation may be that sites of the intralaminar lesions differed across the various studies. This, in turn, could affect separate, and perhaps non-overlapping, target zones of different sectors of the dorsal striatum. Moreau et al. (2013) suggested this stating: “Functional heterogeneity within the striatum and across ILN regions is, however, one complication that is neglected by a simple ILN-striatal perspective”.
Due in part to the close proximity of the anterior nuclei (ATh) and the intralaminar nuclei of the rostral thalamus, several reports (Mair et al., 2003; Bailey and Mair, 2005; Mitchell and Dalrymple-Alford, 2005, 2006, Wolff et al., 2008; Lopez et al., 2009; Moreau et al., 2013) have compared the differing functions of these thalamic regions showing that the anterior nuclei, with connections to the extended hippocampal system, are involved in (allocentric) spatial working memory, whereas the intralaminar nuclei serve no role in spatial memory but instead participate in non-hippocampal-related functions such as delayed response activity and egocentric working memory. Despite this, several studies have described deficits in spatial memory associated with ILt lesions. Lopez et al. (2009) reported that while rostral ILt lesions did not alter acquisition or short term (5 day) retrieval in a hippocampal-dependent spatial memory task in the water maze, such lesions did impair long term (25 day) retrieval on this task – or remote memory. As mentioned above, Hembrook and Mair (2011) recently showed that ILt lesions did not disrupt spatial memory performance on a radial arm task, but altered performance on a striatal dependent visuospatial task. However, they stated that relatively ‘large’ rostral intralaminar lesions produced deficits on a delayed non-match to sample radial arm maze task. Earlier studies which found spatial memory deficits following ILt disruption also involved rather large intralaminar lesions that included the central medial nucleus (Mair et al., 1998; Burk and Mair, 2001). This further suggests that the central medial thalamus is more functionally aligned with the midline thalamus than with other intralaminar nuclei.
4. Conclusion
The midline nuclei of the thalamus, per se, consist of the paraventricular, paratenial, reuniens and rhomboid nuclei. As described, other cell groups lying on or close to the midline of thalamus share properties with the midline nuclei. They notably include the medial division of the medial dorsal nucleus (MDm) and the central medial nucleus. The anatomical/functional characteristics of MDm and CM are much more similar to those of the midline nuclei than to those of lateral divisions of MD or lateral nuclei of the rostral intralaminar complex, respectively (Figs. 12, 13). Although we did not review the properties of the intermediodorsal nucleus (IMD) and the interanteromedial nucleus (IAM), which also lie along the midline of thalamus, previous reports (Van der Werf et al., 2002; Groenewegen and Witter, 2004) have also shown them to be anatomically and functionally linked to the limbic system.
Figure 12.
Summary diagram comparing and contrasting the main projection sites of the paraventricular PVP, paratenial (PT), reuniens (RE) and rhomboid (RH) nuclei of the midline thalamus to the cortex, basal forebrain, amygdala and hypothalamus. See list for abbreviations.
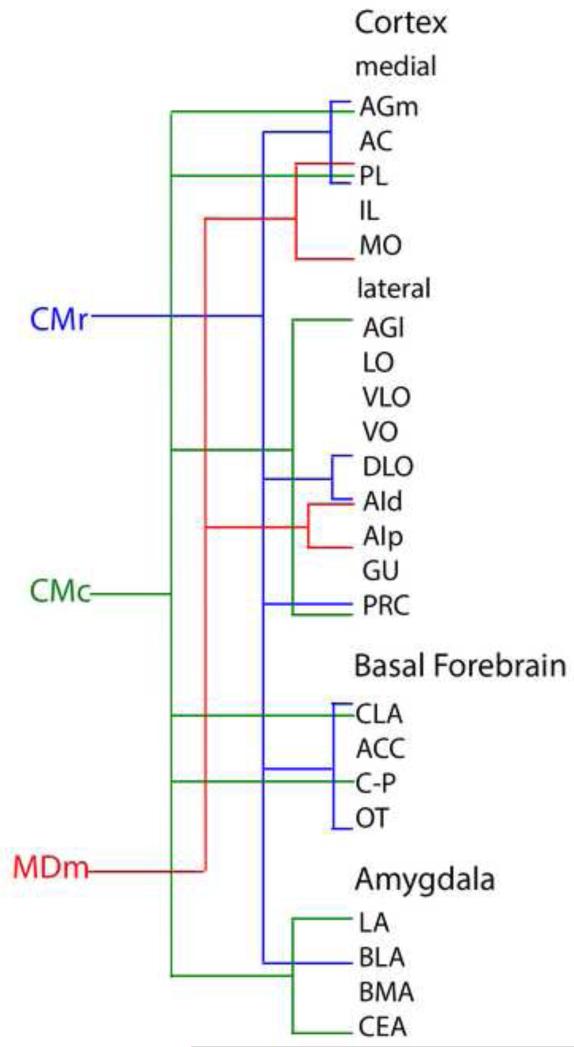
Figure 13.
Summary diagram comparing and contrasting the main projection sites of the rostral (CMr) and caudal (CMc) central medial nucleus and the medial division of the mediodorsal nucleus (MDm) of thalamus to the cortex, basal forebrain and amygdala. See list for abbreviations.
Accordingly, nuclei lying dorsoventrally along the midline/paramidline of the thalamus and extending virtually throughout the thalamus basically constitute the bulk of the “limbic thalamus’ (Fig. 1). This group of ‘limbic’ thalamic nuclei are distinctive in that they receive a wide array of afferents from limbic-related sites of the brainstem and forebrain, project almost exclusively to limbic subcortical and cortical structures (and the dorsal striatum) (Figs. 12, 13) and subserve a range of limbic-associated functions. The dorsal midline nuclei (PV, PT) are primarily involved in affective functions, the ventral midline nuclei (RE, RH) in cognitive functions.
Highlights.
The thalamus was divided into three main groups: sensorimotor nuclei, limbic nuclei and nuclei bridging these two domains.
The reuniens and rhomboid of the midline thalamus were linked to cognitive functions.
The paraventricular and paratenial nuclei of the midline thalamus were linked to affective functions.
