Abstract
Background
This study aimed to show the incidence of distant metastases (DM) in salivary gland cancer as well as the types of histology most commonly associated with it and to identify factors predictive of DM.
Methods
The study identified 301 patients who underwent surgery for cancer of the major salivary glands at Memorial Sloan-Kettering Cancer center between 1985 and 2009. Clinical, tumor, and treatment characteristics were recorded. Tumors were categorized as low-, intermediate-, and high-risk pathology based on histologic subtype and grade. Factors predictive of distant recurrence-free probability (DRFP) were determined by uni- and multivariable analyses.
Results
The primary tumor was parotid in 266 patients (88 %), and 96 tumors (32 %) were clinical T3/T4. For 57 patients (18.9 %), DM developed with a 5-year DRFP of 72.7 %. The most common site of metastasis was the lung (50 %). The clinical predictors were male gender, cT4 stage, cN+ stage, and clinical overall stage. The multivariable analysis of clinical variables showed male gender (p = 0.018), cT4 stage (p < 0.001), and cN+ stage (p = 0.004) to be significant. The pathologic predictors were high-risk and high-grade pathology, vascular invasion, perineural invasion, positive margins, pT4 stage, pN+ stage, and overall stage. The multivariable analysis of pathologic variables showed high-grade pathology (p < 0.001), perineural invasion (p = 0.005), and pN+ stage (p = 0.002) to be significant.
Conclusions
Distant metastases developed in approximately 20 % of the patients with salivary gland cancer. The most common site of metastases was the lung. The significant predictors of DM were cT4, cN+, male gender, high-grade pathology, perineural invasion, and positive nodal disease.
Knowledge of the disease course for distant metastases from salivary gland cancer is limited due to the rarity of salivary gland malignancy, the wide variety of salivary cancer histologic subtypes, and the often long disease course that can lead to loss of patient follow-up evaluation.1,2 According to the World Health Organization (WHO), salivary gland cancer comprises only 0.3 % of all cancers in the United States and only 6 % of all head and neck cancers. Salivary gland cancer exists as 24 different histologic types, all of which can progress in different ways.
Certain types of salivary gland cancer are more common than others. The most common type is mucoepidermoid carcinoma.3 Many of the 24 histologic types contain subtypes, allowing clinicians to distinguish between them even further. For example, the tubular and cribriform variants of adenoid cystic carcinoma (ACC) are somewhat less aggressive than the solid variant.3,4 However, the solid variant also has its own further subtypes, with increased dedifferentiation, resulting in production of anaplastic cells, an extremely aggressive variant that often presents initially with extensive local infiltration and lymph node metastases.4 The tendency toward distant metastasis (DM) also varies by primary location, with distant disease less common with tumors that arise in the parotid gland and more common with tumors that arise in the submandibular gland.5,6
Despite the rarity and wide histologic variety of salivary gland tumors, several generalized tumor characteristics are reported to predict DM, including tumor size, grade, perineural invaston, and genetic mutations.6 This study provides further data collected from the records of patients treated at Memorial Sloan-Kettering Cancer Center between 1985 and 2009 describing the risk factors for distant metastases arising from salivary gland cancer. We show the rate of DM, the most common sites of DM, the histologic subtypes, the primary tumor stage most likely to progress to DM, and several other predictors of 5-year distant recurrence-free probability (DRFP).
METHODS
In a previous article, we presented the results from our data collection and analysis of the clinical, tumor, and the treatment characteristics of the 301 patients who underwent surgery for previously untreated salivary gland cancer at Memorial Sloan Kettering Cancer Center between 1985 and 2009.1 Of these 301 patients, we identified 57 who progressed to DM. Our inclusion criteria for DM specified patients who presented with distant metastases before treatment (M1 stage) (n = 4) and patients who experienced distant recurrence after treatment (n = 53). Patient, tumor, and treatment characteristics were recorded from patient records after an institutional review board (IRB)-approved research waiver. Additionally, data concerning the most common sites for DM were recorded.
Tumors were categorized into different pathology risk groups based on histologic subtype and grade. The low-risk tumors included acinic cell, low-grade mucoepidermoid (MEC), and myoepithelial carcinomas, as well as polymorphous low-grade adenocarcinoma (PLGA). The intermediate-risk tumors included ACC and intermediate grade MEC. The high-risk tumors included salivary duct carcinoma, high-grade MEC, carcinoma expleomorphic adenoma (malignant mixed tumor), and high-grade adenocarcinoma. The histologic subtype “high-grade carcinoma” indicates dedifferentiated carcinoma that does not reflect any subtype. Charts were carefully reviewed to ensure that these patients did not have a cutaneous malignancy.
