Abstract
Surgical resection of metastases to the adrenal gland can improve overall survival of patients with stage IV melanoma, but its relative value with respect to current nonsurgical therapies is unknown. We hypothesized that surgery remains an optimal first-line treatment approach for resectable adrenal metastases. A search of our institution’s prospectively collected melanoma database identified stage IV patients treated for adrenal metastases between January 1, 2000, and August 11, 2014. The 91 study patients had a mean age of 60.3 years at diagnosis of adrenal metastasis and 24 had undergone adrenalectomy. Improved survival was associated with an unknown primary lesion, surgical resection, and nonsurgical therapies. Median overall survival from diagnosis of adrenal metastases was 29.2 months with adrenalectomy versus 9.4 months with nonoperative treatment. Adrenalectomy, either as complete metastasectomy or targeted to lesions resistant to systemic therapy, is associated with improved long-term survival in metastatic melanoma.
The incidence of melanoma in the United States is rising, and metastatic melanoma continues to have a poor overall survival.1, 2 Postmortem evaluation of patients with advanced malignant melanoma revealed metastases to the adrenal gland in almost 50 per cent of cases.3 Our institution has demonstrated that surgical resection of intra-abdominal metastatic melanoma, and in particular adrenal metastases, may prolong overall survival in patients whose disease burden can be managed through resection.4, 5 Further studies have corroborated these findings and illustrated a median overall survival of 16 to 20.7 months after adrenalectomy.6,7 Recent advances in non-surgical therapies8 have more commonly yielded situations in which some disease is responsive, but specific sites are refractory. We hypothesized that in appropriately selected patients, adrenalectomy remains an optimal first-line approach or as an adjunct to partially effective systemic treatment.
Methods
This study was approved through exemption by regulatory review. Query of our prospectively collected melanoma database identified 94 patients diagnosed with metastatic melanoma involving the adrenal gland between January 1, 2000, and August 11, 2014. Three patients were excluded: two had incomplete follow-up records and one did not have adrenal metastasis. Of the remaining 91 patients, 24 had one or both adrenal glands surgically removed. Demographics, tumor characteristics, and treatment modalities were recorded. Nonsurgical therapy was defined as locoregional or systemic treatments other than surgery including chemotherapy, immunotherapy, vaccine treatment, biochemotherapy, treatment with biologic agents, and radiation therapy.
Demographic characteristics were compared between patients who underwent adrenal surgery and those whose adrenal metastases were managed non-operatively. Overall survival was calculated from diagnosis of adrenal metastasis to death or the end of follow-up, and evaluated by age, sex, unknown primary lesion, first site of distant disease, and treatment received for adrenal metastases. Survival curves were generated by the Kaplan–Meier method and the log-rank test was used to evaluate differences. The χ2 statistic was used for comparison of demographic and prognostic factors. Prognostic factors were also evaluated with a multivariate Cox proportional hazard regression model. Analyses were performed using Statistical Analysis Software (SAS), version 9.3, SAS Institute Inc., Cary, North Carolina. A P value of <0.05 was considered statistically significant.
Results
During a 14-year period, 91 patients were treated for metastatic melanoma to the adrenal gland. The average age at diagnosis of adrenal metastasis was 60.2 years with approximately a 3:1 male predominance. At a median follow-up of 11.6 months, 13 patients were alive. The median overall survival after diagnosis of adrenal metastasis was 11.9 months.
Specific site and Clark level of the primary lesion did not significantly affect survival of the 91 patients analyzed. The mean Breslow thickness for the entire cohort was 2.29 mm. The adrenal gland was the initial site of stage IV disease in 35 (38.5%) patients and was associated with a worse overall survival (10.5 months vs 14.7 months in those with other organ site at stage IV diagnosis; P = 0.016). Patients with an unknown primary lesion (n = 21) demonstrated improved overall survival compared with those with known primary sites of disease (n = 70) (median of 22.4 months vs 10.7 months, respectively; P = 0.015).
Of 91 patients, 24 (26.4%) with metastatic melanoma to the adrenal gland had an adrenalectomy. Fifteen (63%) adrenal surgeries were performed at John Wayne Cancer Institute and most in an open manner (71%). Adrenal metastasis in the adrenalectomy group was diagnosed through either CT scans (n = 10) or positron emission tomography scans (n = 10). Three patients underwent bilateral adrenalectomy (two patients with bilateral adrenalectomy during one procedure, one patient with separate adrenalectomies due to recurrence) with a median follow-up of 17 months.
Comparing the adrenalectomy group and non-adrenalectomy group, the mean age at diagnosis and gender ratio of patients, as well as the specific site, Breslow thickness and ulceration status of the primary lesion were similar between groups (Table 1). The adrenal gland was the first site of metastasis in six (25%) adrenalectomy patients versus 29 (43.3%) nonadrenalectomy patients (P = 0.114). The median time from primary diagnosis to stage IV disease at any location was 52.3 months in the adrenalectomy group (n = 16) compared with 47.7 months in the non-adrenalectomy group (n = 54) (P = 0.651).
Table 1.
Characteristics of Patients with Metastatic Melanoma to the Adrenal Gland Managed with Adrenalectomy vs No Adrenalectomy
| Adrenalectomy (n = 24) | No Adrenalectomy (n = 67) | P Value | |
|---|---|---|---|
| Age at diagnosis of adrenal metastases (mean ± SD) |
60.17 ± 13.18 | 60.21 ± 14.56 | 0.99 |
| Gender (%) | |||
| Female | 29.2 | 22.4 | 0.506 |
| Male | 70.8 | 77.6 | |
| Site of primary melanoma (%) | |||
| Extremity | 16.7 | 28.4 | 0.346 |
| Trunk | 37.5 | 31.3 | |
| Other | 12.5 | 20.9 | |
| Unknown | 33.3 | 19.4 | |
| Clark level (%) | |||
| I–III | 20.8 | 28.4 | 0.086 |
| IV | 8.3 | 25.4 | |
| V | 12.5 | 3 | |
| Unknown | 58.3 | 43.3 | |
| Breslow thickness of primary melanoma mm, means ± SD |
2.74 ± 2.92 | 2.16 ± 1.6 | 0.56 |
| Ulceration (%) | |||
| Yes | 12.5 | 17.9 | 0.34 |
| No | 16.67 | 28.4 | |
| Unknown | 70.83 | 53.7 |
Median survival in the adrenalectomy group was 29.2 months versus 9.4 months (P < 0.001) for patients whose adrenal metastases were managed nonoperatively (Fig. 1A). Of those having adrenal surgery, 14 (58%) underwent an operative procedure that rendered the patient free of disease. Interestingly, a survival advantage was noted in both patients without evidence of disease after adrenal surgery and those alive with disease after adrenal surgery versus non-operative management of adrenal metastasis (P = 0.0007 and 0.023, respectively) (Fig. 1B).
Fig. 1.
The association between the treatments of metastatic melanoma to the adrenal gland managed operatively and nonoperatively. (A) Overall survival is enhanced in the group receiving surgical resection (n = 24) of adrenal metastasis versus no adrenalectomy (n = 67). (B) Adrenalectomy rendering patients with no evidence of disease (n = 14) illustrated no difference in survival compared with patients alive with disease after resection (n = 10), (P = 0.56). (C) Operative procedure performed was investigated, and adrenalectomy alone (n = 16) was associated with improved survival compared with patients having resection of the adrenal gland along with other sites of synchronous disease (n = 8). Rxn = resection; Mets = metastasis.
A separate analysis of the adrenalectomy group evaluated the specific type of procedure performed. Of the 24 adrenalectomy patients, 16 (67%) underwent adrenalectomy alone; these patients demonstrated a significantly better survival than the eight patients who underwent adrenalectomy plus concurrent resection of synchronous nonadrenal metastases (P = 0.034) or the 67 nonadrenalectomy patients (P < 0.001) (Fig. 1C). Nine (56%) of 16 patients undergoing adrenalectomy alone were rendered free of disease.