The characteristics of the mediodorsal and central medial nuclei are very similar to those of the midline nuclei.
Nuclei extending dorsoventrally throughout the midline of the thalamus basically constitute the ‘limbic thalamus’.
Acknowledgements
This work was supported by NIMH grant MH099590 to RPV.
Abbreviations
- 3V
third ventricle
- ac
anterior commissure
- AC
anterior cingulate cortex
- ACC
nucleus accumbens
- ACCc
nucleus accumbens, core division
- ACCs
nucleus accumbens, shell division
- ACo
anterior commissure
- AD
anterior dorsal nucleus of thalamus
- AGl
lateral agranular (primary motor) cortex
- AGm
medial agranular (secondary motor) cortex
- AHN
anterior hypothalamic nucleus
- AI
agranular insular cortex
- AId
dorsal agranular insular cortex
- AIp
posterior agranular insular cortex
- AMd
anteromedial nucleus of thalamus, dorsal division
- ATh
anterior thalamus
- AV
anteroventral nucleus of thalamus
- BLA
basolateral nucleus of amygdala
- BMA
basomedial nucleus of amygdala
- BST
bed nucleus of stria terminalis
- CA1
field CA1 of Ammon's horn
- CA3
field CA3 of Ammon's horn
- CB
cingulum bundle
- cc
corpus callosum
- CEA
central nucleus of the amygdala
- CEAc
central nucleus of amygdala, capsular division
- CEAl
central nucleus of amygdala, lateral division
- CL
central lateral nucleus of thalamus
- CLA
claustrum
- CM
central medial nucleus of thalamus
- CMc
caudal central medial nucleus of thalamus
- CMr
rostral central medial nucleus of thalamus
- C-P
caudate-putamen
- DG
dentate gyrus
- DLG
dorsal lateral geniculate nucleus of thalamus
- DLO
dorsal lateral orbital cortex
- DMH
dorsomedial hypothalamic nucleus
- DR
dorsal raphe nucleus
- ECl
entorhinal cortex, lateral division
- fx
fornix
- GP
globus pallidus
- GPi
globus pallidus, internal segment
- GU
gustatory cortex
- HF
hippocampal formation
- IAM
interanteromedial nucleus of thalamus
- ic
internal capsule
- IGL
intergeniculate leaflet
- IL
infralimbic cortex
- ILt
intralaminar thalamus
- IMD
intermediodorsal nucleus of thalamus
- LA
lateral nucleus of amygdala
- LC
locus coeruleus
- LD
lateral dorsal nucleus of thalamus
- LDT
laterodorsal tegmental nucleus
- LGN
lateral geniculate complex
- LH
lateral habenula
- LHA
lateral hypothalamic area
- LO
lateral orbital cortex
- LP
lateral posterior nucleus of thalamus
- LS
lateral septum
- MD
mediodorsal nucleus of thalamus
- MDc
mediodorsal nucleus of thalamus, central division
- MDl
mediodorsal nucleus of thalamus, lateral division
- MDm
mediodorsal nucleus of thalamus, medial division
- MDpl
mediodorsal nucleus of thalamus, paralamellar division
- MGN
medial geniculate nucleus of thalamus
- MO
medial orbital cortex
- mPFC
medial prefrontal cortex
- MR
median raphe nucleus
- MS
medial septum
- mt
mammillothalamic tract
- OC
occipital cortex
- OT
olfactory tubercle
- PAG
periaqueductal gray matter
- PB
parabrachial nucleus
- PC
paracentral nucleus of thalamus
- PF
parafascicular nucleus of thalamus
- PFC
prefrontal cortex
- PL
prelimbic cortex
- PO
posterior nucleus of thalamus
- POST
postsubiculum
- PPT
pedunculopontine tegmental nucleus
- PRC
perirhinal cortex
- PT
paratenial nucleus of thalamus
- PV
paraventricular nucleus of thalamus
- RE
nucleus reuniens of thalamus
- RF
reticular formation
- RH
rhomboid nucleus of thalamus
- RSP
retrosplenial cortex
- RT
reticular nucleus of thalamus
- SC
superior colliculus
- SCN
suprachiasmatic nucleus of hypothalamus
- SI
substantia innominata
- slm
straum lacunosum-moleculare of CA1 of hippocampus
- sm
stria medullaris
- SMT
submedial nucleus of thalamus
- SNc
substantia nigra, pars compacta
- SPF
subparafascicular nucleus of thalamus
- SUB
subiculum
- SUBv
ventral subiculum
- SUM
supramammillary nucleus of hypothalamus
- TE
temporal cortex
- TTd
taenia tecta, dorsal division
- VA
ventral anterior nucleus of thalamus
- VAL
ventral anterior lateral complex of thalamus
- VL
ventral lateral nucleus of thalamus
- VLG
ventral lateral genciulate nucleus of thalamus
- VLO
ventrolateral orbital cortex
- VM
ventral medial nucleus of thalamus
- VO
ventral orbital cortex
- VPL
ventral posterolateral nucleus of thalamus
- VPM
ventral posteromedial nucleus of thalamus
- VTA
ventral tegmental area
- ZI
zona incerta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aggleton JP, Dumont JR, Warburton EC. Unraveling the contributions of the diencephalon to recognition memory: a review. Learn. Mem. 2011;18:384–400. doi: 10.1101/lm.1884611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Mendoza J, Escobar C. Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience. 2007;144:344–355. doi: 10.1016/j.neuroscience.2006.08.064. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Mair RG. Lesions of specific and nonspecific thalamic nuclei affect prefrontal cortex-dependent aspects of spatial working memory. Behav. Neurosci. 2005;119:410–419. doi: 10.1037/0735-7044.119.2.410. [DOI] [PubMed] [Google Scholar]
- Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J. Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J. Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J. Comp. Neurol. 1990;299:187–228. doi: 10.1002/cne.902990206. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Zhang DX. Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neuroscience. 1999;92:15–26. doi: 10.1016/s0306-4522(98)00712-x. [DOI] [PubMed] [Google Scholar]
- Bester H, Bourgeais L, Villanueva L, Besson JM, Bernard JF. Differential projections to the intralaminar and gustatory thalamus from the parabrachial area: a PHAL study in the rat. J. Comp. Neurol. 1999;405:421–499. [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J. Neuroendocrinol. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb. Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- Bokor H, Csáki A, Kocsis K, Kiss J. Cellular architecture of the nucleus reuniens thalami and its putative aspartatergic/glutamatergic projection to the hippocampus and medial septum in the rat. Eur. J. Neurosci. 2002;16:1227–1239. doi: 10.1046/j.1460-9568.2002.02189.x. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Reversible hippocampal lesions disrupt water maze performance during both recent and remote memory tests. Learn. Mem. 2006;13:187–191. doi: 10.1101/lm.134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp. Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Bubser M, Deutch AY. Thalamic paraventricular nucleus neurons collateralize to innervate the prefrontal cortex and nucleus accumbens. Brain Res. 1998;787:304–310. doi: 10.1016/s0006-8993(97)01373-5. [DOI] [PubMed] [Google Scholar]
