Abstract
IMPORTANCE
Postoperative complications after head and neck surgery carry the potential for significant morbidity. Estimating the risk of complications in an individual patient is challenging.
OBJECTIVE
To develop a statistical tool capable of predicting an individual patient’s risk of developing a major complication after surgery for oral cavity squamous cell carcinoma.
DESIGN, SETTING, AND PARTICIPANTS
Retrospective case series derived from an institutional clinical oncologic database, augmented by medical record abstraction, at an academic tertiary care cancer center. Participants were 506 previously untreated adult patients with biopsy-proven oral cavity squamous cell carcinoma who underwent surgery between January 1, 2007, and December 31, 2012.
MAIN OUTCOMES AND MEASURES
The primary end point was a major postoperative complication requiring invasive intervention (Clavien-Dindo classification grades III–V). Patients treated between January 1, 2007, and December 31, 2008 (354 of 506 [70.0%]) comprised the modeling cohort and were used to develop a nomogram to predict the risk of developing the primary end point. Univariable analysis and correlation analysis were used to prescreen 36 potential predictors for incorporation in the subsequent multivariable logistic regression analysis. The variables with the highest predictive value were identified with the step-down model reduction method and included in the nomogram. Patients treated between January 1, 2007, and December 31, 2008 (152 of 506 [30.0%]) were used to validate the nomogram.
RESULTS
Clinical characteristics were similar between the 2 cohorts for most comparisons. Thirty-six patients in the modeling cohort (10.2%) and 16 patients in the validation cohort (10.5%) developed a major postoperative complication. The 6 preoperative variables with the highest individual predictive value were incorporated within the nomogram, including body mass index, comorbidity status, preoperative white blood cell count, preoperative hematocrit, planned neck dissection, and planned tracheotomy. The nomogram predicted a major complication with a validated concordance index of 0.79. Inclusion of surgical operative variables in the nomogram maintained predictive accuracy (concordance index, 0.77).
CONCLUSIONS AND RELEVANCE
A statistical tool was developed that accurately estimates an individual patient’s risk of developing a major complication after surgery for oral cavity squamous cell carcinoma.
Postoperative complications after head and neck surgery carry the potential for functional and aesthetic morbidity, prolonged hospitalization, increased cost of treatment, delay in initiation of postoperative adjuvant therapy, and higher risk of mortality.1,2 Furthermore, because of the inherent implications related to alteration of normal anatomy and physiology, surgery for oral cavity cancer in particular invariably affects physiological function, quality of life, and psychological well-being.3,4 Therefore, gauging and setting appropriate patient expectations as part of preoperative counseling, including anticipation of potential complications, are of paramount importance.
The surgical management of oral cavity cancers is complex, frequently entailing extensive resections and complicated reconstructions. In addition, many patients with oral cavity cancer carry a litany of formidable medical comorbidities.5 The interplay between long, complex operations and preexisting medical conditions can substantially affect an individual’s risk of developing postoperative complications.6 Quantification of risk in an individual patient may allow surgeons to more effectively identify patients at higher risk of complications and develop strategies for prevention, timely recognition, proactive management, and informed consent from the patient.7
While the risk of complications may be obvious in certain situations, it is difficult to precisely quantify in most patients. Experienced physicians base clinical management on their anticipated estimation of the risk of complications in a particular patient. However, consistently precise quantification of this risk is difficult. Consequently, most patients are provided imprecise estimates. More precise estimation of risk may improve the physician’s ability to counsel patients, assist in the allocation of resources (intensive care monitoring, nursing staff, etc), and potentially help normalize outcomes reporting to allow meaningful comparisons of quality of care. Nomograms are advantageous because they provide individualized risk assessment in a user-friendly and dynamic manner.8 The aim of this study was to develop a statistical tool capable of predicting an individual patient’s risk of developing a major complication after surgery for oral cavity squamous cell carcinoma (OCSCC).
Methods
Patients
Eligible for inclusion in the study were 506 patients with previously untreated OCSCC without distant metastasis or unresectable locoregional disease at presentation who underwent surgery using general anesthesia at Memorial Sloan Kettering Cancer Center between January 1, 2007, and December 31, 2012. After approval by Memorial Sloan Kettering Institutional Review Board, the medical records of eligible patients were accessed and reviewed. Patients treated between January 1, 2009, and December 31, 2012 (354 of 506 [70.0%]), herein known as the modeling cohort, were used to create a nomogram to predict the risk of developing major postoperative complications. Complications experienced by the patients in the modeling cohort have been reported elsewhere.9 An independent set of patients treated between January 1, 2007, and December 31, 2008 (152 of 506 [30.0%]), herein known as the validation cohort, was used to validate the nomograms generated by the modeling cohort. In total, 36 potential predictors of developing complications were selected based on a review of the literature and clinical experience. Patient demographics, social habits, oncologic characteristics, preoperative laboratory values, and operative details for both cohorts were retrieved from the medical records. Medical comorbidity status was evaluated with the American Society of Anesthesiologists physical status classification,10 the Karnofsky Performance Status Scale,11 and the Washington University Head and Neck Comorbidity Index.12 Clinical staging was recorded using the seventh edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual.13
Primary Outcome
The method whereby we described and classified postoperative complications within this data set is comprehensively described elsewhere.9 Briefly, the postoperative period was defined as the interval from the date of surgery to either the date of discharge from the hospital or 45 days after surgery, whichever occurred later. A postoperative complication was defined as any deviation from the normal postoperative course not better explained by a previous medical condition, not inherent to the procedure or hospital course, and not reflective of the underlying pathophysiology of the primary diagnosis. Complication severity was graded based on the revised Clavien-Dindo classification.14,15 Minor complications were considered those requiring no or minimal therapeutic intervention and were classified as grades I and II, respectively. Major complications were considered those requiring surgical intervention or intensive care or resulting in death and were classified as grades III, IV, and V, respectively. Hereafter, we report complication severity as complications requiring any intervention (grade ≥II) and complications requiring invasive intervention (grade ≥III). Our primary outcome of interest was the incidence of complications requiring invasive intervention.
Statistical Analysis
A nomogram was developed to predict complications in a dynamic fashion at 2 discrete time points during an individual patient’s treatment cycle. The nomogram was designed for use during presurgical counseling and in the postoperative setting, once operative details become available. The end point of interest for both nomograms was the ability to predict the probability of developing a major complication requiring invasive intervention (grade ≥III).
To preserve our sample size, while limiting selection bias, multivariate imputation by chained equations was used to impute missing values before conducting the multivariable analysis.16 The factors predictive of complications requiring invasive intervention were identified by univariable analysis and correlation analysis. Statistically significant predictors were incorporated in the subsequent multivariable logistic regression analysis. The variables with the highest predictive value were identified with the step-down model reduction method and included in the final nomogram. Validation was performed internally, with bootstrapping to correct for overfitting bias, and externally, with the validation cohort. The concordance indexes were calculated to measure discrimination ability, with values ranging from 0.5 (no discrimination) to 1.0 (perfect discrimination). Calibration was measured by plotting the expected against the observed probabilities of developing a complication.
Clinical characteristics and complication rates were compared between the modeling cohort and the validation cohort. The Pearson χ2 test was used for categorical variables, and the t test was used for continuous variables. P ≤ .05 was considered significant. Statistical analyses were performed using a software program (SPSS, version 21; IBM Corporation).
Results
Modeling Cohort
Thirty-six variables were tested as potential predictors of complications in the modeling cohort (summarized in the Table). In total, 36 of 354 patients (10.2%) developed a major postoperative complication requiring invasive intervention (grade ≥III).
Table.
Potential Predictors of Developing Complications in the Modeling Cohort
| Variable | Q1–Q3 | Median (Range) | Mean (SD) | No. (%) of 354 Patients |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 52–73.2 | 61.7 (26.6–93.7) | 62.2 (14.0) | NA |
| Sex | ||||
| Male | NA | NA | NA | 209 (59.0) |
| Female | NA | NA | NA | 145 (41.0) |
| Smoking | ||||
| Current | NA | NA | NA | 100 (28.2) |
| Former | NA | NA | NA | 123 (34.7) |
| Never | NA | NA | NA | 131 (37.0) |
| Alcohol use ≥5drinks per week | ||||
| Yes | NA | NA | NA | 86 (24.3) |
| No | NA | NA | NA | 268 (75.7) |
| Comorbidities | ||||
| BMI | 24–31.1 | 26.9 (14.9–61.1) | 27.7 (5.9) | NA |
| WUHNCI | 0–1 | 0 (0–10) | 0.7 (1.4) | NA |
| Severe comorbidity, WUHNCI ≥1 | ||||
| Yes | NA | NA | NA | 116 (32.8) |
| No | NA | NA | NA | 238 (67.2) |
| Karnofsky Performance Status Scale score | ||||
| Normal, 100 | NA | NA | NA | 264 (74.6) |
| Symptoms, 90 | NA | NA | NA | 61 (17.2) |
| Difficulty or worse, ≤80 | NA | NA | NA | 29 (8.2) |
| American Society of Anesthesiologists physical status classification | ||||
| No or moderate limitation, ≤2 | NA | NA | NA | 128 (36.2) |
| Severe limitation, >2 | NA | NA | NA | 226 (63.8) |
| TNM Stage | ||||
| Clinical T classification | ||||
| T1 | NA | NA | NA | 152 (42.9) |
| T2 | NA | NA | NA | 121 (34.2) |
| T3 | NA | NA | NA | 26 (7.3) |
| T4a | NA | NA | NA | 55 (15.5) |
| Clinical N classification | ||||
| N0 | NA | NA | NA | 237 (66.9) |
| N1 | NA | NA | NA | 43 (12.1) |
| N2 | NA | NA | NA | 72 (20.3) |
| N3 | NA | NA | NA | 2 (0.6) |
| Overall TNM stage | ||||
| I | NA | NA | NA | 132 (37.3 |
| II | NA | NA | NA | 74 (20.9) |
| III | NA | NA | NA | 47 (13.3) |
| IV | NA | NA | NA | 101 (28.5) |
| Preoperative Laboratory Values | ||||
| White blood cell count, ×103/μL (n = 352) | 5.8–8.3 | 7.1 (1.7–29.9) | 7.3 (2.5) | NA |
| Hemoglobin level, g/dL (n = 352) | 12.9–14.7 | 13.7 (9.8–17.7) | 13.7 (1.4) | NA |
| Hematocrit, % (n = 352) | 38.5–43.3 | 40.9 (29.5–52.0) | 40.7 (3.8) | NA |
| Platelet count, ×103/μL (n = 351) | 207–296 | 248 (71–815) | 258.7 (84.0) | NA |
| International normalized ratio (n = 345) | 1.0–1.1 | 1.0 (0.9–3.1) | 1.1 (0.2) | NA |
| Activated partial thromboplastin time, s (n = 341) | 27.7–31.7 | 29.4 (21.2–56.3) | 29.9 (3.6) | NA |
| Sodium level, mEq/L (n = 352) | 139–142 | 141 (128–147) | 140.3 (2.8) | NA |
| Potassium level, mEq/L (n = 352) | 4.2–4.7 | 4.5 (3.0–5.7) | 4.5 (0.4) | NA |
| Serum creatinine level, mg/dL (n = 352) | 0.8–1.1 | 1.0 (0.5–3.1) | 1.0 (0.3) | NA |
| Albumin level, g/dL (n = 350) | 4.1–4.5 | 4.3 (2.7–5.2) | 4.3 (0.3) | NA |
| Total bilirubin level, mg/dL (n = 350) | 0.4–0.8 | 0.6 (0.2–2.2) | 0.6 (0.3) | NA |
| Aspartate aminotransferase level, U/L (n = 350) | 20–31 | 24 (12–334) | 28.5 (20.2) | NA |
| Alkaline phosphatase level, U/L (n = 350) | 63–93 | 76 (26–480) | 82.4 (40.4) | NA |
| Operation | ||||
| Neck dissection | ||||
| Yes | NA | NA | NA | 247 (69.8) |
| No | NA | NA | NA | 107 (30.2) |
| Bone resectiona | ||||
| Yes | NA | NA | NA | 125 (35.3) |
| No | NA | NA | NA | 229 (64.7) |
| Neck dissection laterality (n = 247) | ||||
| Ipsilateral | NA | NA | NA | 210 (85.0) |
| Bilateral | NA | NA | NA | 37 (15.0) |
| Neck dissection extent (n = 247) | ||||
| Selective | NA | NA | NA | 173 (70.0) |
| Comprehensive | NA | NA | NA | 74 (30.0) |
| Reconstructionb | ||||
| Yes | NA | NA | NA | 139 (39.3) |
| No | NA | NA | NA | 215 (60.7) |
| Tracheotomy | ||||
| Yes | NA | NA | NA | 93 (26.3) |
| No | NA | NA | NA | 261 (73.7) |
| Anesthesia Details | ||||
| Anesthesia time, min | 132–425 | 233.5 (30–1327) | 322.9 (261.3) | NA |
| Volume of colloid transfused, mL | 0–500 | 0 (0–5000) | 243.3 (459.7) | NA |
| Fluid balance, mL | 990–3240 | 1550 (75–11 680) | 2321.8 (1950.0) | NA |
| Estimated blood loss, mL | 20–200 | 50 (0–1150) | 145.2 (180.3) | NA |
| Perioperative antibiotics | ||||
| Yes | NA | NA | NA | 334 (94.4) |
| No | NA | NA | NA | 20 (5.6) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable; Q1, first quintile; Q3, third quintile; WUHNCI, Washington University Head and Neck Comorbidity Index.
SI conversion factors: To convert white blood cell count to ×109/L, multiply by 0.001; hemoglobin level to grams per liter, multiply by 10.0; hematocrit to proportion of 1.0, multiply by 0.01; platelet count to ×109/μL, multiply by1.0; sodium and potassium levels to millimoles per liter, multiply by 1.0; serum creatinine level to micromoles per liter, multiply by 88.4; albumin level to grams per liter, multiply by 10; total bilirubin level to micromoles per liter, multiply by 17.104; aspartate aminotransferase and alkaline phosphatase levels to microkatals per liter, multiply by 0.0167.
Includes partial maxillectomy, marginal mandibulectomy, and segmental mandibulectomy.
Includes split-thickness and full-thickness skin grafts, human acellular tissue matrix graft, local and regional flaps, and microvascular free tissue transfer.
The factors predictive of complications requiring invasive intervention were identified by univariable analysis (eTable 1 in the Supplement). Statistically significant predictors were incorporated in the subsequent multivariable logistic regression analysis, and the variables with the highest individual predictive value were incorporated within the nomogram (eTable 2 in the Supplement). In the preoperative setting, lower body mass index (BMI) and lower hematocrit increased the risk of developing a complication requiring invasive intervention. In addition, higher Washington University Head and Neck Comorbidity Index, elevated preoperative white blood cell count, planned neck dissection, and planned tracheotomy increased the risk of developing a complication requiring invasive intervention. Figure 1 shows the nomogram for use in the preoperative setting, with a concordance index of 0.82.
Figure 1. Preoperative Nomogram Predicting Major Complications.
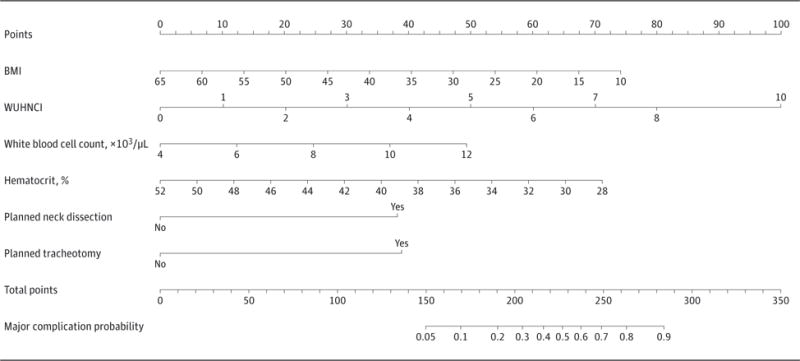
Shown is a nomogram predicting the probability of developing a complication requiring invasive intervention (grade ≥III) based on preoperative variables only. BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); WUHNCI, Washington University Head and Neck Comorbidity Index. To convert white blood cell count to ×109/L, multiply by 0.001; to convert hematocrit to proportion of 1.0, multiply by 0.01.
Figure 2 shows the nomogram for use in the postoperative setting. By adding intraoperative and postoperative variables to the existing preoperative variables, the concordance index of the nomogram remained high, at 0.82. Excellent calibration was maintained between the 2 settings (eFigure 1 in the Supplement).
Figure 2. Postoperative Nomogram Predicting Major Complications.

Shown is a nomogram predicting the probability of developing a complication requiring invasive intervention (grade ≥III) after postoperative variables become available. WUHNCI indicates Washington University Head and Neck Comorbidity Index. To convert white blood cell count to ×109/L, multiply by 0.001; to convert hematocrit to proportion of 1.0, multiply by 0.01.
Validation Cohort
The nomogram was tested on an independent cohort of patients at the preoperative and postoperative time points. In this cohort, 16 of 152 patients (10.5%) developed a major postoperative complication requiring invasive intervention. Clinical characteristics were similar between the modeling and validation cohorts (P > .05 for most comparisons) (eTable 3 in the Supplement). The mean estimated blood loss was significantly greater in the validation cohort (189.1 vs 145.2 mL, P = .03) while complication rates were similar between the 2 cohorts (10.5% [16 of 152] vs 10.2% [35 of 354], P = .90).
At the preoperative time point, the nomogram predicted a major complication requiring invasive intervention (grade ≥III) with a validated concordance index of 0.79. By adding intraoperative and postoperative variables to the existing preoperative variables, the nomogram maintained its predictive value (concordance index, 0.77). Good calibration was observed in both settings (eFigure 2 in the Supplement).
An additional nomogram was created to predict complications requiring any intervention (grade ≥II) to be used at the preoperative (Figure 3) and postoperative (Figure 4) time points. This nomogram was developed and validated using the same method as the aforementioned nomogram. Its concordance indexes at the preoperative and postoperative time points were 0.83 and 0.85, respectively, with excellent calibration. The nomogram maintained its predictive value in the validation cohort.
Figure 3. Preoperative Nomogram Predicting All Complications.
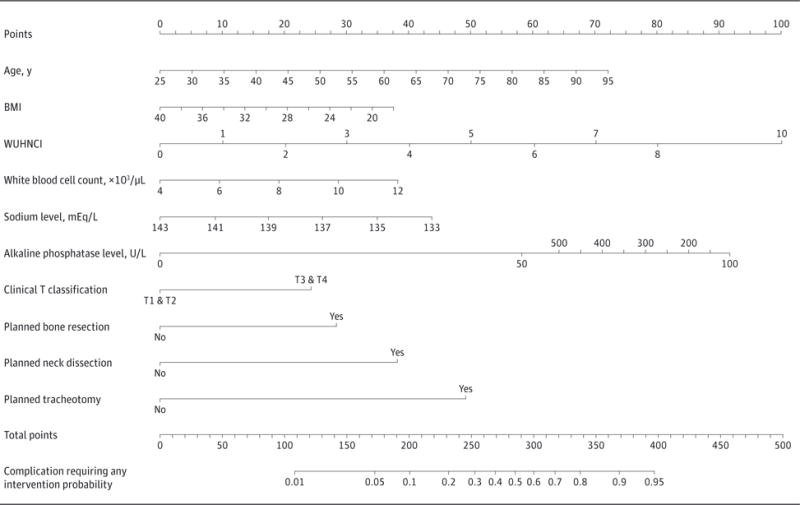
Shown is a nomogram predicting the probability of developing a complication requiring any intervention (grade ≥II) based on preoperative variables only. BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); WUHNCI, Washington University Head and Neck Comorbidity Index. To convert white blood cell count to ×109/L, multiply by 0.001; to convert sodium level to millimoles per liter, multiply by 1.0; and to convert alkaline phosphatase level to microkatals per liter, multiply by 0.0167.
Figure 4. Postoperative Nomogram Predicting All Complications.
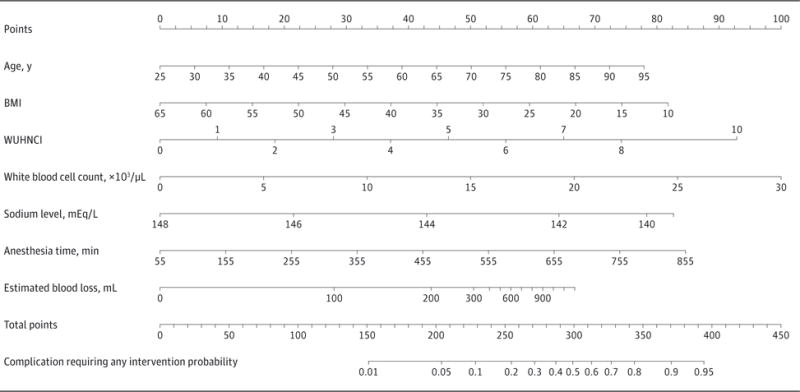
Shown is a nomogram predicting the probability of developing a complication requiring any intervention (grade ≥II) after postoperative variables become available. BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); WUHNCI, Washington University Head and Neck Comorbidity Index. To convert white blood cell count to ×109/L, multiply by 0.001; to convert sodium level to millimoles per liter, multiply by 1.0.
Discussion
We created nomograms that facilitate the accurate prediction of clinically relevant complications after surgical treatment of OCSCC. Although there are many studies in the literature that have investigated predictors of postoperative complications in head and neck surgery, to our knowledge, only one other study17 has presented the data in the form of a validated nomogram (further described and compared 2 paragraphs below). Nomograms are advantageous because they provide individualized risk assessment in a user-friendly and dynamic manner.8 Their judicious use could minimize rates of failure to rescue (or mortality after a major complication), improve morbidity rates, and ultimately enhance quality of care in head and neck oncologic surgery.18
Comparisons with other studies1,6,19–34 in the literature are hampered by variability of tumor site in the head and neck region, sample size, and the surgical procedure performed (ablative or reconstructive), as well as whether or not multimodal treatment was included. Equally important is the pervasive inconsistency in the definition of complications across such studies.9
Our nomogram demonstrated a subtle fall in discriminatory ability after validation, with concordance indexes decreasing from 0.82 to 0.77. In the study by Santoro et al,17 who developed a complication prediction model with a similar study design, a negligible decline in concordance indexes from 0.79 to 0.74 after validation was reported. However, their study was designed to predict any complication, irrespective of severity, and used a predetermined list of possible complications rather than a standardized definition thereof. Furthermore, the study by Santoro et al included a heterogeneous group of patients with SCC of the oropharynx in addition to the oral cavity, as well as patients who received neoadjuvant therapy. Their final model included alcohol consumption, primary site (oropharynx vs oral cavity), cT stage according to sex, and neck dissection. Primary tumors of the oropharynx, as well as the patients with these tumors, vary dramatically from their oral cavity counterparts. Therefore, it is logical that the primary site was a significant predictor of developing complications. Moreover, the advent of transoral surgery and the human papillomavirus epidemic complicate contemporary analysis of surgical complications of oropharyngeal cancer. Consequently, the practical usefulness of such a generalized predictive tool is limited.
At the preoperative time point, our nomogram included the variables that described the anticipated complexity of the operation, such as the need for bone resection, neck dissection, and tracheotomy. These variables were replaced by more specific intraoperative indicators of surgical complexity (anesthesia time and estimated blood loss) once they became available. Inclusion of these operation-related variables still maintained the predictive value of the nomogram. Consistent with our findings, Weber et al6 demonstrated that negative performance indicators, including blood product transfusion, 30-day surgical site infection, return to the operating room, and death, were dependent on procedure acuity, individual surgeon skill, and comorbidity. Other studies have also found that greater procedure complexity increased a patient’s risk of developing complications, including neck dissection,17,20,21 reconstruction,20,22,23 and tracheotomy.24,25 Surrogate operative markers of procedure complexity also increased this risk, including anesthesia time,26–28 volume of intraoperative fluid administered,26,29 and estimated blood loss.20 We included anesthesia time as a representative marker of surgical operative complexity, and this strongly correlated with positive fluid balance (R2 = 0.76), which was therefore not included in our model. Our observations highlight the need to risk adjust for procedure complexity and comorbid conditions when reporting outcomes and predicting complications.
Nomograms that are capable of accurately quantifying the risk of complications may be useful for the purpose of risk adjustment and comparison of treatment outcomes within and across institutions. Accurate and meaningful comparison of surgery-related outcomes is becoming increasingly important as the concept of pay-for-performance gains traction. A reliable method of quantifying an individual patient’s risk of complications may help normalize outcomes reporting by taking into account the expected risk based on preoperative and intraoperative variables so that actual outcomes reported within and across institutions become more meaningful.
The significant effect of comorbidities on the development of complications has been consistent across many other studies,20,24,25,27,28,30,31 as well. Other studies1,25,29 also confirmed our finding that older age was associated with increasing rates of postoperative complications. Our model has an advantage of providing precise risk probability prediction by including preoperative laboratory values. Similarly, investigators from Brazil found the Acute Physiology and Chronic Health Evaluation II score, which includes hematocrit, white blood cell count, serum creatinine and sodium levels, and other variables, to be an independent predictor of complications.20,21 However, because this measure is an aggregate disease severity classification originally designed for patients receiving intensive care, parsing out which variables most significantly affect complication risk is impossible, thereby limiting applicability of such a grading scheme to individualized risk prediction.
Other studies22,25 similarly identified low preoperative hemoglobin level (in addition to other variables) as an independent predictor of complications after microvascular free flap reconstruction of the head and neck. These studies demonstrated that low BMI was predictive of developing complications. Our data showed that lower BMI was indeed associated with higher risk of developing complications, which is likely a reflection of malnutrition and poor general health.
Limitations to our study include its retrospective design and reliance on abstraction of complications from an electronic medical record infrastructure unique to Memorial Sloan Kettering Cancer Center. Our group has previously explored the inherent limitations of data abstraction of postoperative complications in detail.9 The present study was performed at an academic, high-volume, tertiary care cancer referral center, and our findings may not be generalizable to most health care providers practicing in the community. Studies7,35 have demonstrated that rates of failure to rescue are lower at high-volume hospitals, which are believed to be associated with better hospital resources, timely recognition of complications, and proactive management. Although we successfully validated our nomogram using an independent data set, it used a similar patient population in the same setting as the modeling cohort. Therefore, before generalized acceptance of this nomogram, external validation would be desirable.
Conclusions
We developed an accurate nomogram to estimate an individual patient’s risk of developing clinically relevant postoperative complications in a dynamic fashion during his or her treatment cycle for OCSCC. This study provides proof of principle that nomograms can allow clinicians to anticipate which patients are at higher risk of developing complications after surgery, thereby facilitating timely recognition and effective management of postoperative complications.
Supplementary Material
Acknowledgments
Funding/Support: All aspects of the research presented in this article (design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication) were supported by internal funds from Memorial Sloan Kettering Cancer Center.
Role of Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Awad and Patel had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Awad, Palmer, Patel.
Acquisition, analysis, or interpretation of data: Awad, Palmer, Kou, Yu, Montero, Shuman, Patel.
Drafting of the manuscript: Awad, Palmer.
Critical revision of the manuscript for important intellectual content: Kou, Yu, Shuman, Ganly, Shah, Kattan, Patel.
Statistical analysis: Awad, