Abstract
Background
Predictive role of undetectable thyroglobulin (Tg) in patients with poorly differentiated thyroid carcinoma (PDTC) is unclear. Our goal was to report on Tg levels following total thyroidectomy and adjuvant RAI in PDTC patients and to correlate Tg levels with recurrence.
Methods
Forty patients with PDTC with no distant metastases at presentation (M0) and managed by total thyroidectomy and adjuvant RAI were identified from a database of 91 PDTC patients. Of these, 31 patients had Tg values recorded and formed the basis of our analysis. A nonstimulated Tg level <1 ng/ml was used as a cutoff point for undetectable Tg levels. Association of patient and tumor characteristics with Tg levels was examined by χ2 test. Recurrence-free survival (RFS) stratified by postop Tg level was calculated by Kaplan–Meier method and compared by log-rank test.
Results
Twenty patients had undetectable Tg (<1 ng/ml) and 11 had detectable Tg (≥1 ng/ml; range 2–129 ng/ml) following surgery. After adjuvant RAI, 24 patients had undetectable Tg (<1 ng/ml) and 7 had detectable Tg (≥1 ng/ml; range 1–57 ng/ml). Patients with undetectable Tg were less likely to have pathologically positive margins compared to those with detectable Tg (33 vs. 72 % respectively; p = 0.03). Patients with undetectable Tg levels had better 5-year regional control and distant control than patients with detectable Tg level (5-year regional recurrence- free survival 96 vs. 69 %; p = 0.03; 5-year distant recurrence-free survival 96 vs. 46 %, p = 0.11).
Conclusion
Postoperative thyroglobulin levels in subset of patients with PDTC appear to have predictive value for recurrence. Patients with undetectable Tg have a low rate of recurrence.
Thyroglobulin (Tg) is a specific product of thyroid follicular cells. Serum Tg levels have been widely used as a postoperative marker for residual or recurrent tumor in differentiated thyroid carcinoma (DTC). Patients with low-risk DTC with undetectable postoperative Tg values have very low risk of recurrence.1–3 On the other hand, studies on Tg values in poorly differentiated thyroid carcinoma (PDTC) have been limited and the predictive role of an undetectable Tg in PDTC is unknown.4,5 Therefore, the purposes of our study were to report on Tg values in PDTC following total thyroidectomy and adjuvant RAI, correlate Tg values with outcome and determine if undetectable Tg predicts for low risk of recurrence in PDTC.
MATERIALS AND METHODS
Following Institutional Review Board approval, we performed a retrospective review of our thyroid cancer database for patients with PDTC, treated with primary surgery, with or without adjuvant RAI at MSKCC from 1986 to 2009. 91 patients with primary PDTC were identified. Diagnosis was confirmed by two independent pathologists (R.A.G. and D.L.C.) and was based on histological and/or immunohistochemical evidence of follicular cell differentiation with presence of tumor necrosis and/or ≥5 mitoses per 10 high-power fields (400×).6 Of 91 PDTC patients, 67 were M0 (had no distant metastasis at presentation). Of 67 M0 patients, 56 had total thyroidectomy, of whom 43 also received adjuvant RAI. After exclusion of 12 patients (3 due to external radiotherapy and 9 due to unknown Tg values), data on Tg values were available in 31 patients. These 31 PDTC patients formed the basis of our analysis. All cases were considered to express thyroglobulin based upon the histologic identification of colloid on hematoxylin and eosin section and/or the presence of positive thyroglobulin on immunohistochemistry (12 of the cases were stained for thyroglobulin and all were positive for this protein by immunostaining).
Undetectable serum Tg was defined as an unstimulated Tg <1 ng/ml measured at least 5 weeks after total thyroidectomy and at least 3 months (3–11 months; median 6 months) after adjuvant RAI. 26 PDTC patients in our cohort were treated from 2000 to 2009, whereas five patients were treated from 1992 to 1998. Sensitivity of Tg assay ranged from <0.4 to 0.6 ng/ml for the period 2000–2009 and <0.9–1 ng/ml during 1992–1998. Therefore a cutoff point of <1 ng/ml was used to define an undetectable Tg levels. By choosing this level, we are able to encompass all patients from both time periods into our definition. Tg antibodies were routinely performed in conjunction with the Tg assay and samples with Tg antibody interference were excluded from our analysis. In the patient follow-up, TSH levels were maintained at <0.1–0.5 mIU/L. Patient charts were reviewed for patient characteristics, clinical presentation, tumor pathological features, treatment, recurrences, and survival. Staging was classified according to the 7th edition of AJCC Cancer Staging Manual.7 All 31 patients had total thyroidectomy with complete gross tumor removal (29 had total thyroidectomy and 2 had extended total thyroidectomy with removal of all tissues involved by extrathyroidal spread). Select patients also underwent neck dissection for clinically suspicious disease at the time of total thyroidectomy (determined by neck palpation, cross sectional imaging or ultrasound). In our institution, we do not routinely perform elective dissection of either the central compartment or lateral compartment lymph nodes. If clinically suspicious lymph nodes are present in the lateral compartment, dissection of neck levels II–V is performed. For clinically suspicious nodes in the central compartment, bilateral paratracheal lymph node dissections are performed. If small, 1–2 lymph nodes are present in the central compartment, lymph node sampling is performed. In addition to surgical removal of all gross tumor, patients received adjuvant RAI (30–391 mCi; median dose 150 mCi). Only three patients received doses under 100 mCi, i.e., 29.5, 78.4, and 97.7 mCi. Therefore, the overwhelming majority of our patients received therapeutic RAI dose >100 mCi. All patients had diagnostic scan before adjuvant RAI and adjuvant RAI dose was determined based on the diagnostic scan and stage of PDTC.
The association of patient and tumor characteristics with Tg levels (obtained postadjuvant RAI) was examined by using the Pearson χ2 test. Overall survival (OS), disease-specific survival (DSS), recurrence-free survival (RFS), regional recurrence-free survival (RRFS), and distant recurrence-free survival (DRFS) were calculated by the Kaplan–Meier method, stratified by Tg levels (obtained post adjuvant RAI) and compared using the log-rank test. Recurrence was defined as a new local, regional, or distant finding, in a patient clinically free of disease for at least 6 months following initial therapy, that was proven by biopsy or identified on computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), or RAI scanning. Death from disease was defined if it was confirmed by death certificate or hospital summary. A level of significance alpha value p < 0.05 was used to determine significance. Statistical analysis was performed by SPSS (IBM Company Headquarters, 233 S. Wacker Drive, 11th Floor, Chicago, IL 60606).
RESULTS
Tg Values in PDTC Patients and Phenotype of PDTC
Following surgery, 20 patients had undetectable Tg (<1 ng/ml) and 11 had detectable Tg (≥1 ng/ml; range 2–129 ng/ml). After adjuvant RAI, 24 patients had undetectable Tg (<1 ng/ml) and 7 had detectable Tg (≥1 ng/ml; range 1–57 ng/ml). The phenotypes of the 24 patients with undetectable Tg following adjuvant RAI were papillary (n = 15), tall cell variant (n = 5), and mixed phenotype (n = 4). The phenotypes of the seven PDTC patients with detectable Tg were papillary (n = 3), tall cell variant (n = 1), follicular (n = 1), and mixed phenotype (n = 2). Four patients that converted from detectable Tg levels to undetectable Tg levels following adjuvant RAI had papillary phenotype (three patients) and mixed phenotype (papillary, follicular, and Hürthle cell carcinoma; one patient). Three of four patients were older males (≥45 years), whereas one patient was a young female (29 years old). Three of four patients had smaller tumors (≤4 cm), whereas one patient had a larger tumor (>4 cm). Only one patient had extrathyroidal extension (microscopic). Respective staging of the PDTC that converted from detectable Tg levels to undetectable Tg levels were: T1NxM0, T2NxM0, T3NxM0, T3N1bM0.
Association of Tg Levels with Patient and Tumor Characteristics of PDTC
Patients with undetectable Tg (<1 ng/ml) and detectable Tg levels (≥1 ng/ml) post adjuvant RAI showed no significant age or gender differences (Table 1). Patients in both groups were predominantly older (≥45 years): 62 % of patients with undetectable Tg and 71 % of patients with detectable Tg (p = 0.664). Patients who achieved an undetectable Tg postadjuvant RAI were predominantly females (71 %) compared with patients with detectable Tg (43 %; p = 0.173).
TABLE 1.
Association of Tg levels with patient and tumor characteristics
| Variable | Tg < 1 ng/ml No. (%) |
Tg ≥ 1 ng/ml No. (%) |
χ2, p value |
|---|---|---|---|
| Age (years) | |||
| <45 | 9 (38 %) | 2 (29 %) | 0.664 |
| ≥45 | 15 (62 %) | 5 (71 %) | |
| Sex | |||
| M | 7 (29 %) | 4 (57 %) | 0.173 |
| F | 17 (71 %) | 3 (43 %) | |
| pT size (cm) | |||
| ≤4 | 17 (71 %) | 4 (57 %) | 0.495 |
| >4 | 7 (29 %) | 3 (43 %) | |
| pT stage | |||
| T1/T2 | 8 (33 %) | 0 (0 %) | 0.076 |
| T3/T4 | 16 (67 %) | 7 (100 %) | |
| ETE | |||
| No | 12 (50 %) | 1 (14 %) | 0.092 |
| Yes | 12 (50 %) | 6 (86 %) | |
| Minimal | 7 (54 %) | 0 (0 %) | |
| Gross | 6 (46 %) | 7 (100 %) | |
| Margins | |||
| Negative | 15 (63 %) | 1 (14 %) | 0.033 |
| Positive/close | 8 (33 %) | 5 (72 %) | |
| Unknown | 1 (4 %) | 1 (14 %) | |
| pN stage | |||
| pNx/pN0 | 16 (67 %) | 2 (29 %) | 0.072 |
| Nx | 10 (63 %) | 0 (0 %) | |
| N0 | 6 (37 %) | 2 (100 %) | |
| pN+ | 8 (33 %) | 5 (71 %) | |
| N1a | 5 (63 %) | 1 (20 %) | |
| N1b | 3 (37 %) | 3 (60 %) | |
| N1x | 0 (0 %) | 1 (20 %) |
Bold value indicates statistically significant
ETE extrathyroid extension, pNx clinically negative neck
When pathological tumor characteristics were examined, only pathologically positive margins were significantly associated with detectable Tg compared with undetectable Tg (72 vs. 33 % respectively; p = 0.03). Patients with undetectable Tg had less advanced pathological tumor characteristics compared with patients with detectable Tg. Patients with undetectable Tg were less likely to have large primary tumors (>4 cm; 29 vs. 43 %; p = 0.495), higher pT stage (pT3/T4; 67 vs. 100 %; p = 0.076), extrathyroid extension (50 vs. 86 %; p = 0.092), and pathologically positive neck nodes (33 vs. 71 %; p = 0.072), respectively.
Outcome in PDTC Stratified by Tg Levels
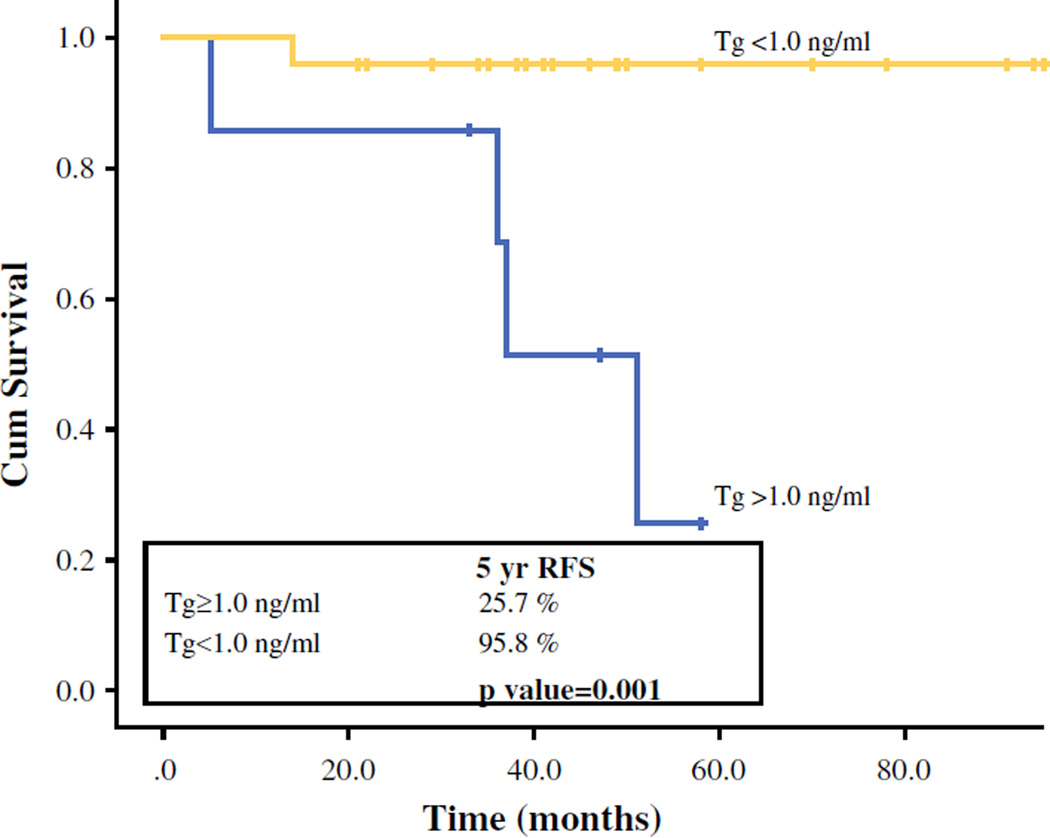
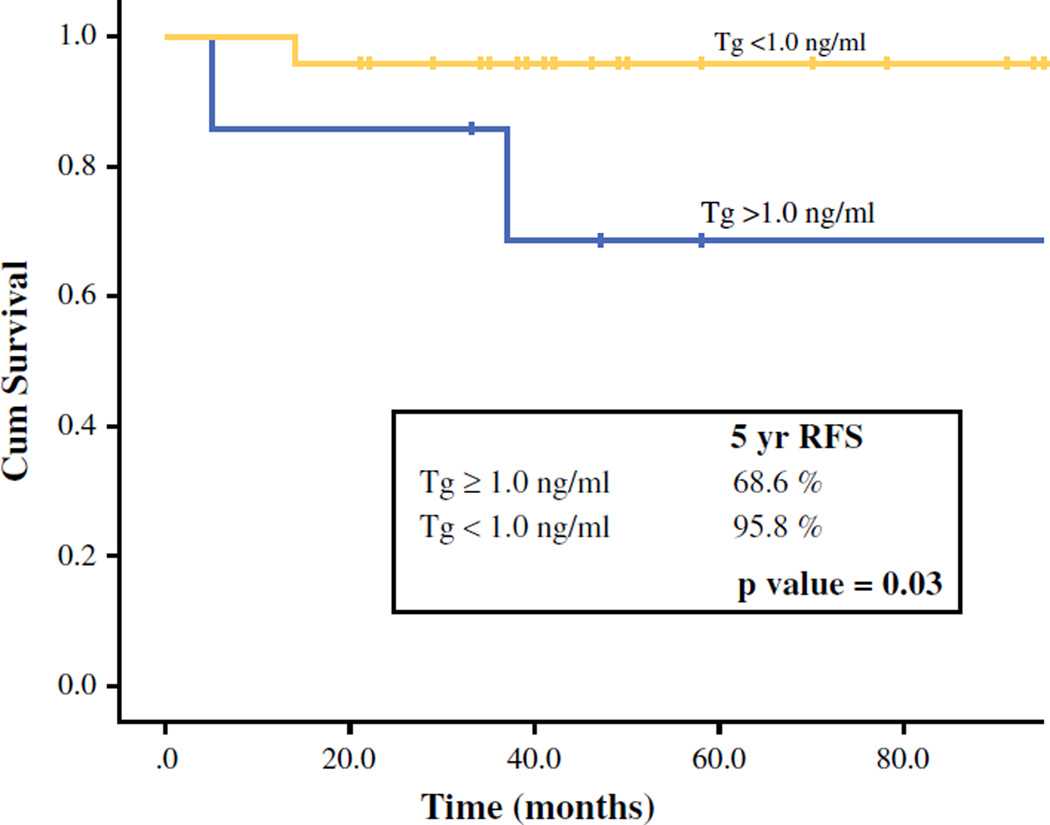
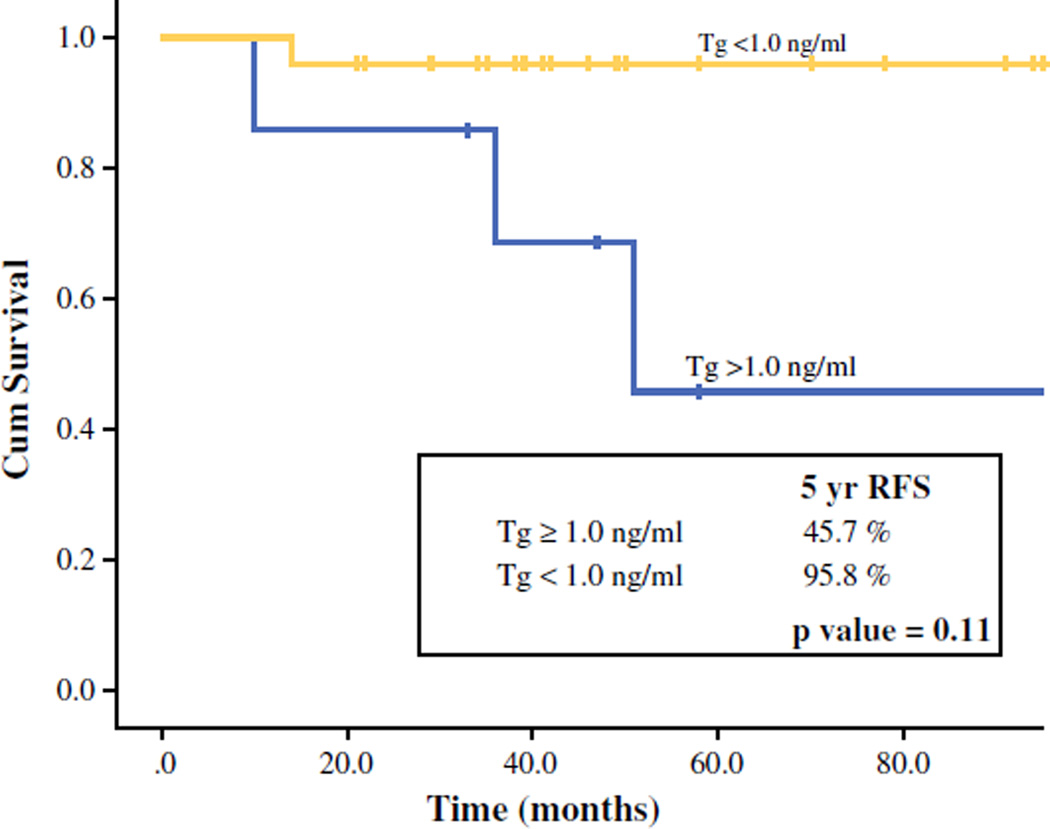
Of 31 PDTC patients in our cohort, 6 patients (19 %) died of whom 4 died of the disease. There were 6 recurrences: 1 regional and 5 distant and regional. With a median follow-up of 49 months (17–143 months), there was no significant difference in 5-year OS or DSS between patients with detectable and undetectable Tg (5-year OS: 85.7 vs. 86.3 %; p = 0.38; 5-year DSS: 100 vs. 95.8 %; p = 0.45). Patients with an undetectable Tg had significantly better 5-year recurrence control (RFS) compared with patients with detectable Tg (96 vs. 26 %; p = 0.001; Fig. 1). Patients with undetectable Tg levels had significantly better 5-year regional control (RRFS: 96 vs. 69 %; p = 0.03; Fig. 2); of 6 patients with regional recurrence during 5-year follow-up, 2 (33 %) had undetectable Tg. Patients with undetectable Tg levels also had better 5-year distant control (DRFS: 96 vs. 46 % vs. p = 0.11; Fig. 3). Of 5 patients with distant recurrence during 5 year follow-up, 2 (40 %) had undetectable Tg. Two of 24 patients with undetectable Tg had distant recurrences: 1 to lung and 1 to lung and bone. Three of seven patients with detectable Tg had distant recurrences: 1 to lung, 1 to lung and bones, and 1 to lung, bones, and liver. Two patients with undetectable Tg that developed both distant and regional recurrence received EBRT (external beam radiation therapy) and EBRT + RAI, respectively. Three patients with detectable Tg that developed both regional and distant recurrences received: (1) neck dissection and RAI; (2) neck dissection and EBRT/experimental systemic therapy; and (3) RAI plus an operation of solitary lung metastasis, respectively. One patient with detectable Tg levels that developed regional recurrence received a neck dissection.
FIG. 1.
Five-year, recurrence-free survival (RFS) stratified by Tg level
FIG. 2.
Five-year, regional, recurrence-free survival (RRFS) stratified by Tg level
FIG. 3.
