Abstract
The genomes of 28 bacterial strains, including mycobacterial species Mycobacterium tuberculosis and Mycobacterium bovis, were analyzed for the presence of a special class of microsatellite, that of trinucleotide repeat sequences (TRS). Results of a search of all 10 possible TRS motifs (i.e., CCT, CGG, CTG, GAA, GAT, GTA, GTC, GTG, GTT, and TAT) with five or more repeating units showed that (CGG)5 was highly represented within the genomic DNA of M. tuberculosis and M. bovis. Most of the (CGG)5 repeats in the genome were within the open reading frames of two large gene families encoding PE_PGRS and PPE proteins that have the motifs Pro-Glu (PE) and Pro-Pro-Glu (PPE). (CGG)5-probed Southern hybridization showed that some mycobacterial species, such as Mycobacterium marinum, Mycobacterium kansasii, and Mycobacterium szulgai, possess many copies of (CGG)5 in their genomes. Analysis of clinical isolates obtained from Tokyo and Warsaw with both IS6110 and (CGG)5 probes showed that there is an association between the fingerprinting patterns and the geographic origin of the isolates and that (CGG)5 fingerprinting patterns were relatively more stable than IS6110 patterns. The (CGG)5 repeat is a unique sequence for some mycobacterial species, and (CGG)5 fingerprinting can be used as an epidemiologic method for these species as well as IS6110 fingerprinting can. If these two fingerprinting methods are used together, the precise analysis of M. tuberculosis isolates will be accomplished. (CGG)5-based fingerprinting is particularly useful for M. tuberculosis isolates with few or no insertion elements and for the identification of other mycobacterial species when informative probes are lacking.
DNA fingerprinting of the inserted IS6110 element specific for the Mycobacterium tuberculosis complex is a powerful epidemiological tool for visualizing DNA restriction fragment length polymorphisms (RFLP) of M. tuberculosis (26). The major limitation of IS6110-based RFLP typing is the difficulty of discriminating genetic polymorphisms of M. tuberculosis isolates with only a few copies of the element. In addition, there are two reports (1, 29) that described IS6110-based RFLP as unstable, although other studies have confirmed a high degree of stability (5, 15). Yeh and colleagues (29) indicated that genotypes with IS6110 were relatively unstable because they changed rapidly compared with those based on another marker. Alito et al. (1) reported that a multidrug-resistant outbreak strain changed rapidly, according to IS6110 RFLP, over a period of a few years.
A number of alternative typing methods for M. tuberculosis isolates that use genetic markers, such as polymorphic GC-rich repetitive sequences (PGRS) (19), tandem repeat sequences of 10 bp found in PPE family proteins (10), the direct repeat (9), a (GTG)5 repeat (28), IS1547 (6), katG (30), and tandem repeats of 40 to 100 bp (14, 24), have been reported.
Trinucleotide repeat sequences (TRS) comprise a class of microsatellites that are involved in human neurodegenerative diseases (27). Studies in Escherichia coli showed that these TRS, such as (CTG)n and (CGG)n, may effect genetic instability during DNA replication, transcription, and repair processes (17). (GAA)12 has been found in a plasmid of Mycoplasma gallisepticum (12, 13), and it positively regulates gene expression in this plasmid. It is not as well known whether bacterial genomes possess tandem repeat sequences. The types, lengths, and distribution of such sequences may serve as valuable markers for phylogenetic or epidemiologic studies of various bacteria.
In the present study, we searched for all possible TRS in various bacterial strains and found that M. tuberculosis and Mycobacterium bovis possess many (CGG)5 repeats. We also analyzed M. tuberculosis clinical isolates obtained from Japan and Poland with (CGG)5-based DNA fingerprinting and show that this method is useful for the genetic analysis of clinical isolates of M. tuberculosis.
MATERIALS AND METHODS
Bacterial strains.
The sources of mycobacterial strains used in this study are listed in Table 1. Clinical isolates were obtained from the International Medical Center of Japan (IMCJ) in Tokyo, Japan, in 2001 and from the National Research Institute of Tuberculosis and Lung Diseases in Warsaw, Poland, in 2000. These clinical isolates were obtained from different patients. Drug susceptibility testing was performed by conventional culture on solid media with a proportion method (Wellpack; Japan BCG Laboratory, Tokyo, Japan) or by a microdilution method with Vit spectrum SR (Kyokuto Pharmaceutical Co., Ltd., Tokyo, Japan). The antituberculosis drugs tested and the concentrations used were as follows: isoniazid, 0.2 and 1.0 μg/ml; rifampin, 40 μg/ml; ethambutol, 2.5 μg/ml; streptomycin, 10 μg/ml; para-aminosalicylic acid, 0.5 μg/ml; cycloserine, 30 μg/ml; ethionamide, 20 μg/ml; kanamycin, 20 μg/ml; enviomycin, 20 μg/ml; and levofloxacin, 1.0 μg/ml. Drug resistance is defined as resistance to at least one drug. Serial cultures were made from M. tuberculosis strain H37Rv and a clinical isolate from Japan (IMCJ 541) and were passaged weekly over 9 weeks.
TABLE 1.
Mycobacterial strains used in this study
| Strain | Property or origina | Source or reference |
|---|---|---|
| M. tuberculosis H37Rv | ATCC 27294 | ATCC |
| M. tuberculosis H37Ra | ATCC 25177 | ATCC |
| M. abscessus | Clinical isolate (IMCJ 268) | IMCJ |
| M. avium | ATCC 25291 | ATCC |
| M. bovis BCG | Japanese strain 172 | Japan BCG Laboratory |
| M. chelonae | JCM 6390 (ATCC 14472) | JCM |
| M. fortuitum | Clinical isolate (IMCJ 531) | IMCJ |
| M. gastri | GTC 610 (ATCC 15754) | GTC |
| M. intracellulare | JCM 6384 (ATCC 13950) | JCM |
| M. kansasii | JCM 6379 (ATCC 12478) | JCM |
| M. marinum | GTC 616 (ATCC 927) | GTC |
| M. nonchromogenicum | JCM 6364 (ATCC 19530) | JCM |
| M. peregrinum | Clinical isolate (IMCJ 460) | IMCJ |
| M. scrofulaceum | JCM 6381 (ATCC 19981) | JCM |
| M. simiae | GTC 620 (ATCC 25275) | GTC |
| M. smegmatis | ATCC 19420 | ATCC |
| M. szulgai | JCM 6383 (ATCC 35799) | JCM |
| M. terrae | GTC 623 (ATCC 15755) | GTC |
| M. xenopi | Clinical isolate (IMCJ 788) | IMCJ |
JCM, Japan Collection of Microorganisms, The Institute of Physical and Chemical Research (RIKEN), Saitama, Japan; GTC, Gifu Type Culture Collection, Department of Microbiology-Bioinformatics, Regeneration and Advanced Medical Science, Gifu University, Graduate School of Medicine, Bacterial Genetic Resources, Gifu, Japan.
Genome sequence.
The genome sequences of 28 bacterial strains were downloaded from the National Center for Biotechnology Information GenBank database (http://www.ncbi.nlm.nih.gov/genomes/MICROBES/Complete.html), The Institute for Genomic Research website (http://www.tigr.org/CMR), the Sanger Center (http://www.sanger.ac.uk), and the DNA Data Bank of Japan (http://www.ddbj.nig.ac.jp).
Isolation and restriction enzyme digestion of mycobacterial DNA.
Chromosomal DNA of the mycobacterial strains and M. tuberculosis clinical isolates were prepared as described previously (16, 26) with slight modifications. Briefly, for isolation of genomic DNA, M. tuberculosis strains were grown on egg-based Ogawa solid medium (Kyokuto Pharmaceutical Co., Ltd.) for 3 to 5 weeks. All bacterial cells from one slant were transferred to 400 μl of TE buffer (0.01 M Tris-HCl, 0.001 M EDTA [pH 8.0]), and the solution was heated at 80°C for 20 min to kill the bacteria. Fifty microliters of lysozyme (10 mg/ml) was added, and the tube was incubated overnight at 37°C. Seventy microliters of sodium dodecyl sulfate (10%) and 5 μl of proteinase K (10 mg/ml) were added, and the mixture was incubated for 10 min at 65°C. A 100-μl volume of 5 M NaCl and the same volume of an N-cetyl-N,N,N-trimethylammonium bromide (CTAB)-NaCl solution (4.1 g of NaCl and 10 g of CTAB per 100 ml) were added together. The tubes were vortexed and incubated for 10 min at 65°C. An equal volume of chloroform-isoamylalcohol (24:1) was added, the mixture was centrifuged for 5 min at 12,000 × g, and the aqueous supernatant was carefully transferred to a fresh tube. The total DNA was precipitated in isopropanol and was redissolved in 20 μl of 0.1× TE buffer. All restriction enzymes used in this study, AatII, AfaI, AluI, EcoRI, HinfI, MluI, NruI, NsbI, PstI, PvuII, SacI, Sau3AI, SalI, SmaI, XhoI, and XspI, were purchased from Takara Bio Inc. (Shiga, Japan). Chromosomal DNA was digested overnight with each restriction enzyme (1 U/μg of DNA) under the conditions specified by the manufacturer. The digested fragments were separated by electrophoresis on horizontal 1% agarose gel at 15 V for 20 h (14-cm gel) in 1× TAE buffer (0.04 M Tris-acetate, 0.001 M EDTA). A 1-kb DNA ladder and λ DNA restricted with HindIII (Promega Corp., Madison, Wis.) were used as size markers. The gels were then stained with ethidium bromide, and the results were recorded photographically.
Southern blotting.
Gels were depurinated in 0.25 M HCl for 30 min and then denatured in 0.5 M NaOH and 1.5 M NaCl for 30 min. DNA fragments were transferred to an N+ Hybond membrane (Amersham Biosciences, Little Chalfont, Buckinghamshire, United Kingdom) overnight, and the DNA was fixed to the membrane by UV irradiation.
The IS6110 probe used in this study was a 245-bp DNA fragment amplified by PCR as described previously (26). Briefly, oligonucleotides INS1 (5′-CGTGAGGGCATCGAGGTGGC-3′) and INS2 (5′-GCGTAGGCGTCGGTGACAAA-3′) were used to amplify a 245-bp fragment from purified chromosomal M. bovis BCG DNA by PCR. The 15-mer oligonucleotide (CGG)5, 5′-CGGCGGCGGCGGCGG-3′, was synthesized (Nippn TechnoCluster, Inc., Tokyo, Japan). These probes were labeled with horseradish peroxidase by the ECL direct system (Amersham Biosciences). Hybridization and detection were performed according to the recommendations of the manufacturer. Autoradiographs were obtained by exposing the membrane to X-ray film.
Analysis.
IS6110- and (CGG)5-based fingerprinting patterns were analyzed with Molecular Analyst Fingerprinting Plus software, version 1.6 (Bio-Rad Laboratories, Inc., Hercules, Calif.). To facilitate the comparison of the fingerprinting patterns, normalization was carried out with the use of molecular weight standards and the IS6110- or (CGG)5-fingerprinting patterns of two clinical isolates, IMCJ 541 and a Poland-derived isolate, no. 28 (P 28), on each gel. Each dendrogram was calculated with the unweighted pair group method with average linkage according to the supplier's instructions.
RESULTS
Presence of TRS in mycobacterial strains and other bacterial species.
To detect TRS among bacterial genomes and to determine the types of TRS and their repeat sizes, we searched for all 10 possible TRS motifs (i.e., CCT, CGG, CTG, GAA, GAT, GTA, GTC, GTG, GTT, and TAT) of five or more repeating units with the BLASTN algorithm (2). Among 28 bacterial strains, the numbers of TRS displayed large variation, with values ranging from zero to 38 (shown in the extreme right column in Table 2). M. tuberculosis strains H37Rv and CDC1551 and M. bovis possessed markedly more TRS copies than other species examined. The majority of the other species possessed fewer than 10 copies. Five strains, Listeria innocua, Listeria monocytogenes, Staphylococcus aureus N315, Thermoplasma acidophilum, and Thermoplasma volcanium, did not possess any TRS. The types of TRS varied (Table 2). (CCT)5 did not exist in any of the bacteria examined in this study. CGG repeats, predominantly (CGG)5, existed with high frequency in the genomes of M. tuberculosis strains H37Rv and CDC1551 and M. bovis; the frequencies of the appearance of CGG with five or more repeats were one per 150 to 200 kb. Neisseria meningitidis MC58 and Pseudomonas aeruginosa possessed six copies of (CGG)5 with a frequency of one copy per 380 kb and five copies with a frequency of one copy per 1,250 kb, respectively. Few (CGG)5 repeats were found in E. coli K12-MG1655, E. coli O157:H7 EDL933, E. coli O157:H7 VT2-Sakai, N. meningitidis serogroup A Z2491, Salmonella enterica, and S. enterica serovar Typhimurium. There were no (CGG)5 repeats in Clostridium acetobutylicum, Clostridium perfringens, Helicobacter pylori 26695, H. pylori J99, L. innocua, L. monocytogenes, Mycobacterium leprae, Mycoplasma genitalium, Mycoplasma pneumoniae, Mycoplasma pulmonis, Rickettsia conorii, Rickettsia prowazekii, S. aureus Mu50, S. aureus N315, T. acidophilum, T. volcanium, and Yersinia pestis. Other possible repeats of CTG, GAA, GAT, GTA, GTC, GTG, GTT, and TAT were found sporadically among various bacterial strains. However, only a few copies of these TRS were found. For example, one copy of (CTG)5 was found in C. acetobutylicum, one (CTG)10 was found in S. enterica serovar Typhi, two (CTG)5 repeats were found in S. enterica serovar Typhimurium, and one (CTG)5 and one (CTG)6 repeat were found in Y. pestis. Relatively large TRS with 21 or 16 repeats were detected in M. leprae and Mycoplasma genitalium, respectively. M. genitalium possessed three types of TRS repeats (GAA, GTA, and GTT) and different numbers of repeats [(GAA)5, (GAA)6, and (GAA)16; (GTA)5, (GTA)7, (GTA)8, (GTA)9, (GTA)10, (GTA)11, and (GTA)16; and (GTT)11].
TABLE 2.