The patients with available slides had a histology review. For the patients without slides (30 % of cases), the histology was considered to be that reported in the pathology report.
In the univariate analysis, clinical and pathologic factors predictive of DRFP were calculated using the Kaplan–Meier method. This analysis was performed with patients who experienced distant metastases, excluding the four patients who presented with distant metastases.
In the multivariable analysis, the Cox proportional hazards model was used to determine appropriate hazard ratios. Statistical analysis was performed using SPSS (IBM Company Headquarters, Chicago, IL, USA).
RESULTS
Of the 301 patients who presented with salivary gland cancer between 1985 and 2009, 156 (52 %) were men and 145 (48 %) were women. The median age of these patients was 62 years (range 9–89 years). For the patients in this data set, the most common sites of primary tumor were parotid (n = 266, 88 %), submandibular (n = 30, 10 %), and sublingual (n = 5, 2 %) glands. The clinical T stage was T1 for 54 patients (18 %), T2 for 129 patients (43 %), T3 for 64 patients (21 %), and T4 for 32 patients (11 %). More details on the clinical presentation and treatment of these patients have been reported previously.1
The median follow-up period was 43 months (range 1–264 months). Overall, DM developed in 57 patients (18.9 %), including four patients who were M1 at presentation.
The most common sites of metastases were lung (49 %), bone (40 %), liver (19 %), soft tissue (9 %), distant lymph nodes (8 %), brain (7 %), kidney (2 %), orbit (2 %), and pancreas (2 %). Subcutaneous soft tissue metastases developed in five patients (in three patients with parotid cancer and in two patients with submandibular cancer). All five patients had T3T4 tumors, high-risk pathology, and positive neck disease. Two of the patients had multiple sites of metastases as well as subcutaneous metastases. The remaining three patients had subcutaneous metastases without any other distant metastases. Our cohort of 301 salivary gland patients presented with 9 of the 24 salivary gland cancer types recognized by WHO. Of these nine subtypes, only seven progressed to DM.
In addition to the WHO-recognized histologic subtypes, DM also developed in a group of highly dedifferentiated tumors designated in this study as “high-grade carcinoma.” The two histologic subtypes in which DM was most likely to develop were salivary duct carcinoma (53 %) and adenocarcinoma (42 %). The remaining five histologies and the percentage of patients in each who experienced DM were adenoid cystic carcinoma (14 %), acinic cell carcinoma (16 %), carcinoma expleomorphic adenoma (20 %), mucoepidermoid carcinoma (7 %), myoepithelial carcinoma (6 %), and high-grade carcinoma (23 %) (Table 1).
TABLE 1.
Distant metastases according to histology of the primary tumor
| Histology group | Total count |
Distant recurrence |
% |
|---|---|---|---|
| Salivary duct carcinoma | 17 | 9 | 53 |
| Adenocarcinoma | 33 | 14 | 42 |
| High-grade carcinoma | 13 | 3 | 23 |
| Carcinoma expleomorphic adenoma |
59 | 12 | 20 |
| Acinic cell carcinoma | 37 | 6 | 16 |
| Adenoid cystic carcinoma | 28 | 4 | 14 |
| Mucoepidermoid carcinoma | 94 | 7 | 7 |
| Myoepithelial carcinoma | 17 | 1 | 6 |
| Other | 3 | 0 | 0 |
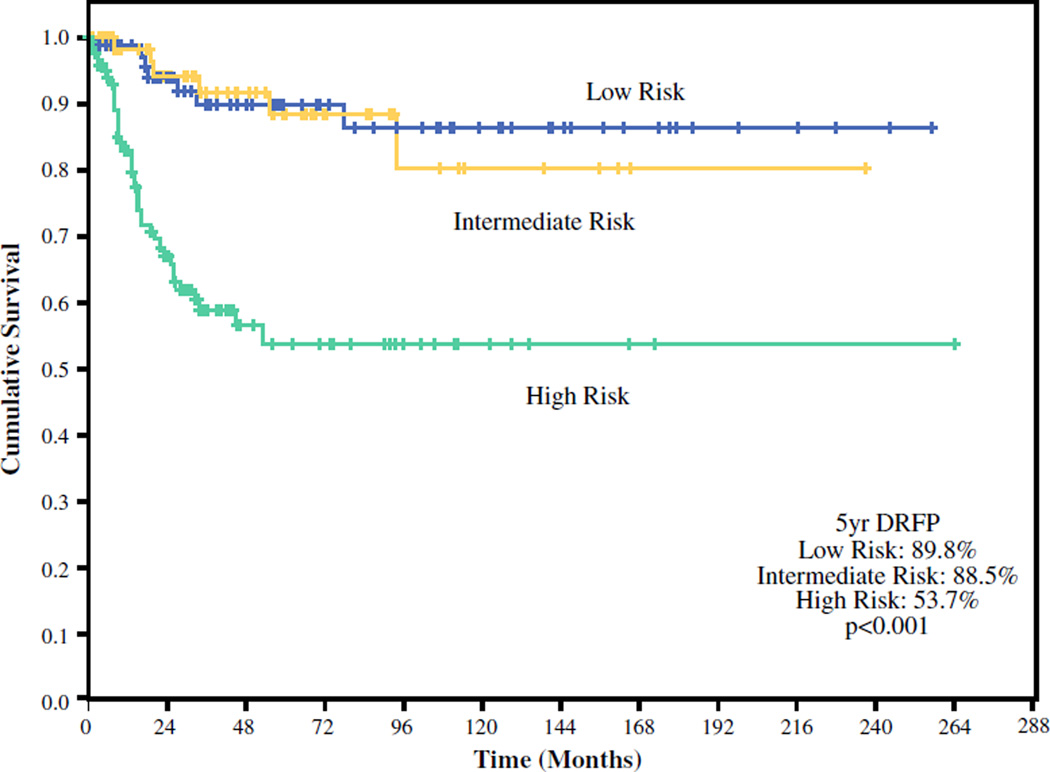
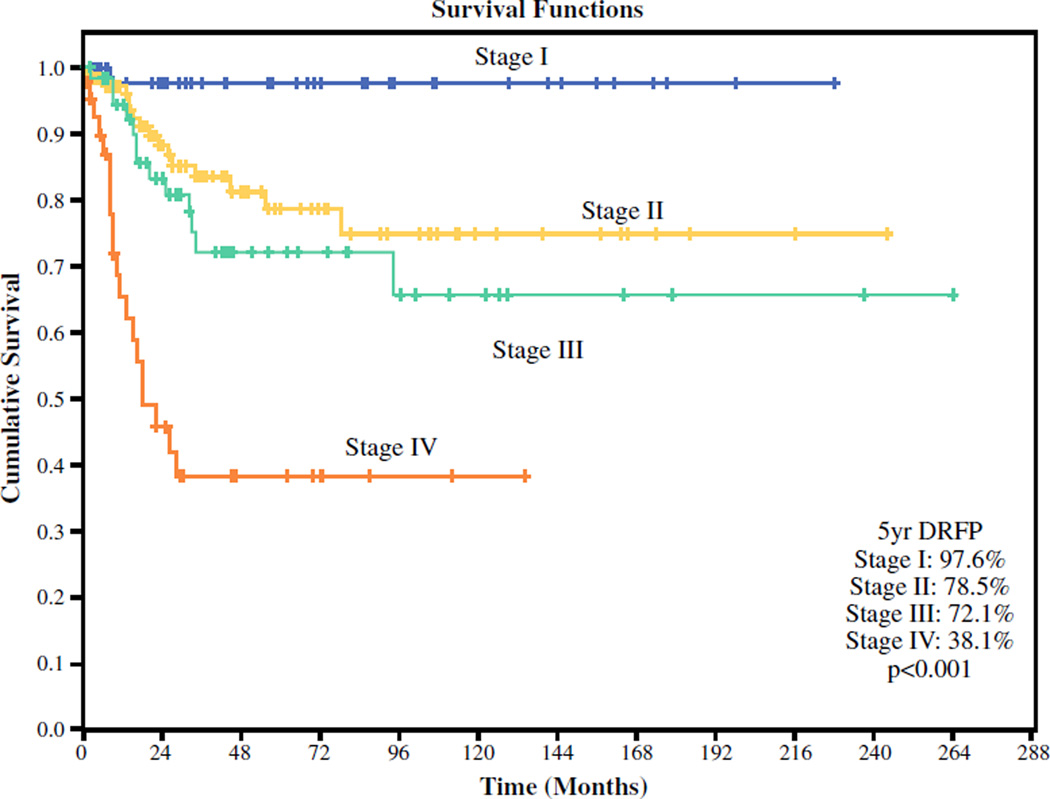
Kaplan–Meier estimates showed an overall 5-year DRFP of 72.7 %. Stratified by risk group, the 5-year DRFP was 89.8 % for the low-risk, 88.5 % for the intermediate-risk, and 53.7 % for the high-risk tumors (Fig. 1). Stratified by clinical overall stage, the 5-year DRFP was 97.8 %for stage 1, 78.5 % for stage 2, 72.1 % for stage 3, and 38.1 % for stage 43 disease (Fig. 2).
FIG. 1.
Distant recurrence-free probability stratified by risk category
FIG. 2.
Distant recurrence-free probability stratified by clinical overall stage
The factors predictive of DRFP in the univariable analysis are shown in Table 2. The clinical predictors of DM were male gender, cT4 stage, cN+ stage, and clinical overall stage. The multivariable analysis of a model of four clinical predictors (cT stage, cN+ stage, gender, and age; Table 3) showed that cT4 stage, cN+ stage, and gender remained significant. The patients with cT4 stage tumor were four times more likely to have DM than the patients with cT1, cT2, or cT3 stage tumor (p < 0.001). The patients with cN+ stage tumor were 2.85 times more likely to have DM than the patients with Cn0 stage tumor (p = 0.004). The male patients were 2.1 times more likely to have DM than the female patients (p = 0.018).
TABLE 2.
Factors predictive of distant recurrence-free survival (DRFP) in univariable analysis
| Variable | n | 5-year DRFP |
p Value |
|---|---|---|---|
| Gender | |||
| Female | 144 | 83.1 | 0.002 |
| Male | 153 | 65.1 | |
| Age (years) | |||
| <60 | 139 | 78.5 | 0.283 |
| ≥60 | 158 | 69.2 | |
| Facial nerve paralysis | |||
| Absent | 262 | 79.5 | <0.0001 |
| Present | 35 | 31.9 | |
| Skin involvement | |||
| Absent | 286 | 74.5 | 0.013 |
| Present | 11 | 50.0 | |
| Tobacco | |||
| Never | 119 | 77.7 | 0.331 |
| Ever | 155 | 70.4 | |
| Alcohol | |||
| Never | 80 | 74.7 | 0.694 |
| Ever | 175 | 71.3 | |
| cT stage | |||
| T1 | 54 | 97.7 | <0.0001 |
| T2 | 128 | 76.3 | |
| T3 | 63 | 71.0 | |
| T4 | 31 | 30.5 | |
| cN stage | |||
| N0 | 263 | 78.8 | <0.0001 |
| N+ | 34 | 39.7 | |
| cTNM stage | |||
| 1 | 52 | 97.6 | <0.0001 |
| 2 | 118 | 78.5 | |
| 3 | 63 | 72.1 | |
| 4 | 47 | 38.1 | |
| Gland | |||
| Parotid | 263 | 73.9 | 0.683 |
| Submandibular | 29 | 69.6 | |
| Sublingual | 5 | 100.0 | |
| Histology | |||
| Other | 3 | 100.0 | <0.0001 |
| Myoepithelial carcinoma | 16 | 100.0 | |
| Mucoepidermoid carcinoma | 94 | 87.5 | |
| Adenoid Cystic carcinoma | 28 | 82.9 | |
| Acinic cell carcinoma | 36 | 79.3 | |
| Carcinoma-expleomorphic adenoma | 58 | 69.3 | |
| High-grade carcinoma | 13 | 75.0 | |
| Adenocarcinoma (NOS) | 32 | 38.1 | |
| Salivary duct carcinoma | 17 | 26.9 | |
| Risk group | |||
| Low risk | 92 | 89.8 | <0.0001 |
| Intermediate risk | 68 | 88.5 | |
| High risk | 137 | 53.7 | |
| Grade | |||
| Low grade | 85 | 97.8 | <0.0001 |
| Intermediate Grade | 23 | 92.9 | |
| High grade | 112 | 48.3 | |
| Vascular invasion | |||
| Absent | 153 | 93.5 | <0.0001 |
| Present | 63 | 36.8 | |
| Perineural invasion | |||
| Absent | 126 | 95.7 | <0.0001 |
| Present | 103 | 48.5 | |
| Margins | |||
| Negative | 125 | 88.8 | <0.0001 |
| Close/positive | 145 | 63.2 | |
| pT stage | |||
| T1 | 106 | 96.0 | <0.0001 |
| T2 | 89 | 69.7 | |
| T3 | 21 | 83.0 | |
| T4 | 75 | 42.0 | |
| pN stage | |||
| NX/N0 | 236 | 88.9 | <0.0001 |
| N+ | 61 | 25.9 | |
| pTNM stage | |||
| 1 | 100 | 98.3 | <0.0001 |
| 2 | 75 | 83.9 | |
| 3 | 18 | 92.9 | |
| 4 | 100 | 38.3 | |
| PORT | |||
| No | 139 | 95.7 | <0.0001 |
| Yes | 158 | 59.3 |
Univariable analysis for each variable was calculated only with patients who were M0 at presentation (n = 53). M1 patients were excluded from this analysis
TNM tumor-node-metastasis, NOS not otherwise specified, PORT post operative radiotherapy
TABLE 3.
Factors predictive of distant recurrence-free probability (DRFP) in multivariable analysis
| Variable | HR | 95 % CI | p Value |
|---|---|---|---|
| Clinical model (4 variables) | |||
| cT stage (1, 2, 3 vs. 4) | 4.0 | 2–8 | <0.001 |
| cN stage (N0 vs. N+) | 2.85 | 1.4–5.79 | 0.004 |
| Gender (female vs. male) | 2.1 | 1.13–5.79 | 0.018 |
| Age (continuous) | 1.01 | 0.99–1.03 | 0.411 |
| Pathologic model (4 variables) | |||
| Pathology grade (low/intermediate vs. high) |
7.54 | 1.71–33.35 | 0.008 |
| Perineural invasion | 5.04 | 1.65–15.42 | 0.005 |
| pT stage(1, 2, 3 vs. 4) | 1.28 | 0.59–2.77 | 0.539 |
| pN (N0/Nx vs. N+) | 3.45 | 1.55–7.66 | 0.002 |
HR hazard ratio, CI confidence interval
The pathologic predictors of DM were high-risk pathology, high-grade pathology, vascular invasion, perineural invasion, positive surgical margins, pT4 stage, pN+ stage, and overall stage. The multivariable analysis of a model of four pathologic predictors (pathologic grade, perineural invasion, pathologic T stage, pathologic N stage; Table 3) showed that pathologic grade, perineural invasion, and pathologic N stage remained significant. The patients with high-grade pathology were 7.5 times more likely to have DM than the patients with low- or intermediate-grade pathology (p = 0.008). The patients with perineural invasion were five times more likely to have DM than the patients with no perineural invasion (p = 0.005). The patients with pathologic positive nodes were 3.4 times more likely to have DM than the patients with no positive neck nodes (p = 0.002).
DISCUSSION
This report describes the risk factors predicting DM for the various histologic subtypes of carcinoma of the major salivary gland treated at a single institution from 1985 to 2009. We report that in our cohort of patients, 18.9 % of the salivary gland cancers progressed to DM. The most common site of metastasis was the lung. Outcome was highly dependent on overall clinical stage, with patients who had stage 4 disease having a 22-fold increased risk of DM compared with stage 1 patients. Outcome also was dependent on histology and grade, with high-risk tumors such as salivary duct cancer having the poorest outcome.
The DM rate of 18.9 % during a 5-year follow-up period for our study group is similar to a result from Teo et al.,7 who reported a rate of 22 % during a 5-year follow-up period in their series of 50 patients, who also had mixed histopathology and mixed primary tumor sites. A study by Yu and Ma8 reported a DM rate of 11.1 % after a 3-year follow-up period for a similar group of 405 patients. The variation in the incidence of distant metastases in these studies likely reflects the differences in referral patterns at the respective institutions.