Five-year, distant, recurrence-free survival (DRFS) stratified by Tg level
DISCUSSION
Poorly differentiated thyroid carcinoma (PDTC) is a rare type of thyroid cancer with biologically and histologically intermediate characteristics on a progression scale from DTC to undifferentiated or anaplastic thyroid carcinoma (AC).8,9 Despite loss of some of the well-differentiated features, PDTC still contains colloid and produces Tg.10,11 This is due to tumor heterogeneity within the primary tumor mass. However, due to the rarity of PDTC, data on Tg production and the possible predictive role in PDTC are unclear.4,5 The objective of our study was to report on Tg values in PDTC following total thyroidectomy and adjuvant RAI, correlate Tg values with outcome in PDTC, and determine if an undetectable Tg level predicted low-risk for recurrence.
Based on the observations from our study, thyroglobulin appears to have a prognostic role in the prediction of recurrence in patients with PDTC. In our study, we found that PDTC patients who were free of macroscopic disease after initial treatment and had undetectable Tg showed significantly better 5-year RFS compared with patients with detectable Tg (96 vs. 26 %; p = 0.001). In particular, 5-year regional control and distant control was better for our patients with undetectable Tg levels (5-year RRFS 96 vs. 69 %; p = 0.03; 5-year DRFS 96 vs. 46 % vs. p = 0.11). Similar to our study of PDTC, the study by van Dijk et al. of DTC found that after initial surgery and adjuvant RAI therapy, patients with detectable Tg and negative posttherapeutic WBS had significant earlier and more recurrences than patients without detectable Tg.12 Survival in both DTC groups with detectable and undetectable Tg was comparable as we found in our PDTC cohort.
As in DTC, we also report that patients who had detectable Tg were more likely to have larger tumors, ETE, positive neck disease, and also positive margins following thyroidectomy. These observations mirror those found in differentiated thyroid cancer.
Our data suggest that measurement of serum Tg is valuable in the follow-up of both DTC and PDTC. If an undetectable Tg level is obtained after initial surgery and adjuvant RAI, then the risk of recurrence is low. However, this is not absolute, because two of our patients with undetectable Tg levels had recurrence (2/24). This is most likely due to tumor heterogeneity and the presence of a less differentiated tumor component. Therefore, if undetectable Tg level is obtained after initial treatment, the physician must still be aware that a small subset of PDTC patients might recur due to the presence of a less differentiated tumor component.
Our study is not without its limitations however. The study is retrospective and therefore susceptible to the deficiencies associated with retrospective data collection. In addition, our sample size was small (31 patients). However, PDTC is a rare type of thyroid cancer and our cohort was created from one of the largest available series of PDTC patients (91 patients) who have been treated at a single tertiary care center. It is unlikely that other centers will have any more detailed information on the role of Tg in PDTC. Another strength of the cohort was that all had pathology review and that a single diagnostic criteria based on high grade features (mitosis and necrosis) was used to classify the patients. These diagnostic criteria have been shown to define a biologically more homogenous group of tumors.6 Selection bias is another clear limitation to the data. The 5-year OS for our cohort (86 %) was better than most reports on 5-year OS in PDTC, which range from 62 to 85 %.13–16 This reflects the fact that our 31 PDTC patients represent a cohort that was free of distant metastases at presentation (M0) and free of macroscopic disease after initial treatment with surgery and adjuvant RAI. In addition, it is possible that the selected cohort of tumors has heterogeneity with areas of differentiated carcinoma coexisting with PDTC that still secrete Tg. Lastly, although we stated that there was no difference in OS and DSS between patients with detectable and undetectable Tg levels, the follow-up of our cohort was limited to 49 months. Even with PDTC, recurrences and deaths can occur after our median follow-up time of 49 months.
Despite these limitations, we conclude that many PDTC, who are M0 at presentation, retain the ability to produce Tg after initial treatment with surgery and adjuvant RAI. Those PDTC patients with undetectable Tg levels show lower rates of distant recurrence and significantly lower rates of regional recurrence. Tg therefore may serve as a predictor for recurrence in PDTC. However, in a small subset of PDTC patients with undetectable Tg after initial treatment, undetectable levels may not be a reliable indicator for recurrence due to presence of a more aggressive and less differentiated component in a heterogenous tumor. We emphasize the need for future studies with a larger sample size to reach a definitive conclusion. Further studies, most likely with multi-institutional collaboration due to the rarity of these tumors, may help to confirm our observations.
Footnotes
Presented at the IFHNOS 5th World Congress/AHNS meeting 2014, New York as an oral podium presentation.