- Bubser M, Deutch AY. Stress induces Fos expression in neurons of the thalamic paraventricular nucleus that innervate limbic forebrain sites. Synapse. 1999;32:13–22. doi: 10.1002/(SICI)1098-2396(199904)32:1<13::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Burk JA, Mair RG. Effects of intralaminar thalamic lesions on sensory attention and motor intention in the rat: A comparison with lesions involving frontal cortex and hippocampus. Behav. Brain Research. 2001;123:49–63. doi: 10.1016/s0166-4328(01)00202-9. [DOI] [PubMed] [Google Scholar]
- Burk JA, Mair RG. Thalamic amnesia reconsidered: excitotoxic lesions of the intralaminar nuclei, but not the mediodorsal nucleus, disrupt place delayed matching-to-sample performance in rats (Rattus norvegicus). Behav. Neurosci. 1998;112:54–67. [PubMed] [Google Scholar]
- Canteras NS, Goto M. Connections of the precommissural nucleus. J. Comp. Neurol. 1999;408:23–45. doi: 10.1002/(sici)1096-9861(19990524)408:1<23::aid-cne3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J. Comp. Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J. Comp. Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J. Comp. Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cassel JC, Pereira de Vasconcelos A, Loureiro M, Cholvin T, Dalrymple-Alford JC, Vertes RP. The reuniens and rhomboid nuclei: neuroanatomy, electrophysiological characteristics and behavioral implications. Prog. Neurobiol. 2013;111:34–52. doi: 10.1016/j.pneurobio.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavdar S, Onat FY, Cakmak YO, Yananli HR, Gülçebi M, Aker R. The pathways connecting the hippocampal formation, the thalamic reuniens nucleus and the thalamic reticular nucleus in the rat. J. Anat. 2008;212:249–256. doi: 10.1111/j.1469-7580.2008.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastrette N, Pfaff DW, Gibbs RB. Effects of daytime and nighttime stress on Fos like immunoreactivity in the paraventricular nucleus of the hypothalamus, the habenula, and the posterior paraventricular nucleus of the thalamus. Brain Res. 1991;563:339–344. doi: 10.1016/0006-8993(91)91559-j. [DOI] [PubMed] [Google Scholar]
- Chen S, Su H-S. Afferent connections of the thalamic paraventricular and parataenial nuclei in the rat—A retrograde tracing study with iontophoretic application of fluoro-gold. Brain Res. 1990;522:1–6. doi: 10.1016/0006-8993(90)91570-7. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167:11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC. Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience. 2012;210:243–248. doi: 10.1016/j.neuroscience.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholvin T, Loureiro M, Cassel R, Cosquer B, Geiger K, De Sa Nogueira D, Raingard H, Robelin L, Kelche C, Pereira de Vasconcelos A, Cassel JC. The ventral midline thalamus contributes to strategy shifting in a memory task requiring both prefrontal cortical and hippocampal functions. J. Neurosci. 2013;33:8772–8783. doi: 10.1523/JNEUROSCI.0771-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav. Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. Hippocampus and remote spatial memory in rats. Hippocampus. 2005;15:260–272. doi: 10.1002/hipo.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde F, Audinat E, Maire-Lepoivre E, Crepel F. Afferent connections of the medial frontal cortex of the rat: A study using retrograde transport of fluorescent dyes. I. Thalamic afferents. Brain Res. Bull. 1990;24:341–354. doi: 10.1016/0361-9230(90)90088-h. [DOI] [PubMed] [Google Scholar]
- Condé F, Maire-Lepoivre E, Audinat E, Crépel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J. Comp. Neurol. 1995;352:567, 593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- Cornwall J, Phillipson OT. Afferent projections to the dorsal thalamus of the rat as shown by retrograde lectin transport. II. The midline nuclei. Brain Res. Bull. 1988;21:147–161. doi: 10.1016/0361-9230(88)90227-4. [DOI] [PubMed] [Google Scholar]
- Cross L, Brown MW, Aggleton JP, Warburton EC. The medial dorsal thalamic nucleus and the medial prefrontal cortex of the rat function together to support associative recognition and recency but not item recognition. Learn. Mem. 2012;20:41–50. doi: 10.1101/lm.028266.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Zaborszky L. Organization of ascending hypothalamic projections to the rostral forebrain with special reference to the innervation of cholinergic projection neurons. J. Comp. Neurol. 1991;306:631–667. doi: 10.1002/cne.903060408. [DOI] [PubMed] [Google Scholar]
- Davoodi FG, Motamedi F, Naghdi N, Akbari E. Effect of reversible inactivation of the reuniens nucleus on spatial learning and memory in rats using Morris water maze task. Behav. Brain Res. 2009;198:130–135. doi: 10.1016/j.bbr.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Davoodi FG, Motamedi F, Akbari E, Ghanbarian E, Jila B. Effect of reversible inactivation of reuniens nucleus on memory processing in passive avoidance task. Behav. Brain Res. 2011;221:1–6. doi: 10.1016/j.bbr.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Menetrey A, Thierry AM. Indirect nucleus accumbens input to the prefrontal cortex via the substantia nigra pars reticulata: A combined anatomical and electrophysiological study in the rat. Neuroscience. 1994;61:533–545. doi: 10.1016/0306-4522(94)90432-4. [DOI] [PubMed] [Google Scholar]
- Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. Eur. J. Neurosci. 2000;12:4457–4466. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, Witter MP. Projections from the nucleus reuniens thalami to the entorhinal cortex, hippocampal field CA1, and the subiculum in the rat arise from different populations of neurons. J. Comp. Neurol. 1996;364:637–650. doi: 10.1002/(SICI)1096-9861(19960122)364:4<637::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Lopes da Silva FH, Witter MP. Nucleus reuniens thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. J. Neurosci. 1997;17:5640–5650. doi: 10.1523/JNEUROSCI.17-14-05640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, Morris RG, Witter MP. Neurotoxic lesions of the thalamic reuniens or mediodorsal nucleus in rats affect non-mnemonic aspects of watermaze learning. Brain Struct. Funct. 2009;213:329–342. doi: 10.1007/s00429-008-0200-6. [DOI] [PubMed] [Google Scholar]
- Eleore L, López-Ramos JC, Guerra-Narbona R, Delgado-García JM. Role of reuniens nucleus projections to the medial prefrontal cortex and to the hippocampal pyramidal CA1 area in associative learning. PLoS One. 2011;6:e23538. doi: 10.1371/journal.pone.0023538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behav. Brain Res. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- Erro ME, Lanciego JL, Gimenez-Amaya JM. Re-examination of the thalamostriatal projections in the rat with retrograde tracers. Neurosci. Res. 2002;42:45–55. doi: 10.1016/s0168-0102(01)00302-9. [DOI] [PubMed] [Google Scholar]
- Ferino F, Thierry AM, Glowinski J. Anatomical and electrophysiological evidence for a direct projection from Ammon's horn to the medial prefrontal cortex in the rat. Exp. Brain Res. 1987;65:421–426. doi: 10.1007/BF00236315. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Goto M, Swanson LW. Axonal projections from the parasubthalamic nucleus. J. Comp. Neurol. 2004;469:581–607. doi: 10.1002/cne.11036. [DOI] [PubMed] [Google Scholar]
- Goto M, Swanson LW, Canteras NS. Connections of the nucleus incertus. Journal of Comparative Neurology. 2001;438:86–122. doi: 10.1002/cne.1303. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Witter MP. Thalamus. In: Paxinos G, editor. The Rat Nervous System. third ed. Academic Press; San Diego: 2004. pp. 408–441. [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AHM. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: Evidence for a parallel organization. Prog. Brain Res. 1990;85:95–118. doi: 10.1016/s0079-6123(08)62677-1. [DOI] [PubMed] [Google Scholar]
- Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. Journal of Comparative Neurology. 1987;262:105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- Hallock HL, Wang A, Shaw CL, Griffin AL. Transient inactivation of the thalamic nucleus reuniens and rhomboid nucleus produces deficits of a working-memory dependent tactile-visual conditional discrimination task. Behav. Neurosci. 2013;127:860–866. doi: 10.1037/a0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur. J. Neurosci. 2009;29:802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Haroian AJ, Massopust LC, Young PA. Cerebellothalamic projections in the rat: An autoradiographic and degeneration study. J. Comp. Neurol. 1981;197:217–236. doi: 10.1002/cne.901970205. [DOI] [PubMed] [Google Scholar]
- Hembrook JR, Mair RG. Lesions of reuniens and rhomboid thalamic nuclei impair radial maze win-shift performance. Hippocampus. 2011;21:815–826. doi: 10.1002/hipo.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembrook JR, Onos KD, Mair RG. Inactivation of ventral midline thalamus produces selective spatial delayed conditional discrimination impairment in the rat. Hippocampus. 2012;22:853–860. doi: 10.1002/hipo.20945. [DOI] [PubMed] [Google Scholar]
- Herkenham M. The connections of the nucleus reuniens thalami: Evidence for a direct thalamo-hippocampal pathway in the rat. J. Comp. Neurol. 1978;177:589–610. doi: 10.1002/cne.901770405. [DOI] [PubMed] [Google Scholar]
- Heydendael W, Sharma K, Iyer V, Luz S, Piel D, Beck S, Bhatnagar S. Orexins/hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology. 2011;152:4738–4752. doi: 10.1210/en.2011-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks RR, Huerta MF. Differential thalamic connectivity of rostral and caudal parts of cortical area Fr2 in rats. Brain Res. 1991;568:325–329. doi: 10.1016/0006-8993(91)91420-6. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Projections of the medial orbital and ventral orbital cortex in the rat. J. Comp. Neurol. 2011;519:3766–3801. doi: 10.1002/cne.22733. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Collateral projections from nucleus reuniens of thalamus to hippocampus and medial prefrontal cortex in the rat: a single and double retrograde fluorescent labeling study. Brain Struct. Funct. 2012;217:191–209. doi: 10.1007/s00429-011-0345-6. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Kirouac GJ, Zubieta JK, Bhatnagar S. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front. Behav. Neurosci. 2014;8:73. doi: 10.3389/fnbeh.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PR, Aggleton JP. Neurotoxic lesions of the dorsomedial thalamus impair the acquisition but not the performance of delayed matching to place by rats: a deficit in shifting response rules. J. Neurosci. 1998;18:10045–10052. doi: 10.1523/JNEUROSCI.18-23-10045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J. Comp. Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Igelstrom KM, Herbison AE, Hyland BI. Enhanced c-Fos expression in superior colliculus, paraventricular thalamus and septum during learning of cue-reward association. Neuroscience. 2010;168:706–714. doi: 10.1016/j.neuroscience.2010.04.018. [DOI] [PubMed] [Google Scholar]
- James MH, Dayas CV. What about me...? The PVT: a role for the paraventricular thalamus (PVT) in drug-seeking behavior. Front. Behav. Neurosci. 2013;7:18. doi: 10.3389/fnbeh.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Charnley JL, Jones E, Levi EM, Yeoh JW, Flynn JR, Smith DW, Dayas CV. Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS One. 2010;5:e12980. doi: 10.1371/journal.pone.0012980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV. Propensity to “relapse” following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience. 2011;199:235–242. doi: 10.1016/j.neuroscience.2011.09.047. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Burkey AR, Granato A, Ohara PT. Rostral agranular insular cortex and pain areas of the central nervous system: a tract-tracing study in the rat. J. Comp. Neurol. 2004;468:425–440. doi: 10.1002/cne.10978. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J. Comp. Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J. Comp. Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Kawano J, Krout KE, Loewy AD. Suprachiasmatic nucleus projections to the paraventricular thalamic nucleus of the rat. Thal. Rel. Syst. 2001;1:197–202. [Google Scholar]