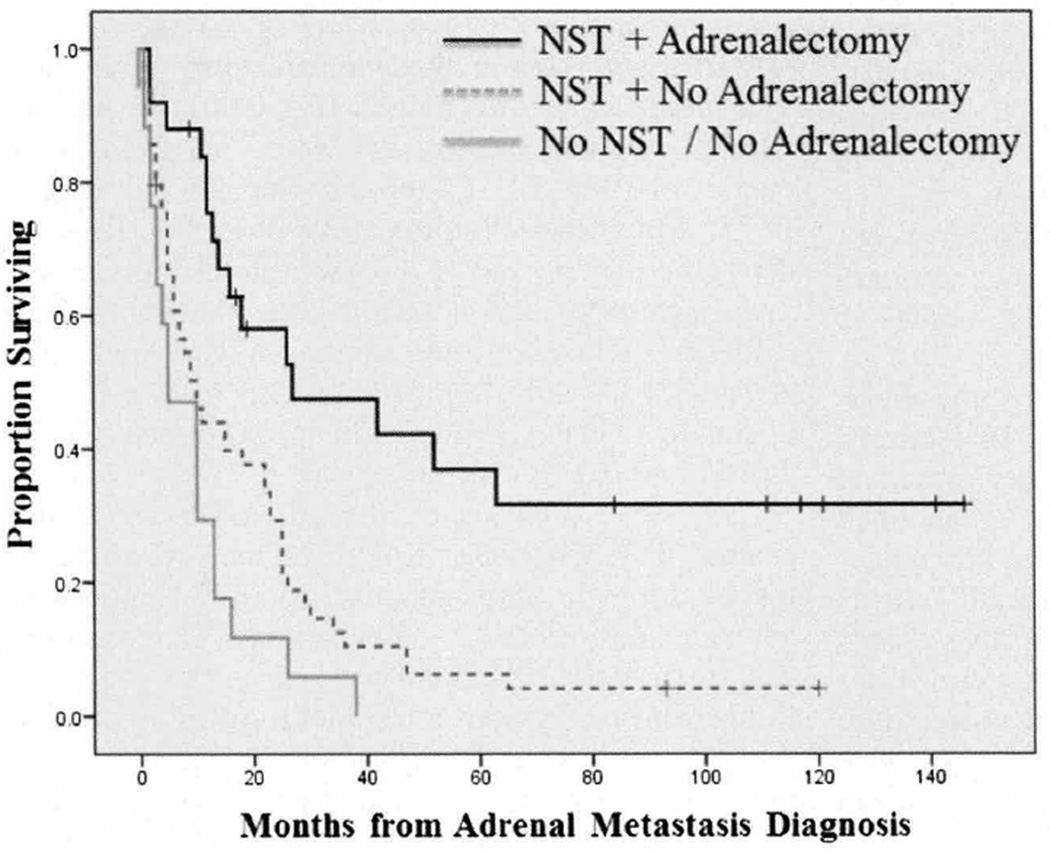
Median overall survival was 15 months for 74 patients (81.3%) patients receiving nonsurgical therapy and five months for those not receiving any nonsurgical therapy (P = 0.002). All patients who had adrenal surgery also received nonsurgical therapies, while 74.6 per cent of nonadrenalectomy patients were treated with nonsurgical therapy (P = 0.006). In the adrenalectomy group, 50 per cent of patients received nonsurgical therapy before operative intervention, 20.8 per cent after, and 29.2 per cent had nonsurgical therapy before and after surgical resection of their adrenal gland. Median overall survival was 29.2 months in patients receiving nonsurgical therapy and adrenalectomy but only 9.4 months in those receiving nonsurgical therapy without adrenalectomy (P = 0.0005) and five months in patients receiving neither nonsurgical therapy nor adrenalectomy (P < 0.0001) (Fig. 2).
Fig. 2.
The association of nonsurgical therapy with surgery and overall survival in patients with metastatic melanoma to the adrenal gland. Patients treated with both nonsurgical therapy and adrenalectomy was associated with enhanced survival compared with patients treated only with nonsurgical therapy and patients treated with neither nonsurgical therapy nor adrenalectomy. NST = nonsurgical therapy.
Prognostic factors analyzed for overall survival were evaluated in a multivariate Cox model. Unknown primary lesions [hazard ratio (HR) = 0.535 (0.296–0.967); P = 0.0384], nonadrenal involvement at stage IV diagnosis [HR = 0.566 (0.347–0.925); P = 0.0233], and adrenalectomy alone compared with adrenalectomy plus resection of synchronous metastases and no surgery [HR = 0.233 (0.079–0.692); P = 0.0087 and HR = 0.216 (0.097–0.482); P = 0.0002, respectively] were independent predictors of improved survival in patients with stage IV melanoma metastatic to the adrenal gland.
Discussion
Past studies have advocated an aggressive surgical approach in patients with operable metastatic disease due to the recognized paucity of effective systemic treatments available for metastatic melanoma.4, 9 Our institutional approach toward metastatic melanoma has been to render the patient free of macroscopic disease through surgical resection in appropriately selected candidates.5 The presented data reaffirms the survival benefit gained through surgical resection of metastatic melanoma to the adrenal gland. Our management of adrenal metastasis in the current therapeutic environment also illustrates enhanced survival through the combination of surgery and nonsurgical therapy as compared with nonsurgical treatment alone.
When treating stage IV melanoma with nonsurgical therapy, isolated metastatic sites with phenotypic aberrancies may grow in the setting of otherwise stable disease. Enhanced survival after targeted resection of single refractory sites of disease in the setting of otherwise stable disease has been demonstrated in carefully selected patients.10 Previous studies examining the treatment of adrenal metastasis from melanoma suggested survival benefit only in patients undergoing complete metastasectomy with adrenalectomy.4, 5 Our current series finds that patients undergoing incomplete resection also experienced favorable outcomes. This may be due to either selection bias in operative candidates or to increasing effectiveness of nonsurgical therapy at controlling residual disease. In our institutional practice, patients undergoing systemic therapy who demonstrate stability or partial response on sequential imaging (every 6–12 weeks) but progression at a selected site are considered for metastasectomy of that problematic site. We further demonstrated no survival benefit in patients undergoing resection of multiple sites of synchronous disease plus adrenalectomy, which may be an indication that widespread metastasectomy in the setting of generalized progression is of limited or no value.
Regarding operative approach, four patients underwent laparoscopic adrenalectomy. Although small, this number is reflective of recent changes in institutional approach as three of the last four adrenalectomies performed for metastatic melanoma at our institution were accomplished laparoscopically. Operations were almost entirely nonemergent because metastases were diagnosed through screening.
The incidence of melanoma of unknown primary (MUP) in stage IV disease has been reported by our institution to be as high as 17.7 per cent.11 Twenty-three per cent of stage IV melanoma cases reviewed in this study originated from an unknown primary source and were associated with enhanced survival. Previously, our institution demonstrated survival benefit with MUP in M1c disease, and concluded these patients may possess innate survival advantages making them candidates for aggressive management strategies.11 This study has a frequency of MUP above that previously reported. The initial site of stage IV disease is an important factor, as a worse overall outcome was demonstrated if the initial site of metastasis was the adrenal gland. The first site of metastatic disease and the location status of the primary lesion are prognostic factors that should be considered when evaluating a patient with adrenal metastases for operative intervention.
This study was limited due to its retrospective nature with respects to treatment intent, as selection criteria used in the decision to operate was not known in all cases. An institutional bias may also exist as our institution is a known referral center for advanced cases of melanoma that get treated aggressively.
Conclusions
Patients with metastatic melanoma to the adrenal gland are being treated with an evolving armamentarium of nonsurgical therapy. Targeted metastasectomy of adrenal metastasis refractory to nonsurgical therapy is a reasonable treatment strategy for patients with otherwise stable systemic disease.
Footnotes
Presented at the Annual Scientific Meeting of the Southern California Chapter of the American College of Surgeons, Santa Barbara, California, January 16–18, 2015.
REFERENCES
- 1.Chang C, Murzaku EC, Penn L, et al. More skin, more sun, more tan, more melanoma. Am J Public Health. 2014;104:e92–e99. doi: 10.2105/AJPH.2014.302185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 3.Patel JK, Didolkar MS, Pickren JW, et al. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–810. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 4.Wood TF, DiFronzo LA, Rose DM, et al. Does complete resection of melanoma metastatic to solid intra-abdominal organs improve survival? Ann Surg Oncol. 2001;8:658–662. doi: 10.1007/s10434-001-0658-4. [DOI] [PubMed] [Google Scholar]
- 5.Haigh PI, Essner R, Wardlaw JC, et al. Long-term survival after complete resection of melanoma metastatic to the adrenal gland. Ann Surg Oncol. 1999;6:633–639. doi: 10.1007/s10434-999-0633-z. [DOI] [PubMed] [Google Scholar]
- 6.Collinson FJ, Lam TK, Bruijn WM, et al. Long-term survival and occasional regression of distant melanoma metastases after adrenal metastasectomy. Ann Surg Oncol. 2008;15:1741–1749. doi: 10.1245/s10434-008-9836-y. [DOI] [PubMed] [Google Scholar]
- 7.Mittendorf EA, Lim SJ, Schacherer CW, et al. Melanoma adrenal metastasis: natural history and surgical management. Am J Surg. 2008;195:363–368. doi: 10.1016/j.amjsurg.2007.12.018. discussion 368–9. [DOI] [PubMed] [Google Scholar]
- 8.Gyorki DE, Spillane J, Speakman D, et al. Current management of advanced melanoma: a transformed landscape. ANZ J Surg. 2014;84:612–617. doi: 10.1111/ans.12673. [DOI] [PubMed] [Google Scholar]
- 9.Branum GD, Epstein RE, Leight GS, et al. The role of resection in the management of melanoma metastatic to the adrenal gland. Surgery. 1991;109:127–131. [PubMed] [Google Scholar]
- 10.Gyorki DE, Yuan J, Mu Z, et al. Immunological insights from patients undergoing surgery on ipilimumab for metastatic melanoma. Ann Surg Oncol. 2013;20:3106–3111. doi: 10.1245/s10434-013-2999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CC, Faries MB, Wanek LA, et al. Improved survival for stage IV melanoma from an unknown primary site. J Clin Oncol. 2009;27:3489–3495. doi: 10.1200/JCO.2008.18.9845. [DOI] [PMC free article] [PubMed] [Google Scholar]