Although the men and women in our group presented with salivary gland cancer in roughly equal numbers, we observed that the men were significantly more likely to have salivary gland cancer that proceeded to DM. This appeared to be due to the fact that the men were more likely to present with clinical stage 4 disease (22 vs. 13 %; p = 0.04) and more likely to present with high-risk pathology (57 vs. 34 %; p < 0.001) than the women. As is commonly known, many cancers show increased risk for DM with increased tumor size and the presence of lymph node metastasis at presentation, 9 and this proved true also with our study group.
Both the histology and grade predicted DM. By combining these two variables into risk categories (low, intermediate, and high risk), we were able to show that high-risk tumors had an almost threefold increased risk of DM compared with low-risk tumors. Other studies also have identified the different rates of survival and DM for both different histologic subtypes6,10 and different grades.2,6 For example, salivary duct carcinoma of the parotid gland is inherently high grade and prone to DM,3 and a review reported DM in 52–82 % of cases.10
In our series, the rate of DM was similar, at 53 %. Salivary duct carcinoma often results in unfavorable prognoses due to its rapid proliferation and increase in size. Reports show that most patients present with stage 3 or 4 disease,11 59 % present with positive lymph nodes, 60 % present with perineural invasion, and 31 % present with intravascular tumor thrombi. Acinic cell carcinoma is by definition histologically high grade.3 Among the patients who present with no clinical evidence of nodal disease, 50 % are found to have positive nodes on histology after neck dissection.11 By contrast, myoepithelial carcinoma typically presents as aggressive local disease without progression to DM.3 Myoepithelial carcinoma had the lowest rate of DM (6 %) of any histologic subtype in our study group. Acinic cell cancer usually is a low-grade cancer, although some forms can behave more aggressively,12 and thus it falls into the intermediate-risk group.
The importance of grade was described in an analysis by Renehan et al.,2 who reported the rate of DM to be 2 % for low-grade, 44 % for intermediate-grade, and 36 % for high-grade tumors (p < 0.001). This analysis showed quite different results for intermediate-grade tumors compared with our series, in which the intermediate-grade tumors had a rate of DM similar to that of the low-grade tumors. These differences highlight the complexity in the pathologic grading associated with salivary gland cancer due to the heterogeneity of the histologic types.
Other potential predictors of DRFP are under investigation but less well understood and less widely used in prognosis. For example, the specific genetic features of a cancer in an individual also may have prognostic value. A higher expression of H3K9me3 is correlated with an increased probability of DM (p = 0.001) in adenoid cystic carcinoma,13 but also, a higher expression of TMPRSS4 was associated with a higher tendency toward both lymph node metastasis (p = 0.002) and distant metastasis in (p < 0.001) in adenoid cystic carcinoma.14 In one study of salivary duct cancer, p53 expression correlated with local disease recurrence (p < 0.013), distant metastasis (p < 0.049), and 5-year survival (p < 0.008), and HER-2/neu overexpression correlated with both distant metastasis (p < 0.034) and 5-year survival (p < 0.0239).15
Our study was not without its limitations. First, the study was retrospective and therefore susceptible to the limitations associated with such data. In particular, studies can never completely account for the selection bias due to physician- and patient-related factors in determining treatment, especially with relatively rare histologies that constitute salivary gland cancer. The different histologies and the rate of distant metastases that we report also are reflective of our own institution’s referral practice.
Second, because the median follow-up period was only 43 months, we could report only 5-year outcome figures. However, other studies have shown that certain tumors take a more protracted course, eventually resulting in distant recurrence after a longer period.10 For example, our study showed that 14 % of patients with adenoid cystic carcinoma experienced distant disease after 5 years. This correlates well with a study by Jones et al.16 in which 13 % of 108 patients with ACC at mixed primary sites experienced DM after 5 years. However, another study by Spiro17 investigating DM in 196 patients with ACC at various primary sites showed a DM rate of 38 % during a follow-up period of 10 years. Thus, more research into the long-term behavior of salivary gland cancer is desirable.
In conclusion, we report that approximately 20 % of our study cohort with carcinoma of the major salivary glands experienced distant metastases. The most common site of metastasis was the lung. The multivariable analysis of clinical variables showed that clinical T4 stage, clinical N+ stage, and male gender were significant predictors of DM. The multivariable analysis of pathologic variables showed that high-grade pathology, perineural invasion, and pathologic positive neck disease were significant predictors of DM.
REFERENCES
- 1.Ali S, Palmer FL, Yu C, et al. Postoperative nomograms predictive of survival after surgical management of malignant tumors of the major salivary glands. Ann Surg Oncol. 2014;21:637–642. doi: 10.1245/s10434-013-3321-y. [DOI] [PubMed] [Google Scholar]
- 2.Renehan AG, Gleave EN, Slevin NJ, McGurk M. Clinicopathological and treatment-related factors influencing survival in parotid cancer. Br J Cancer. 1999;80:1296–1300. doi: 10.1038/sj.bjc.6990501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes LEJ, Reichart P, Sidransky D. Pathology and Genetics of Head and Neck Tumors. World Health Organization classification of tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 4.Ettl T, Schwarz-Furlan S, Gosau M, Reichert TE. Salivary gland carcinomas. Oral Maxillofac Surg. 2012;16:267–283. doi: 10.1007/s10006-012-0350-9. [DOI] [PubMed] [Google Scholar]
- 5.Schwentner I, Obrist P, Thumfart W, Sprinzl G. Distant metastasis of parotid gland tumors. Acta Otolaryngol. 2006;126:340–345. doi: 10.1080/00016480500401035. [DOI] [PubMed] [Google Scholar]
- 6.Speight PM, Barrett AW. Prognostic factors in malignant tumours of the salivary glands. Br J Oral Maxillofac Surg. 2009;47:587–593. doi: 10.1016/j.bjoms.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Teo PM, Chan AT, Lee WY, Leung SF, Chan ES, Mok CO. Failure patterns and factors affecting prognosis of salivary gland carcinoma: retrospective study. Hong Kong Med J. 2000;6:29–36. [PubMed] [Google Scholar]
- 8.Yu GY, Ma DQ. Carcinoma of the salivary gland: a clinicopathologic study of 405 cases. Semin Surg Oncol. 1987;3:240–244. doi: 10.1002/ssu.2980030405. [DOI] [PubMed] [Google Scholar]
- 9.Sobin LH. TNM: evolution and relation to other prognostic factors. Semin Surg Oncol. 2003;21:3–7. doi: 10.1002/ssu.10014. [DOI] [PubMed] [Google Scholar]
- 10.Bradley PJ. Distant metastases from salivary glands cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:233–242. doi: 10.1159/000055748. [DOI] [PubMed] [Google Scholar]
- 11.Hosal A, Fan C, Barnes L, Myers E. Salivary duct carcinoma. Otolaryngol Head Neck Surg. 2003;129:720–725. doi: 10.1016/S0194-59980301386-X. [DOI] [PubMed] [Google Scholar]
- 12.Skalova A, Sima R, Vanecek T, et al. Acinic cell carcinoma with high-grade transformation: a report of 9 cases with immunohistochemical study and analysis of TP53 and HER-2/neu genes. Am J Surg Pathol. 2009;33:1137–1145. doi: 10.1097/PAS.0b013e3181a38e1c. [DOI] [PubMed] [Google Scholar]
- 13.Xia R, Zhou R, Tian Z, et al. High expression of H3K9me3 is a strong predictor of poor survival in patients with salivary adenoid cystic carcinoma. Arch Pathol Lab Med. 2013;137:1761–1769. doi: 10.5858/arpa.2012-0704-OA. [DOI] [PubMed] [Google Scholar]
- 14.Dai W, Zhou Q, Xu Z, Zhang E. Expression of TMPRSS4 in patients with salivary adenoid cystic carcinoma: correlation with clinicopathological features and prognosis. Med Oncol. 2013;30:749. doi: 10.1007/s12032-013-0749-7. [DOI] [PubMed] [Google Scholar]
- 15.Jaehne M, Roeser K, Jaekel T, Schepers JD, Albert N, Loning T. Clinical and immunohistologic typing of salivary duct carcinoma: a report of 50 cases. Cancer. 2005;103:2526–2533. doi: 10.1002/cncr.21116. [DOI] [PubMed] [Google Scholar]
- 16.Jones AS, Hamilton JW, Rowley H, Husband D, Helliwell TR. Adenoid cystic carcinoma of the head and neck. Clin Otolaryngol Allied Sci. 1997;22:434–443. doi: 10.1046/j.1365-2273.1997.00041.x. [DOI] [PubMed] [Google Scholar]
- 17.Spiro RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg. 1997;174:495–498. doi: 10.1016/s0002-9610(97)00153-0. [DOI] [PubMed] [Google Scholar]