- Kirouac GJ, Parsons MP, Li S. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2005;1059:179–188. doi: 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S. Innervation of the paraventricular nucleus of the thalamus from cocaine- and amphetamine-regulated transcript (CART) containing neurons of the hypothalamus. J. Comp. Neurol. 2006;497:155–165. doi: 10.1002/cne.20971. [DOI] [PubMed] [Google Scholar]
- Kolmac CI, Mitrofanis J. Organisation of the reticular thalamic projection to the intralaminar and midline nuclei in rats. J. Comp. Neurol. 1997;377:165–178. doi: 10.1002/(sici)1096-9861(19970113)377:2<165::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J. Comp. Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Krout KE, Loewy AD. Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol. 2000a;428:475–494. doi: 10.1002/1096-9861(20001218)428:3<475::aid-cne6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Krout KE, Loewy AD. Periaqueductal gray matter projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol. 2000b;424:111–141. doi: 10.1002/1096-9861(20000814)424:1<111::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Krout KE, Loewy AD, Westby GW, Redgrave P. Superior colliculus projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol. 2001;431:198–216. doi: 10.1002/1096-9861(20010305)431:2<198::aid-cne1065>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Krout KE, Belzer RE, Loewy AD. Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol. 2002;448:53–101. doi: 10.1002/cne.10236. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Price JL. Synaptic organization of projections from basal forebrain structures to the mediodorsal thalamic nucleus of the rat. J. Comp. Neurol. 1991a;303:513–533. doi: 10.1002/cne.903030402. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Price JL. Ultrastructure and synaptic organization of axon terminals from brainstem structures to the mediodorsal thalamic nucleus of the rat. J. Comp. Neurol. 1991b;313:539–552. doi: 10.1002/cne.903130313. [DOI] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–446. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Leonard CM. The prefrontal cortex of the rat. I. Cortical projections of the mediodorsal nucleus. II. Efferent connections. Brain Res. 1969;12:321–343. doi: 10.1016/0006-8993(69)90003-1. [DOI] [PubMed] [Google Scholar]
- Leonard CM. The connections of the dorsomedial nuclei. Brain Behav. Evol. 1972;6:524–541. doi: 10.1159/000123730. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J. Comp. Neurol. 2008;506:263–287. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct. Funct. 2012;217:257–273. doi: 10.1007/s00429-011-0360-7. [DOI] [PubMed] [Google Scholar]
- Lopez J, Wolff M, Lecourtier L, Cosquer B, Bontempi B, Dalrymple-Alford J, Cassel JC. The intralaminar thalamic nuclei contribute to remote spatial memory. J. Neurosci. 2009;29:3302–3306. doi: 10.1523/JNEUROSCI.5576-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J, Herbeaux K, Cosquer B, Engeln M, Muller C, Lazarus C, Kelche C, Bontempi B, Cassel JC, Pereira de Vasconcelos AP. Context-dependent modulation of hippocampal and cortical recruitment during remote spatial memory retrieval. Hippocampus. 2012;22:827–841. doi: 10.1002/hipo.20943. [DOI] [PubMed] [Google Scholar]
- Loureiro M, Cholvin T, Lopez J, Merienne N, Latreche A, Cosquer B, Geiger K, Kelche C, Cassel JC, Pereira de Vasconcelos A. The ventral midline thalamus (reuniens and rhomboid nuclei) contributes to the persistence of spatial memory in rats. J. Neurosci. 2012;32:9947–9959. doi: 10.1523/JNEUROSCI.0410-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailly P, Aliane V, Groenewegen HJ, Haber SN, Deniau JM. The rat prefrontostriatal system analyzed in 3D: evidence for multiple interacting functional units. J. Neurosci. 2013;33:5718–5727. doi: 10.1523/JNEUROSCI.5248-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair RG, Hembrook JR. Memory enhancement with event-related stimulation of the rostral intralaminar thalamic nuclei. J. Neurosci. 2008;28:14293–14300. doi: 10.1523/JNEUROSCI.3301-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair RG, Burk JA, Porter MC. Lesions of the frontal cortex, hippocampus, and intralaminar thalamic nuclei have distinct effects on remembering in rats. Behav. Neurosci. 1998;112:772–792. doi: 10.1037//0735-7044.112.4.772. [DOI] [PubMed] [Google Scholar]
- Mair RG, Koch JK, Newman JB, Howard JR, Burk JA. A double dissociation within striatum between serial reaction time and radial maze delayed nonmatching performance in rats. J. Neurosci. 2002;22:6756–6765. doi: 10.1523/JNEUROSCI.22-15-06756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair RG, Burk JA, Porter MC. Impairment of radial maze delayed nonmatching after lesions of anterior thalamus and parahippocampal cortex. Behav. Neurosci. 2003;117:596–605. doi: 10.1037/0735-7044.117.3.596. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Furlong TM, McNally GP. Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. J. Neurosci. 2010;30:14102–14115. doi: 10.1523/JNEUROSCI.4079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Boutrel B. Orexin/hypocretin (Orx/Hcrt) transmission and drug-seeking behavior: is the paraventricular nucleus of the thalamus (PVT) part of the drug seeking circuitry? Front. Behav. Neurosci. 2012;6:75. doi: 10.3389/fnbeh.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Leos BN, Kerr TM, Weiss F. Administration of orexin/hypocretin (orx/hert) in the paraventricular nucleus of the thalamus (PVT) produces cocaine-seeking: comparison with natural reward-seeking. Soc. Neurosci. Abstr. 2011;69.10 [Google Scholar]
- Matzeu A, Zamora-Martinez ER, Martin-Fardon R. The paraventricular nucleus of the thalamus is recruited by both natural rewards and drugs of abuse: recent evidence of a pivotal role for orexin/hypocretin signaling in this thalamic nucleus in drug-seeking behavior. Front. Behav. Neurosci. 2014;8:117. doi: 10.3389/fnbeh.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan GM, Robbins TW, Everitt BJ. Effects of medial dorsal thalamic and ventral pallidal lesions on the acquisition of a conditioned place preference: further evidence for the involvement of the ventral striatopallidal system in reward-related processes. Neuroscience. 1993;52:605–620. doi: 10.1016/0306-4522(93)90410-h. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav. Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Vertes RP. Afferent projections to nucleus reuniens of the thalamus. J. Comp. Neurol. 2004;480:115–42. doi: 10.1002/cne.20342. [DOI] [PubMed] [Google Scholar]
- Menzie JM, Hoover WB, Vertes RP. Afferent projections to the paraventricular nucleus of the dorsal midline thalamus. Soc. Neurosci. Abstr. 2010;404.13 [Google Scholar]
- Meredith GE, Wouterlood FG. Hippocampal and midline thalamic fibers and terminals in relation to the choline acetyltransferase-immunoreactive neurons in nucleus accumbens of the rat: a light and electron microscopic study. J. Comp. Neurol. 1990;296:204–221. doi: 10.1002/cne.902960203. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Dalrymple-Alford JC. Dissociable memory effects after medial thalamus lesions in the rat. Eur. J. Neurosci. 2005;22:973–985. doi: 10.1111/j.1460-9568.2005.04199.x. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Dalrymple-Alford JC. Lateral and anterior thalamic lesions impair independent memory systems. Learn. Mem. 2006;13:388–396. doi: 10.1101/lm.122206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS, Chakraborty S. What does the mediodorsal thalamus do? Front. Syst. Neurosci. 2013;7:37. doi: 10.3389/fnsys.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JA, Hall G. Caudate-putamen lesions in the rat may impair or potentiate maze learning depending upon availability of stimulus cues and relevance of response cues. Q. J. Exp. Psychol. B. 1988;40:243–258. [PubMed] [Google Scholar]
- Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J. Comp. Neurol. 1995;359:221–238. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- Moore RY, Weis R, Moga MM. Efferent projections of the intergeniculate leaflet and the ventral lateral geniculate nucleus in the rat. J. Comp. Neurol. 2000;420:398–418. doi: 10.1002/(sici)1096-9861(20000508)420:3<398::aid-cne9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Moreau PH, Tsenkina Y, Lecourtier L, Lopez J, Cosquer B, Wolff M, Dalrymple-Alford J, Cassel JC. Lesions of the anterior thalamic nuclei and intralaminar thalamic nuclei: place and visual discrimination learning in the water maze. Brain Struct. Funct. 2013;218:657–667. doi: 10.1007/s00429-012-0419-0. [DOI] [PubMed] [Google Scholar]
- Morin LP, Meyer-Bernstein EL. The ascending serotonergic system in the hamster: comparison with projections of the dorsal and median raphe nuclei. Neuroscience. 1999;91:81–105. doi: 10.1016/s0306-4522(98)00585-5. [DOI] [PubMed] [Google Scholar]
- Naber PA, Witter MP. Subicular efferents are organized mostly as parallel projections: a double-labeling, retrograde-tracing study in the rat. J. Comp. Neurol. 1998;393:284–297. [PubMed] [Google Scholar]
- Nakahara K, Fukui K, Murakami N. Involvement of thalamic paraventricular nucleus in the anticipatory reaction under food restriction in the rat. J. Vet. Med. Science. 2004;66:1297–1300. doi: 10.1292/jvms.66.1297. [DOI] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Impaired object recognition with increasing levels of feature ambiguity in rats with perirhinal cortex lesions. Behav. Brain Res. 2004;148:79–91. doi: 10.1016/s0166-4328(03)00176-1. [DOI] [PubMed] [Google Scholar]
- Novak CM, Harris JA, Smale L, Nunez AA. Suprachiasmatic nucleus projections to the paraventricular thalamic nucleus in nocturnal rats (Rattus norvegicus) and diurnal nile grass rats (Arviacanthis niloticus). Brain Res. 2000;874:147–157. doi: 10.1016/s0006-8993(00)02572-5. [DOI] [PubMed] [Google Scholar]
- Ohtake T, Yamada H. Efferent connections of the nucleus reuniens and the rhomboid nucleus in the rat: an anterograde PHA-L tracing study. Neurosci. Res. 1989;6:556–568. doi: 10.1016/0168-0102(89)90044-8. [DOI] [PubMed] [Google Scholar]
- Olucha-Bordonau FE, Teruel V, Barcia-González J, Ruiz-Torner A, Valverde Navarro AA, Martínez-Soriano F. Cytoarchitecture and efferent projections of the nucleus incertus of the rat. J. Comp. Neurol. 2003;464:62–97. doi: 10.1002/cne.10774. [DOI] [PubMed] [Google Scholar]
- Otake K. Cholecystokinin and substance P immunoreactive projections to the paraventricular thalamic nucleus in the rat. Neurosci. Res. 2005;51:383–394. doi: 10.1016/j.neures.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Otake K, Nakamura Y. Sites of origin of corticotropin-releasing factor-like immunoreactive projection fibers to the paraventricular thalamic nucleus in the rat. Neurosci. Lett. 1995;201:84–86. doi: 10.1016/0304-3940(95)12148-w. [DOI] [PubMed] [Google Scholar]
- Otake K, Nakamura Y. Single midline thalamic neurons projecting to both the ventral striatum and the prefrontal cortex in the rat. Neuroscience. 1998;86:635–649. doi: 10.1016/s0306-4522(98)00062-1. [DOI] [PubMed] [Google Scholar]
- Otake K, Ruggiero DA, Nakamura Y. Adrenergic innervation of forebrain neurons that project to the paraventricular thalamic nucleus in the rat. Brain Res. 1995;697:17–26. doi: 10.1016/0006-8993(95)00749-g. [DOI] [PubMed] [Google Scholar]
- Owens MA. Master`s thesis. Florida Atlantic Univ; 2005. Afferent projections to rhomboid nucleus of thalamus. [Google Scholar]
- Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav. Neurosci. 1992;106:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ. The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse. 2006;59:480–490. doi: 10.1002/syn.20264. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ. Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. J. Comp. Neurol. 2007;500:1050–1063. doi: 10.1002/cne.21224. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. seventh ed. Academic Press; San Diego: 2014. [Google Scholar]
- Peng ZC, Bentivoglio M. The thalamic paraventricular nucleus relays information from the suprachiasmatic nucleus to the amygdala: a combined anterograde and retrograde tracing study in the rat at the light and electron microscopic levels. J. Neurocytol. 2004;33:101–116. doi: 10.1023/B:NEUR.0000029651.51195.f9. [DOI] [PubMed] [Google Scholar]
- Peschanski M, Besson JM. A spino-reticulo-thalamic pathway in the rat: An anatomical study with reference to pain transmission. Neurosci. 1984;12:165–178. doi: 10.1016/0306-4522(84)90145-3. [DOI] [PubMed] [Google Scholar]
- Pinto A, Jankowski M, Sesack SR. Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens shell: ultrastructural characteristics and spatial relationships with dopamine afferents. J. Comp. Neurol. 2003;459:142–155. doi: 10.1002/cne.10596. [DOI] [PubMed] [Google Scholar]
- Porter MC, Mair RG. The effects of frontal cortical lesions on remembering depend on the procedural demands of tasks performed in the radial arm maze. Behav. Brain Res. 1997;87:115–125. doi: 10.1016/s0166-4328(96)02272-3. [DOI] [PubMed] [Google Scholar]
- Porter MC, Burk JA, Mair RG. A comparison of the effects of hippocampal or prefrontal cortical lesions on three versions of delayed non-matching-to-sample based on positional or spatial cues. Behav. Brain Res. 2000;109:69–81. doi: 10.1016/s0166-4328(99)00161-8. [DOI] [PubMed] [Google Scholar]
- Power BD, Mitrofanis J. Zona incerta: Substrate for contralateral interconnectivity in the thalamus of rats. J. Comp. Neurol. 2001;436:52–63. [PubMed] [Google Scholar]
- Power BD, Kolmac CI, Mitrofanis J. Evidence for a large projection from the zona incerta to the dorsal thalamus. J. Comp. Neurol. 1999;404:554–565. [PubMed] [Google Scholar]
- Prasad JA, Macgregor EM, Chudasama Y. Lesions of the thalamic reuniens cause impulsive but not compulsive responses. Brain Struct. Funct. 2013;218:85–96. doi: 10.1007/s00429-012-0378-5. [DOI] [PubMed] [Google Scholar]
- Price JL. Thalamus. In: Paxinos G, editor. The Rat Nervous System. second ed. Academic Press; San Diego: 1995. pp. 629–648. [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J. Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JP, Price JL. The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain–prefrontal cortex topography. J. Comp. Neurol. 1992;323:167–197. doi: 10.1002/cne.903230204. [DOI] [PubMed] [Google Scholar]
- Reep RL, Winans SS. Efferent connections of dorsal and ventral agranular insular cortex in the hamster, Mesocricetus auratus. Neuroscience. 1982;7:2609–2635. doi: 10.1016/0306-4522(82)90087-2. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV. Topographic organization of the striatal and thalamic connections of rat medial agranular cortex. Brain Res. 1999;841:43–52. doi: 10.1016/s0006-8993(99)01779-5. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, Hashimoto A, Watson RT. Efferent connections of the rostral portion of medial agranular cortex in rats. Brain Res. Bulletin. 1987;19:203–221. doi: 10.1016/0361-9230(87)90086-4. [DOI] [PubMed] [Google Scholar]
- Rich EL, Shapiro M. Rat prefrontal cortical neurons selectively code strategy switches. J. Neurosci. 2009;29:7208–7219. doi: 10.1523/JNEUROSCI.6068-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold PY, Canteras NS, Swanson LW. Organization of projections from the anterior hypothalamic nucleus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J. Comp. Neurol. 1994;348:1–40. doi: 10.1002/cne.903480102. [DOI] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res. Rev. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Rotaru DC, Barrionuevo G, Sesack SR. Mediodorsal thalamic afferents to layer III of the rat prefrontal cortex: synaptic relationships to subclasses of interneurons. J. Comp. Neurol. 2005;490:220–238. doi: 10.1002/cne.20661. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Anwar S, Kim J, Glickstein SB. Visceral afferent pathways to the thalamus and olfactory tubercle: Behavioral implications. Brain Res. 1998;799:159–171. doi: 10.1016/s0006-8993(98)00442-9. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographic organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J. Comp. Neurol. 1998;399:440–468. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Shin JW, Geerling JC, Loewy AD. Inputs to the ventrolateral bed nucleus of the stria terminalis. J. Comp. Neurol. 2008;511:628–657. doi: 10.1002/cne.21870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroyama T, Kayahara T, Yasui Y, Nomura J, Nakano K. Projections of the vestibular nuclei to the thalamus in the rat: A Phaseolus vulgaris leucoagglutinin study. Journal of Comparative Neurology. 1999;407:318–332. [PubMed] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav. Neurosci. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- Su HS, Bentivoglio M. Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. J. Comp. Neurol. 1990;297:582–593. doi: 10.1002/cne.902970410. [DOI] [PubMed] [Google Scholar]
- Swanson LW. A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–154. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: Structure of the Rat Brain. third ed. Elsevier; New York: 2004. [Google Scholar]
- Takada M, Campbell KJ, Moriizumi T, Hattori T. On the origin of the dopaminergic innervation of the paraventricular thalamic nucleus. Neurosci. Lett. 1990;115:33–36. doi: 10.1016/0304-3940(90)90513-9. [DOI] [PubMed] [Google Scholar]
- Teixeira CM, Pomedli SR, Maei HR, Kee N, Frankland PW. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J. Neurosci. 2006;26:7555–7564. doi: 10.1523/JNEUROSCI.1068-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Prensa L, Mengual E. Axonal branching patterns of ventral pallidal neurons in the rat. Brain Struct. Funct. 2012;218:1133–1157. doi: 10.1007/s00429-012-0451-0. [DOI] [PubMed] [Google Scholar]
- Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: A test of the amygdala‘s role in sensory processing. J. Comp. Neurol. 1991;313:295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- Uylings HMB, Van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in the rat and in primates, including humans. Prog. Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Varela C, Kumar S, Yang JY, Wilson MA. Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct. Funct. 2014;219:911–929. doi: 10.1007/s00429-013-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. A PHA L analysis of ascending projections of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 1991;313:643. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP. PHA-L analysis of projections from the supramammillary nucleus in the rat. J. Comp. Neurol. 1992;326:595–622. doi: 10.1002/cne.903260408. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J. Comp. Neurol. 2002;442:163–187. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Martin GF. Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe nucleus in the rat. J. Comp. Neurol. 1988;275:511–541. doi: 10.1002/cne.902750404. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J. Comp. Neurol. 2008;508:212–237. doi: 10.1002/cne.21679. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Martin GF, Waltzer R. An autoradiographic analysis of ascending projections from the medullary reticular formation in the rat. Neurosci. 1986;19:873–898. doi: 10.1016/0306-4522(86)90305-2. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Crane AM, Colom LV, Bland BH. Ascending projections of the posterior nucleus of the hypothalamus: PHA-L analysis in the rat. J. Comp. Neurol. 1995;359:90–116. doi: 10.1002/cne.903590107. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J. Comp. Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, do Valle AC, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J. Comp. Neurol. 2006;499:768–796. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res. Bull. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. Pattern of distribution of serotonergic fibers to the thalamus of the rat. Brain Struct. Funct. 2010;215:1–28. doi: 10.1007/s00429-010-0249-x. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Rodriguez JJ. Projections of the central medial nucleus of the thalamus in the rat: node in cortical, striatal and limbic forebrain circuitry. Neuroscience. 2012;219:120–136. doi: 10.1016/j.neuroscience.2012.04.067. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Groenewegen HJ, Witter MP. Thalamus. In: Paxinos G, editor. The Rat Nervous System. fourth ed. Academic Press; San Diego: 2014. in press. [Google Scholar]
- Viana Di Prisco G, Vertes RP. Excitatory actions of the ventral midline thalamus (rhomboid/reuniens) on the medial prefrontal cortex in the rat. Synapse. 2006;60:45–55. doi: 10.1002/syn.20271. [DOI] [PubMed] [Google Scholar]
- Villanueva L, Desbois C, Le Bars D, Bernard JF. Organization of diencephalic projections from the medullary subnucleus reticularis dorsalis and the adjacent cuneate nucleus: A retrograde and anterograde tracer study in the rat. J. Comp. Neurol. 1998;390:133–160. [PubMed] [Google Scholar]
- Wang CC, Shyu BC. Differential projections from the mediodorsal and centrolateral thalamic nuclei to the frontal cortex in rats. Brain Res. 2004;995:226–235. doi: 10.1016/j.brainres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Witter MP, Ostendorf RH, Groenewegen HJ. Heterogeneity in the dorsal subiculum of the rat: Distinct neuronal zones project to different cortical and subcortical targets. Eur. J. Neurosci. 1990;2:718–725. doi: 10.1111/j.1460-9568.1990.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Wolff M, Gibb SJ, Cassel JC, Dalrymple-Alford JC. Anterior but not intralaminar thalamic nuclei support allocentric spatial memory. Neurobiol. Learn. Mem. 2008;90:71–80. doi: 10.1016/j.nlm.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG. Innervation of entorhinal principal cells by neurons of the nucleus reuniens thalami. Anterograde PHA-L tracing combined with retrograde fluorescent tracing and intracellular injection with lucifer yellow in the rat. Eur. J. Neurosci. 1991;3:641–647. doi: 10.1111/j.1460-9568.1991.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Saldana E, Witter MP. Projection from the nucleus reuniens thalami to the hippocampal region: Light and electron microscopic tracing study in the rat with the anterograde tracer phaseolus vulgaris-leucoagglutinin. J. Comp. Neurol. 1990;296:179–203. doi: 10.1002/cne.902960202. [DOI] [PubMed] [Google Scholar]
- Wyder MT, Massoglia DP, Stanford TR. Quantitative assessment of the timing and tuning of visual-related, saccade-related, and delay period activity in primate central thalamus. J. Neurophysiol. 2003;90:2029–2052. doi: 10.1152/jn.00064.2003. [DOI] [PubMed] [Google Scholar]
- Wyder MT, Massoglia DP, Stanford TR. Contextual modulation of central thalamic delay-period activity: representation of visual and saccadic goals. J. Neurophysiol. 2004;91:2628–2648. doi: 10.1152/jn.01221.2003. [DOI] [PubMed] [Google Scholar]
- Yamasaki DS, Krauthamer GM, Rhoades RW. Superior collicular projection to intralaminar thalamus in rat. Brain Res. 1986;378:223–233. doi: 10.1016/0006-8993(86)90925-x. [DOI] [PubMed] [Google Scholar]
- Young WS, Alheid GF, Heimer L. The ventral pallidal projection to the mediodorsal thalamus: a study with fluorescent retrograde tracers and immunohistofluorescence. J. Neurosci. 1984;4:1626–1638. doi: 10.1523/JNEUROSCI.04-06-01626.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci. Biobehav. Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Williams E, Wohltmann C. Ventral striatopallidothalamic projection: IV. Relative involvements of neurochemically distinct subterritories in the ventral pallidum and adjacent parts of the rostroventral forebrain. J. Comp. Neurol. 1996;364:340–362. doi: 10.1002/(SICI)1096-9861(19960108)364:2<340::AID-CNE11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]