Distribution among bacterial genomes of TRS with five or more repeats
| Microorganism (genome size [bp]) (GenBank accession no.) | No. of triplet repeats (no. of TRS copies per genome)
|
Total no. of TRS copies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CCT | CGG | CTG | GAA | GAT | GTA | GTC | GTG | GTT | TAT | ||
| C. acetobutylicum ATCC 824 (3940880) (NC_003030) | 5 (1) | 5 (1) | 5 (1) | 5 (2) | 7 | ||||||
| 6 (2) | |||||||||||
| C. perfringens 13 (3031430) (NC_003366) | 5 (3) | 4 | |||||||||
| 6 (1) | |||||||||||
| E. coli K12-MG1655 (4639221) (NC_000913) | 5 (2) | 2 | |||||||||
| E. coli O157:H7 EDL933 (5528445) (NC_002655) | 5 (1) | 5 (1) | 2 | ||||||||
| E. coli O157:H7 VT2-Sakai (5498450) (NC_002695) | 5 (1) | 5 (1) | 2 | ||||||||
| H. pylori 26695 (1667867) (NC_000915) | 8 (1) | 5 (1) | 2 | ||||||||
| H. pylori J99 (1643831) (NC_000921) | 5 (1) | 8 (1) | 5 (1) | 5 (1) | 4 | ||||||
| L. innocua CLIP 11262 (3011208) (NC_003212) | 0 | ||||||||||
| L. monocytogenes EGD-e (2944528) (NC_003210) | 0 | ||||||||||
| M. bovis AF2122/97 (4345492) (NC_002945) | 5 (22) | 5 (1) | 5 (1) | 5 (4) | 28 | ||||||
| M. leprae TN (3268203) (NC_002677) | 21 (1) | 5 (2) | 5 (3) | 5 (1) | 9 | ||||||
| 9 (1) | 6 (1) | ||||||||||
| M. tuberculosis CDC1551 (4403836) (NC_002755) | 5 (32) | 5 (1) | 5 (1) | 5 (3) | 38 | ||||||
| 6 (1) | |||||||||||
| M. tuberculosis H37Rv lab strain (4411529) (NC_000962) | 5 (27) | 5 (1) | 5 (1) | 5 (3) | 34 | ||||||
| 6 (1) | |||||||||||
| 7 (1) | |||||||||||
| M. genitalium G-37 (580074) (NC_000908) | 5 (1) | 5 (1) | 11 (1) | 11 | |||||||
| 6 (1) | 7 (1) | ||||||||||
| 16 (1) | 8 (1) | ||||||||||
| 9 (1) | |||||||||||
| 10 (1) | |||||||||||
| 11 (1) | |||||||||||
| 16 (1) | |||||||||||
| M. pneumoniae M129 (816394) (NC_000912) | 5 (1) | 2 | |||||||||
| 7 (1) | |||||||||||
| M. pulmonis UAB CTIP (963879) (NC_002771) | 5 (2) | 6 (1) | 3 | ||||||||
| N. meningitidis MC58 (2272351) (NC_003112) | 5 (6) | 6 | |||||||||
| N. meningitidis serogroup A Z2491 (2184406) (NC_003116) | 5 (2) | 3 | |||||||||
| 6 (1) | |||||||||||
| P. aeruginosa PA01 (6264403) (NC_002516) | 5 (5) | 5 (2) | 8 | ||||||||
| 14 (1) | |||||||||||
| R. conorii Malish 7 (1268755) (NC_003103) | 5 (1) | 1 | |||||||||
| R. prowazekii Madrid E (1111523) (NC_000963) | 5 (1) | 5 (1) | 2 | ||||||||
| S. enterica serovar Typhi CT18 (4809037) (NC_003198) | 5 (2) | 10 (1) | 5 (1) | 5 | |||||||
| 6 (1) | |||||||||||
| S. enterica serovar Typhimurium LT2 SGSC1412 (4857432) (NC_003197) | 5 (4) | 5 (2) | 6 | ||||||||
| S. aureus Mu50 (2878040) (NC_002758) | 5 (1) | 1 | |||||||||
| S. aureus N315 (2160837) (NC_002745) | 0 | ||||||||||
| T. acidophilum DSM 1728 (1564906) (NC_002578) | 0 | ||||||||||
| T. volcanium GSS1 (1584804) (NC_002689) | 0 | ||||||||||
| Y. pestis CO92 (4653728) (NC_003143) | 5 (1) | 5 (2) | 4 | ||||||||
| 6 (1) | |||||||||||
Positions of (CGG)5, (CGG)6, and (CGG)7 in the genome.
The M. tuberculosis and M. bovis genomes consist of 4.4 and 4.3 Mb, respectively. All (CGG)5, (CGG)6, and (CGG)7 repeats in both M. tuberculosis strains H37Rv and CDC1551 were located between 0.05 and 4.0 Mb (Table 3). These repeats appeared to be distributed randomly. In strain H37Rv, one (CGG)7 was located at 0.05 Mb, and one (CGG)6 was located at 2.4 Mb. Five (CGG)5 repeats were between 0.1 and 1.0 Mb, six were between 1.0 and 2.0 Mb, eight were between 2.0 and 3.0 Mb, and eight were between 3.0 and 4.4 Mb. In strain CDC1551, one (CGG)6 repeat was located at 0.05 Mb. Six (CGG)5 repeats were between 0.1 and 1.0 Mb, 6 were between 1.0 and 2.0 Mb, 11 were between 2.0 and 3.0 Mb, and 9 were between 3.0 and 4.4 Mb. In M. bovis, four (CGG)5 repeats were located between 0.26 and 1.0 Mb, five were between 1.0 and 2.0 Mb, seven were between 2.0 and 3.0 Mb, and six were between 3.0 and 4.3 Mb (Table 3). Almost all of the (CGG)5, (CGG)6, and (CGG)7 repeats in M. tuberculosis and M. bovis were located within the open reading frame (ORF), with the exception of six (CGG)5 repeats that were located between 1.1 and 3.96 Mb in strain CDC1551. Among these, the four (CGG)5 repeats at 1.09, 3.74, 3.76, and 3.96 Mb were in the putative ORF with authentic frameshift or point mutation (Table 3).
TABLE 3.
Position of (CGG)5, (CGG)6, and (CGG)7 within the genome in three mycobacterial strains
| Strain and position (bp) | No. of repeats | Gene no. | Product | Domain | Translation |
|---|---|---|---|---|---|
| M. tuberculosis | |||||
| 55532 | 7 | Rv0050 | Probable penicillin-binding protein, PonA | ORF | poly(Pro) |
| 261808 | 5 | Rv0218 | Hypothetical protein | ORF | poly(Ala) |
| 340616 | 5 | Rv0280 | PPE family protein | ORF | poly(Ala) |
| 362891 | 5 | Rv0297 | PE_PGRS family protein | ORF | poly(Gly) |
| 672720 | 5 | Rv0578c | PE_PGRS family protein | ORF | poly(Gly) |
| 968964 | 5 | Rv0872c | PE_PGRS family protein | ORF | poly(Gly) |
| 1091589 | 5 | Rv0977 | PE_PGRS family protein | ORF | poly(Gly) |
| 1189183 | 5 | Rv1067c | PE_PGRS family protein | ORF | poly(Gly) |
| 1189430 | 5 | Rv1068c | PE_PGRS family protein | ORF | poly(Gly) |
| 1191358 | 5 | Rv1068c | PE_PGRS family protein | ORF | poly(Gly) |
| 1213387 | 5 | Rv1087 | PE_PGRS family protein | ORF | poly(Gly) |
| 1631645 | 5 | Rv1450c | PE_PGRS family protein | ORF | poly(Gly) |
| 2357161 | 5 | Rv2098c | PE_PGRS family protein | ORF | poly(Gly) |
| 2357267 | 6 | Rv2098c | PE_PGRS family protein | ORF | poly(Gly) |
| 2387312 | 5 | Rv2126c | PE_PGRS family protein | ORF | poly(Gly) |
| 2423539 | 5 | Rv2126c | PE_PGRS family protein | ORF | poly(Gly) |
| 2639030 | 5 | Rv2356c | PPE family protein | ORF | poly(Ala) |
| 2639330 | 5 | Rv2356c | PPE family protein | ORF | poly(Ala) |
| 2639442 | 5 | Rv2356 | PPE family protein | ORF | poly(Ala) |
| 2802267 | 5 | Rv2490c | PE_PGRS family protein | ORF | poly(Gly) |
| 2922778 | 5 | Rv2591 | PE_PGRS family protein | ORF | poly(Gly) |
| 3528969 | 5 | Rv3159c | PPE family protein | ORF | poly(Ala) |
| 3752989 | 5 | Rv3347c | PPE family protein | ORF | poly(Ala) |
| 3766907 | 5 | Rv3350c | PPE family protein | ORF | poly(Ala) |
| 3802146 | 5 | Rv3388 | PE_PGRS family protein | ORF | poly(Gly) |
| 3803514 | 5 | Rv3388 | PE_PGRS family protein | ORF | poly(Gly) |
| 3969420 | 5 | Rv3532 | PPE family protein | ORF | poly(Ala) |
| 3972241 | 5 | Rv3533c | PPE family protein | ORF | poly(Ala) |
| 4029032 | 5 | Rv3587c | Hypothetical protein | ORF | poly(Pro) |
| M. tuberculosis CDC1551 | |||||
| 55478 | 6 | MT0056 | Penicillin-binding protein | ORF | poly(Pro) |
| 261924 | 5 | MT0228 | Hypothetical protein | ORF | poly(Ala) |
| 340680 | 5 | MT0292 | PPE family protein | ORF | poly(Ala) |
| 362955 | 5 | MT0311 | PE_PGRS family protein | ORF | poly(Gly) |
| 674173 | 5 | MT0607 | PE_PGRS family protein | ORF | poly(Gly) |
| 927976 | 5 | MT0855 | PE_PGRS family protein | ORF | poly(Gly) |
| 968979 | 5 | MT0894 | PE_PGRS family protein | ORF | poly(Gly) |
| 1091604 | 5 | MT1004 | Putative; PE_PGRS family protein, authentic frame shift | ORF | poly(Gly) |
| 1189231 | 5 | MT1096.1 | PE_PGRS family protein | ORF | poly(Gly) |
| 1189478 | 5 | MT1096.1 | PE_PGRS family protein | ORF | poly(Gly) |
| 1191406 | 5 | MT1097 | PE_PGRS family protein | ORF | poly(Gly) |
| 1213545 | 5 | MT1118.1 | UTR | poly(Gly) | |
| 1631528 | 5 | MT1497.1 | PE_PGRS family protein | ORF | poly(Gly) |
| 2359430 | 5 | MT2159 | PE family-related protein | ORF | poly(Gly) |
| 2359536 | 5 | MT2159 | PE family-related protein | ORF | poly(Gly) |
| 2385890 | 5 | MT2184 ? | Conserved hypothetical protein ? | Terminator ? | poly(Gly) |
| 2422232 | 5 | MT2220 | PE_PGRS family protein | ORF | poly(Gly) |
| 2633780 | 5 | MT2423 | PPE family protein | ORF | poly(Ala) |
| 2634080 | 5 | MT2423 | PPE family protein | ORF | poly(Ala) |
| 2636362 | 5 | MT2425 | PPE family protein | ORF | poly(Ala) |
| 2636662 | 5 | MT2425 | PPE family protein | ORF | poly(Ala) |
| 2636774 | 5 | MT2425 | PPE family protein | ORF | poly(Ala) |
| 2797756 | 5 | MT2564 | PE_PGRS family protein | ORF | poly(Gly) |
| 2918923 | 5 | MT2668.1 | PE_PGRS family protein | ORF | poly(Gly) |
| 3524456 | 5 | MT3247 | PPE family protein | ORF | poly(Ala) |
| 3526605 | 5 | MT3248 | PPE family protein | ORF | poly(Ala) |
| 3745224 | 5 | MT3453 | Putative; PPE family protein, authentic frame shift | ORF | poly(Ala) |
| 3759134 | 5 | MT3458 | Putative; PPE family protein, authentic frame shift | ORF | poly(Ala) |
| 3793024 | 5 | MT3495 | PE_PGRS family protein | ORF | poly(Gly) |
| 3794392 | 5 | MT3495 | PE_PGRS family protein | ORF | poly(Gly) |
| 3961566 | 5 | MT3636 | Putative; PPE family protein, authentic point mutation | ORF | poly(Gly) |
| 3964387 | 5 | MT3637 | PPE family protein | ORF | poly(Ala) |
| 4021174 | 5 | MT3693 | Hypothetical protein | ORF | poly(Pro) |
| M. bovis | |||||
| 262035 | 5 | Mb0224 | Probable conserved transmembrane protein | ORF | poly(Ala) |
| 341620 | 5 | Mb0288 | PPE family protein | ORF | poly(Ala) |
| 363940 | 5 | Mb0305 | PE_PGRS family protein | ORF | poly(Gly) |
| 673964 | 5 | Mb0593c | PE_PGRS family protein | ORF | poly(Gly) |
| 1092029 | 5 | Mb1002 | PE_PGRS family protein | ORF | poly(Gly) |
| 1189891 | 5 | Mb1096c | PE_PGRS family protein | ORF | poly(Gly) |
| 1192527 | 5 | Mb1097c | PE_PGRS family protein | ORF | poly(Gly) |
| 1214721 | 5 | Mb1116 | PE_PGRS family protein | ORF | Poly(Gly) |
| 1627966 | 5 | Mb1485c | PE_PGRS family protein | ORF | Poly(Gly) |
| 2339003 | 5 | Mb2125c | Conserved hypothetical protein PE_PGRS family protein | ORF | Poly(Gly) |
| 2339109 | 5 | Mb2125c | Conserved hypothetical protein PE_PGRS family protein | ORF | Poly(Gly) |
| 2367710 | 5 | Mb2150c | Conserved hypothetical protein PE_PGRS family protein | ORF | Poly(Gly) |
| 2604931 | 5 | Mb2376c | PPE family protein | ORF | Poly(Ala) |
| 2607401 | 5 | Mb2377c | PPE family protein | ORF | Poly(Ala) |
| 2607513 | 5 | Mb2377c | PPE family protein | ORF | Poly(Ala) |
| 2769065 | 5 | Mb2517c | PE_PGRS family protein | ORF | Poly(Gly) |
| 3706526 | 5 | Mb3380c | PPE family protein | ORF | Poly(Ala) |
| 3720437 | 5 | Mb3385c | PPE family protein | ORF | Poly(Ala) |
| 3755777 | 5 | Mb3420 | PE_PGRS family protein | ORF | Poly(Gly) |
| 3912639 | 5 | Mb3562 | PPE family protein | ORF | Poly(Ala) |
| 3915460 | 5 | Mb3563c | PPE family protein | ORF | Poly(Ala) |
| 3972250 | 5 | Mb3618c | Probable conserved membrane protein | ORF | Poly(Pro) |
In strain H37Rv, the genes containing (CGG)5 and (CGG)6 encoded the PPE and PE_PGRS families of proteins. A gene containing (CGG)7, PonA, encoded a penicillin-binding protein (Table 3). In strain CDC1551, the genes containing (CGG)5 encoded the PPE, PE_PGRS, and PE families of proteins. A gene containing (CGG)6 encoded a penicillin-binding protein (Table 3). In M. bovis, all genes containing (CGG)5 encoded PPE and PE_PGRS family proteins, with the exception of two genes that encoded probable conserved membrane proteins (Table 3). In all three strains, the (CGG)5 in the PPE genes translated to poly(Ala), and the (CGG)5 and (CGG)6 in the PE_PGRS and PE genes translated to poly(Gly). In both M. tuberculosis strains, the (CGG)6 and (CGG)7 in genes encoding penicillin-binding proteins translated to poly(Pro) (Table 3). In M. bovis, the two (CGG)5 repeats in genes encoding probable conserved membrane proteins translated to poly(Ala) and poly(Pro) (Table 3). Most of the (CGG)5 repeats within the PPE genes were located in the N-terminal PPE domain of the genes (data not shown). All (CGG)5 and (CGG)6 repeats within the PE_PGRS genes consisting of PE and PGRS domains were located in the PGRS domain (data not shown). Two (CGG)5 repeats within the PE family-related gene (MT2159) in strain CDC1551 were located in the C-terminal domain of the genes (data not shown).
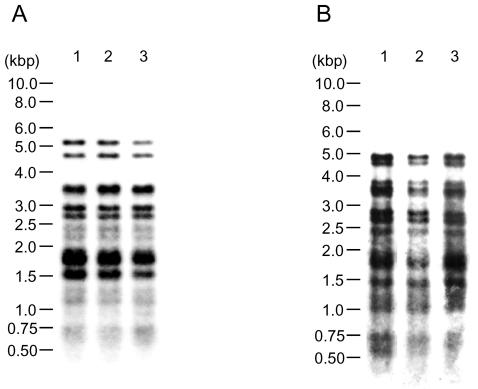
Genomic stability.
To examine whether (CGG)5 repeats in the genome are stable, two M. tuberculosis strains (H37Rv and IMCJ 541) were analyzed for (CGG)5- and IS6110-probed fingerprints. The fingerprint patterns among culture periods were identical for strain H37Rv (Fig. 1A). These findings were confirmed with strain IMCJ 541 (Fig. 1B). The data indicate that (CGG)5 repeats are stable in the genome for at least a few months. In the IS6110-probed fingerprints, the patterns did not change during the 9 weeks of culture of strain H37Rv or strain IMCJ 541 (data not shown), indicating that IS6110 inserts are also stable over a few months.
FIG. 1.
(CGG)5 fingerprinting of M. tuberculosis H37Rv (A) and clinical isolate IMCJ 541 (B), which were cultured and serially passaged weekly. The bacteria were harvested at 0 (lane 1), 3 (lane 2), and 9 (lane 3) weeks after culture.
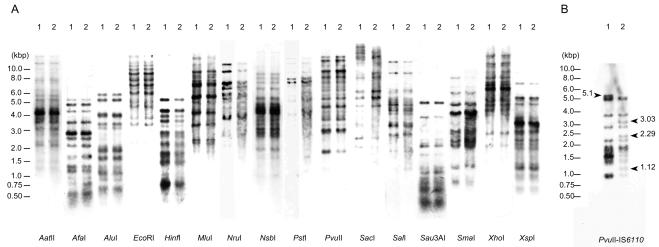
Comparison of fingerprints between M. tuberculosis strains H37Rv and H37Ra.
The virulent M. tuberculosis strain H37Rv and its avirulent derivative strain H37Ra were originally derived from the same strain, H37 (22, 23). It was reported that there are distinct differences between these strains with respect to IS6110-probed fingerprint patterns (3, 11). We investigated whether differences exist between these strains with respect to (CGG)5-probed fingerprint patterns. DNA derived from the H37Rv and H37Ra strains were digested with 16 restriction enzymes as described in Materials and Methods. Unexpectedly, the patterns of (CGG)5-based hybridization showed no differences between the H37Rv and H37Ra strains (Fig. 2A). For example, the (CGG)5-based RFLP patterns of PvuII-digested fragments of H37Rv were identical to those of H37Ra (Fig. 2A, PvuII). However, the IS6110-based RFLP patterns of H37Rv were markedly different from those of H37Ra, which were analyzed with the use of the same blot of PvuII-digested fragments used in the (CGG)5-based RFLP analysis (Fig. 2B). In the IS6110-based RFLP patterns, H37Rv showed 9 bands, and H37Ra showed 11 bands. Strain H37Rv but not H37Ra showed one band of 5.1 kb. Strain H37Ra but not H37Rv showed three bands of 1.1, 2.3, and 3.0 kb.
FIG. 2.
(CGG)5 (A) and IS6110 (B) fingerprinting of M. tuberculosis strains H37Rv (lane 1) and H37Ra (lane 2). Genomic DNA was digested with 16 restriction enzymes. The digested fragments were separated by electrophoresis.
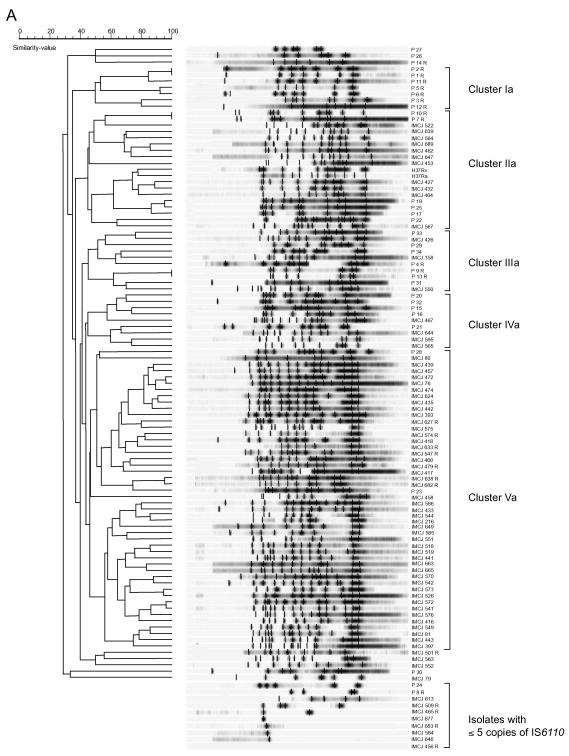
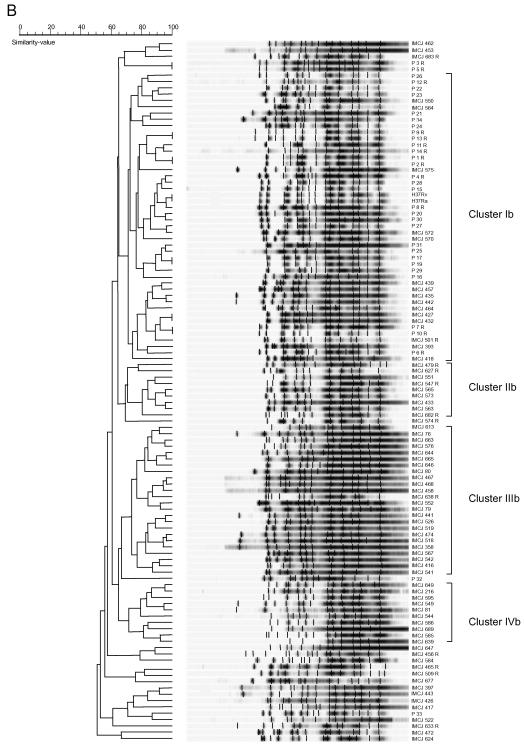
IS6110- and (CGG)5-probed DNA fingerprinting of M. tuberculosis clinical isolates.
To assess the potential usefulness of (CGG)5 as an epidemiologic marker for M. tuberculosis, 109 clinical isolates obtained from Tokyo (76 isolates) and Warsaw (33 isolates) and the H37Rv and H37Ra strains were analyzed by the IS6110- and (CGG)5-probed fingerprint methods. For IS6110-probed hybridization, DNA of these isolates was digested with PvuII according to a standardized protocol (26). For (CGG)5-probed hybridization, DNA of the isolates was digested with AluI. When DNA of the H37Rv and H37Ra strains was digested with AatII, EcoRI, MluI, NruI, NsbI, PstI, PvuII, SacI, SalI, or XhoI, relatively higher-molecular-weight DNA fragments were visualized by the probe with a minimum size of 1 to 3.5 kb and a maximum size of more than 10 kb (Fig. 2A). When digested with AfaI, AluI, HinfI, Sau3AI, SmaI, or XspI, DNA fragments of sizes of 0.5 to 8 kb were visualized. When DNA of five clinical isolates selected at random were digested with AluI, clear (CGG)5 fingerprint patterns with 10 to 14 copies of DNA fragments of 0.75 to 8 kb were detected (data not shown). Although we used AluI for this fingerprinting method, other enzymes may also be used.
IS6110 fingerprint patterns obtained from clinical isolates and the corresponding dendrogram are shown in Fig. 3A. IS6110 copies were detected in 110 of 111 isolates. One isolate from Japan had no copy. As indicated in Fig. 3A, 10 of 111 isolates (9.0% of tested isolates), including 8 isolates from Japan and 2 from Poland, possessed fewer than 6 copies of IS6110, which was insufficient to distinguish polymorphisms. Except for these 10 isolates with fewer than 6 copies of IS6110, the IS6110 fingerprint patterns of 101 isolates showed ≥28% similarity; 98 patterns were found (Fig. 3A). Five clusters with ≥44% similarity, including clusters Ia, IIa, IIIa, IVa, and Va, were detected (Fig. 3A). Cluster Ia was composed of seven Poland-derived isolates. Cluster IIa was composed of two H37 variants and 11 Japan- and 6 Poland-derived isolates. Cluster IIIa was composed of three Japan- and seven Poland-derived isolates. Cluster IVa was composed of four Japan- and five Poland-derived isolates. Cluster Va was composed predominantly of Japan-derived isolates (46 isolates from Japan and 2 from Poland). The majority of Japan-derived isolates (61%) and Poland-derived isolates (76%) belonged to cluster Va and to clusters Ia to IVa, respectively.
FIG. 3.
IS6110- and (CGG)5-probed DNA fingerprinting patterns of M. tuberculosis clinical isolates from Japan and Poland and the respective corresponding dendrograms. The fingerprint patterns are ordered by similarity. The corresponding dendrograms are to the left of the patterns. The position of each IS6110 (A) or (CGG)5 (B) band is normalized so that the patterns for all strains are comparable. The scale depicts the similarity of patterns calculated as described in Materials and Methods. In IS6110-probed DNA fingerprint patterns, five clusters showing a similarity of more than 44% were designated clusters Ia, IIa, IIIa, IVa, and Va. Isolates with five or less than five copies are indicated in panel A. In (CGG)5-probed DNA fingerprint patterns, four clusters showing a similarity of more than 70% were designated clusters Ib, IIb, IIIb, and IVb. The isolates are named according to their origin as IMCJ (Japan) or P (Poland); the suffix R indicates drug resistance For example, IMCJ 627 R is a Japan-derived drug-resistant isolate.
(CGG)5 fingerprint patterns and the corresponding dendrogram are shown in Fig. 3B. (CGG)5 copies were detected in all clinical isolates tested. The copy number ranged from 8 to 16, with a mean of 13.0 ± 1.5 per isolate. The number of (CGG)5 copies of Japan- and Poland-derived isolates ranged from 8 to 16, with a mean of 12.9 ± 1.5 per isolate and from 11 to 15, with a mean of 13.2 ± 1.3 per isolate, respectively. A total of 104 (CGG)5 fingerprint patterns were found with ≥50% similarity (Fig. 3B). Four clusters with ≥70% similarity, including clusters Ib to IVb, were detected (Fig. 3B). Cluster Ib was composed of two H37 variants and 15 Japan- and 29 Poland-derived isolates. Clusters IIb, IIIb, and IVb were composed of 9, 24, and 10 Japan-derived isolates, respectively. Over half of the Japan-derived isolates (57%) and the majority of the Poland-derived isolates (88%) belonged to clusters IIb to IVb and to cluster Ib, respectively (Fig. 3B).
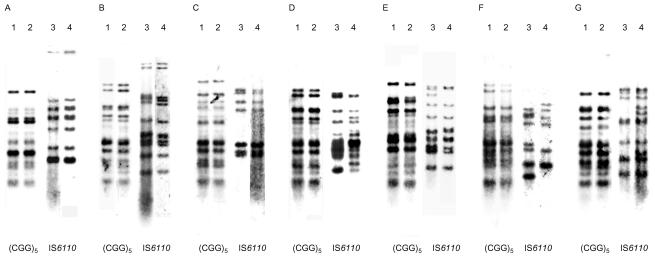
Both the IS6110 and (CGG)5 fingerprint analyses showed an association between fingerprint pattern and geographic origin, indicating a correlation between them. Ten isolates that were indistinguishable by IS6110 RFLP because of the presence of few copies of the marker could be analyzed by (CGG)5 marker. Three and seven pairs of isolates were identical to each other in the IS6110 and (CGG)5 fingerprint patterns, respectively (Fig. 4). The three pairs P 1 and P 2, P 7 and P 10, and P 9 and P 13 were identical to each other in the IS6110 and (CGG)5 fingerprint patterns (Fig. 4A to C, respectively). The four pairs H37Rv and H37Ra, IMCJ 427 and IMCJ 432, P 3 and P 5, and P 17 and P 19 were identical to each other in the (CGG)5 fingerprint pattern but different in the IS6110 fingerprint pattern (Fig. 4D, E, F, and G, respectively). The data suggest that the (CGG)5 fingerprint patterns are more stable than the IS6110 patterns.
FIG. 4.
(CGG)5- and IS6110-probed DNA fingerprinting patterns of M. tuberculosis isolates that shared identical (CGG)5 fingerprinting. (A) Lanes 1 and 3, P1; lanes 2 and 4, P2. (B) Lanes 1 and 3, P 7; lanes 2 and 4, P 10. (C) Lanes 1 and 3, P 9; lanes 2 and 4, P 13. (D) Lanes 1 and 3, H37Rv; lanes 2 and 4, H37Ra. (E) Lanes 1 and 3, IMCJ 427; lanes 2 and 4, IMCJ 432. (F) Lanes 1 and 3, P 3; lanes 2 and 4, P 5. (G) Lanes 1 and 3, P 17; lanes 2 and 4, P 19.
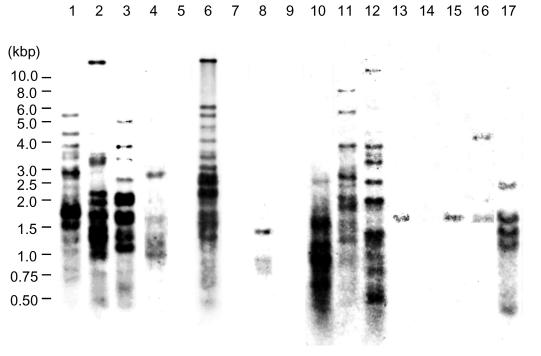
Occurrence of (CGG)5 among various mycobacterial strains.
We investigated the presence of (CGG)5 repeat sequences in mycobacterial species. (CGG)5 hybridization patterns from various mycobacterial species are shown in Fig. 5. Bands ranging from 0 to 20 in number were seen. Mycobacterium szulgai possessed 20 bands. M. bovis BCG, Mycobacterium marinum, and Mycobacterium kansasii possessed 16 bands. Mycobacterium nonchromogenicum, Mycobacterium terrae, Mycobacterium gastri, Mycobacterium simiae, Mycobacterium smegmatis, and Mycobacterium intracellulare possessed 14, 12, 8, 5, 5, and 3 bands, respectively. Mycobacterium peregrinum possessed two bands. Mycobacterium fortuitum and Mycobacterium chelonae possessed one band. Mycobacterium scrofulaceum, Mycobacterium avium, Mycobacterium xenopi, and Mycobacterium abscessus showed no bands.
FIG. 5.
(CGG)5-probed fingerprinting of AluI-digested DNA from various mycobacterial species. Lane 1, M. bovis BCG; lane 2, M. marinum; lane 3, M. kansasii; lane 4, M. simiae; lane 5, M. scrofulaceum; lane 6, M. szulgai; lane 7, M. avium; lane 8, M. intracellulare; lane 9, M. xenopi; lane 10, M. gastri; lane 11, M. terrae; lane 12, M. nonchromogenicum; lane 13, M. fortuitum; lane 14, M. abscessus; lane 15, M. chelonae; lane 16, M. peregrinum; lane 17, M. smegmatis.
DISCUSSION
In this study, we found that various bacterial strains contain TRS in their genomes. In humans, TRS are associated with hereditary neurologic and neuromuscular disorders, including myotonic dystrophy, Huntington's disease, Fragile X syndrome, and Friedreich's ataxia (27). These diseases result from TRS expansion such as (CTG)n, (CGG)n, and (GAA)n (27). The TRS sizes associated with these diseases are usually quite large. For example, 80 to 3,000 repeats of CTG have been found in myotonic dystrophy, 230 to 2,000 repeats of CGG have been found in Fragile X syndrome, and 200 to 900 repeats of GAA have been found in Friedreich's ataxia (21). These expanded TRS can form hairpin structures or intramolecular triplex structures that result in genetic instability (21). The TRS sizes found in bacteria were relatively small. The largest size TRS identified was 21 repeats of GAA in M. leprae. The most frequently identified TRS was five repeats of CGG in M. tuberculosis and M. bovis. TRS found in bacteria are not likely to be linked to genetic instability because of the lower repeat number.
The (CGG)5 TRS found in two strains of M. tuberculosis (H37Rv and CDC1551) and in one strain of M. bovis existed in genes encoding PE protein families, including a PE_PGRS subfamily and PPE protein families comprising 88 to 101 and 61 to 69 kinds of proteins, respectively, which occupy approximately 8% of the genome (4, 7, 8). The functional properties of (CGG)5 in these genes are unknown, but (CGG)5 should not play an important role in the development of the variations among different strains. (CGG)5 in the PPE genes was located in the conserved N-terminal domain PPE but not in the C-terminal variable domain containing the major polymorphic tandem repeats with the consensus sequence of GCCGGTGTTG (10, 18). (CGG)5 in the PE_PGRS genes was within the C-terminal variable domain containing the PGRS with the consensus sequence of CGGCGGCAA (18, 19). (CGG)5 in the PE_PGRS genes did not comprise part of the consensus sequence of PGRS. (CGG)5 was contained in 13 and 12 PE_PGRS genes in H37Rv and CDC1551, respectively. Among these genes, deletion or insertion was detected at one site of Rv1068c, two sites of Rv1087, and two sites of Rv1450c compared with their orthologs, MT1097, MT1118.1, and MT1497.1, respectively (data not shown). However, (CGG)5 was not near these sites, indicating that it did not directly affect the deletion and insertion of PE_PGRS genes. (CGG)5 in PPE, PE, and PE_PGRS genes translated to neutral-charged amino acids of poly(Ala) and poly(Gly), respectively, with no special substitution, indicating that these regions do not participate in the formation of unique structures within these proteins. Thus, the (CGG)5 sequences in these genes will likely not have characteristic properties regarding function.
It is unclear whether TRS in bacteria, particularly (CGG)5 in M. tuberculosis and M. bovis, participate in their pathogenesis. There was no difference between virulent strain H37Rv and the derived avirulent strain H37Ra in (CGG)5-probed fingerprinting (Fig. 2). No correlation was found between the virulency of mycobacterial species and the numbers of bands in (CGG)5-probed fingerprinting or copies of (CGG)5 (Table 2 and Fig. 5). For example, M. leprae had no (CGG)5 repeats (Table 2). Some rare etiologic agents of nontuberculous mycobacteria, such as M. smegmatis and M. szulgai (20), did possess several copies of (CGG)5 in their genomes (Fig. 5), whereas some common etiologic agents, such as M. avium, M. xenopi, and M. abscessus (20), possessed no (CGG)5 repeats (Fig. 5). These results indicate that (CGG)5 repeats do not participate directly in the virulency of mycobacterial species.
Whereas fingerprinting analysis showed that both (CGG)5 and IS6110 were sufficiently stable epidemiologic markers, (CGG)5 appeared to be more stable than IS6110 (Fig. 1). We were unable to find any differences between strains H37Rv and H37Ra in (CGG)5-probed fingerprinting by extensive studies with various restriction enzymes. However, four different bands were detected between these strains with PvuII-IS6110 fingerprinting (Fig. 2B). Lari et al. (11) compared H37Rv and H37Ra strains maintained at their institution by IS6110 fingerprinting with EcoNI, PstI, and PvuII and found different patterns between these strains. Bifani et al. (3) compared the PvuII-IS6110 fingerprints of 15 and 3 different catalogued variants of H37Rv and H37Ra, respectively. Ten distinct fingerprint patterns, making up nine H37Rv variants and one H37Ra variant, were identified. A discrepancy between IS6110- and (CGG)5-probed fingerprints of laboratory strains was observed in three pairs of clinical isolates (Fig. 4). In these cases, each isolate was identical in (CGG)5 fingerprinting pattern but differed in its IS6110 fingerprinting pattern. Our recent epidemiological case report of intrafamilial tuberculosis transmission showed that two clinical isolates from a father and son were identical in (CGG)5-probed fingerprinting patterns, whereas one different band was detected between them by IS6110-probed fingerprinting (25). Collectively, IS6110-probed fingerprint patterns changed more rapidly than did (CGG)5-probed patterns, suggesting that there are different mechanisms by which these patterns change. In other terms, although (CGG)5-probed fingerprinting will hardly detect a few mutations in a clone of M. tuberculosis, it will easily detect an origin among the clones. The (CGG)5-probed fingerprinting combined with IS6110-probed fingerprinting will provide more powerful information about tuberculosis epidemiology.
We collected and analyzed the isolates in this study in Japan and Poland. If isolates could be collected worldwide, it would provide more exact epidemiological data. In conclusion, the (CGG)5 repeat is a useful probe for DNA fingerprinting of M. tuberculosis, because all strains tested here possessed more than eight copies. In addition, (CGG)5-probed fingerprinting will be a useful tool for the investigation of M. bovis, M. marinum, M. kansasii, and M. szulgai.
Acknowledgments
We thank M. Nakano (Jichi Medical School, Japan) for comments on the manuscript and A. S. Swierzko (Centre for Microbiology and Virology, Polish Academy of Sciences, Poland) for coordinating the international collaborative study.
The study was supported by the Health Sciences Research grants from the Ministry of Health, Labour and Welfare and by the Research on Health Sciences focusing on Drug Innovation (KH11008) from the Japan Health Sciences Foundation.
REFERENCES
- 1.Alito, A., N. Morcillo, S. Scipioni, A. Dolmann, M. I. Romano, A. Cataldi, and D. van Soolingen. 1999. The IS6110 restriction fragment length polymorphism in particular multidrug-resistant Mycobacterium tuberculosis strains may evolve too fast for reliable use in outbreak investigation. J. Clin. Microbiol. 37:788-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Bifani, P., S. Moghazeh, B. Shopsin, J. Driscoll, A. Ravikovitch, and B. N. Kreiswirth. 2000. Molecular characterization of Mycobacterium tuberculosis H37Rv/Ra variants: distinguishing the mycobacterial laboratory strain. J. Clin. Microbiol. 38:3200-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 5.de Boer, A. S., M. W. Borgdorff, P. E. de Haas, N. J. Nagelkerke, J. D. van Embden, and D. van Soolingen. 1999. Analysis of rate of change of IS6110 RFLP patterns of Mycobacterium tuberculosis based on serial patient isolates. J. Infect. Dis. 180:1238-1244. [DOI] [PubMed] [Google Scholar]
- 6.Fang, Z., C. Doig, N. Morrison, B. Watt, and K. J. Forbes. 1999. Characterization of IS1547, a new member of the IS900 family in the Mycobacterium tuberculosis complex, and its association with IS6110. J. Bacteriol. 181:1021-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleischmann, R. D., D. Alland, J. A. Eisen, L. Carpenter, O. White, J. Peterson, R. DeBoy, R. Dodson, M. Gwinn, D. Haft, E. Hickey, J. F. Kolonay, W. C. Nelson, L. A. Umayam, M. Ermolaeva, S. L. Salzberg, A. Delcher, T. Utterback, J. Weidman, H. Khouri, J. Gill, A. Mikula, W. Bishai, W. R. Jacobs, Jr., J. C. Venter, and C. M. Fraser. 2002. Whole-genome comparison of Mycobacterium tuberculosis clinical and laboratory strains. J. Bacteriol. 184:5479-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garnier, T., K. Eiglmeier, J. C. Camus, N. Medina, H. Mansoor, M. Pryor, S. Duthoy, S. Grondin, C. Lacroix, C. Monsempe, S. Simon, B. Harris, R. Atkin, J. Doggett, R. Mayes, L. Keating, P. R. Wheeler, J. Parkhill, B. G. Barrell, S. T. Cole, S. V. Gordon, and R. G. Hewinson. 2003. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. USA 100:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermans, P. W., D. van Soolingen, E. M. Bik, P. E. de Haas, J. W. Dale, and J. D. van Embden. 1991. Insertion element IS987 from Mycobacterium bovis BCG is located in a hot-spot integration region for insertion elements in Mycobacterium tuberculosis complex strains. Infect. Immun. 59:2695-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermans, P. W., D. van Soolingen, and J. D. van Embden. 1992. Characterization of a major polymorphic tandem repeat in Mycobacterium tuberculosis and its potential use in the epidemiology of Mycobacterium kansasii and Mycobacterium gordonae. J. Bacteriol. 174:4157-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lari, N., L. Rindi, C. Lami, and C. Garzelli. 1999. IS6110-based restriction fragment length polymorphism (RFLP) analysis of Mycobacterium tuberculosis H37Rv and H37Ra. Microb. Pathog. 26:281-286. [DOI] [PubMed] [Google Scholar]
- 12.Liu, L., K. Dybvig, V. S. Panangala, V. L. van Santen, and C. T. French. 2000. GAA trinucleotide repeat region regulates M9/pMGA gene expression in Mycoplasma gallisepticum. Infect. Immun. 68:871-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, L., V. S. Panangala, and K. Dybvig. 2002. Trinucleotide GAA repeats dictate pMGA gene expression in Mycoplasma gallisepticum by affecting spacing between flanking regions. J. Bacteriol. 184:1335-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazars, E., S. Lesjean, A. L. Banuls, M. Gilbert, V. Vincent, B. Gicquel, M. Tibayrenc, C. Locht, and P. Supply. 2001. High-resolution minisatellite-based typing as a portable approach to global analysis of Mycobacterium tuberculosis molecular epidemiology. Proc. Natl. Acad. Sci. USA 98:1901-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niemann, S., E. Richter, and S. Rüsch-Gerdes. 1999. Stability of Mycobacterium tuberculosis IS6110 restriction fragment length polymorphism patterns and spoligotypes determined by analyzing serial isolates from patients with drug-resistant tuberculosis. J. Clin. Microbiol. 37:409-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niemann, S., S. Rüsch-Gerdes, and E. Richter. 1997. IS6110 fingerprinting of drug-resistant Mycobacterium tuberculosis strains isolated in Germany during 1995. J. Clin. Microbiol. 35:3015-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parniewski, P., A. Bacolla, A. Jaworski, and R. D. Wells. 1999. Nucleotide excision repair affects the stability of long transcribed (CTG*CAG) tracts in an orientation-dependent manner in Escherichia coli. Nucleic Acids Res. 27:616-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poulet, S., and S. T. Cole. 1995. Repeated DNA sequences in mycobacteria. Arch. Microbiol. 163:79-86. [DOI] [PubMed] [Google Scholar]
- 19.Ross, B. C., K. Raios, K. Jackson, and B. Dwyer. 1992. Molecular cloning of a highly repeated DNA element from Mycobacterium tuberculosis and its use as an epidemiological tool. J. Clin. Microbiol. 30:942-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salfinger, M. 1996. Characteristics of the various species of mycobacteria, p. 161-170. In N. R. William and M. G. Stuart (ed.), Tuberculosis. Little, Brown and Company, New York, N.Y.
- 21.Sinden, R. R. 1999. Biological implications of the DNA structures associated with disease-causing triplet repeats. Am. J. Hum. Genet. 64:346-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steenken, W., W. H. Oatway, and S. A. Petroff. 1934. Biological studies of the tubercle bacillus. J. Exp. Med. 60:515-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steenken, W. J., and L. U. Garner. 1946. History of H37 strain of tubercle bacillus. Am. Rev. Tuberc. 79:62-66. [DOI] [PubMed] [Google Scholar]
- 24.Supply, P., S. Lesjean, E. Savine, K. Kremer, D. van Soolingen, and C. Locht. 2001. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J. Clin. Microbiol. 39:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahara, M., Y. Yajima, S. Miyazaki, M. Aiyoshi, T. Fujino, Y. Otsuka, J. Sekiguchi, K. Saruta, T. Kuratsuji, and T. Kirikae. 2003. Molecular epidemiology of intra-familial tuberculosis transmission. Jpn. J. Infect. Dis. 56:132-133. [PubMed] [Google Scholar]
- 26.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, and T. M. Shinnick. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells, R. D., M. Sarmiento, and S. T. Warren. 1998. Genetic instabilities and hereditary neurological diseases. Academic Press, New York, N.Y.
- 28.Wiid, I. J., C. Werely, N. Beyers, P. Donald, and P. D. van Helden. 1994. Oligonucleotide (GTG)5 as a marker for Mycobacterium tuberculosis strain identification. J. Clin. Microbiol. 32:1318-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeh, R. W., A. Ponce de Leon, C. B. Agasino, J. A. Hahn, C. L. Daley, P. C. Hopewell, and P. M. Small. 1998. Stability of Mycobacterium tuberculosis DNA genotypes. J. Infect. Dis. 177:1107-1111. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, Y., B. Heym, B. Allen, D. Young, and S. Cole. 1992. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 358:591-593. [DOI] [PubMed] [Google Scholar]