DISCLOSURE Authors have nothing to disclose.
REFERENCES
- 1.Shaha AR, Loree TR, Shah JP. Intermediate-risk group for differentiated carcinoma of thyroid. Surgery. 1994;116(6):1036–1040. discussion 1040–1. [PubMed] [Google Scholar]
- 2.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1114. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahimpasic T, Nixon IJ, Palmer FL, et al. Undetectable thyroglobulin after total thyroidectomy in patients with low- and intermediate-risk papillary thyroid cancer: is there a need for radioactive iodine therapy? Surgery. 2012;152(6):1096–1105. doi: 10.1016/j.surg.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Robbins RJ, Srivastava S, Shaha A, et al. Factors influencing the basal and recombinant human thyrotropin-stimulated serum thyroglobulin in patients with metastatic thyroid carcinoma. J Clin Endocrinol Metab. 2004;89(12):6010–6016. doi: 10.1210/jc.2003-031573. [DOI] [PubMed] [Google Scholar]
- 5.Tuttle RM, Grewal RK, Larson SM. Radioactive iodine therapy in poorly differentiated thyroid cancer. Nat Clin Pract Oncol. 2007;4(11):665–668. doi: 10.1038/ncponc0979. [DOI] [PubMed] [Google Scholar]
- 6.Hiltzik D, Carlson D, Tuttle RM, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006;106(6):1286–1295. doi: 10.1002/cncr.21739. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti AE. AJCC cancer staging manual 7th edn. New york: Springer; 2010. [Google Scholar]
- 8.Sanders EM, Jr, LiVolsi VA, Brierley J, Shin J, Randolph GW. An evidence-based review of poorly differentiated thyroid cancer. World J Surg. 2007;31(5):934–945. doi: 10.1007/s00268-007-9033-3. [DOI] [PubMed] [Google Scholar]
- 9.Kakudo K, Bai Y, Katayama S, et al. Classification of follicular cell tumors of the thyroid gland: analysis involving Japanese patients from one institute. Pathol Int. 2009;59(6):359–367. doi: 10.1111/j.1440-1827.2009.02378.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosai J, Saxen EA, Woolner L. Undifferentiated and poorly differentiated carcinoma. Sem Diagn Pathol. 1985;2(2):123–136. [PubMed] [Google Scholar]
- 11.Killeen RM, Barnes L, Watson CG, Marsh WL, Chase DW, Schuller DE. Poorly differentiated (“insular”) thyroid carcinoma. Report of two cases and review of the literature. Arch Otolaryngol Head Neck Surg. 1990;116(9):1082–1086. doi: 10.1001/archotol.1990.01870090098018. [DOI] [PubMed] [Google Scholar]
- 12.van Dijk D, Plukker JT, van der Horst-Schrivers AN, et al. The value of detectable thyroglobulin in patients with differentiated thyroid cancer after initial (1)(3)(1)I therapy. Clin Endocrinol (Oxf) 2011;74(1):104–110. doi: 10.1111/j.1365-2265.2010.03885.x. [DOI] [PubMed] [Google Scholar]
- 13.Jung TS, Kim TY, Kim KW, et al. Clinical features and prognostic factors for survival in patients with poorly differentiated thyroid carcinoma and comparison to the patients with the aggressive variants of papillary thyroid carcinoma. Endocr J. 2007;54(2):265–274. doi: 10.1507/endocrj.k06-166. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto A, Kasai N, Sugano H. Poorly differentiated carcinoma of the thyroid. A clinicopathologic entity for a high-risk group of papillary and follicular carcinomas. Cancer. 1983;52(10):1849–1855. doi: 10.1002/1097-0142(19831115)52:10<1849::aid-cncr2820521015>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Volante M, Landolfi S, Chiusa L, et al. Poorly differentiated carcinomas of the thyroid with trabecular, insular, and solid patterns: a clinicopathologic study of 183 patients. Cancer. 2004;100(5):950–957. doi: 10.1002/cncr.20087. [DOI] [PubMed] [Google Scholar]
- 16.Asioli S, Erickson LA, Righi A, et al. Poorly differentiated carcinoma of the thyroid: validation of the Turin proposal and analysis of IMP3 expression. Mod Pathol. 2010;23(9):1269–1278. doi: 10.1038/modpathol.2010.117. [DOI] [PubMed] [Google Scholar]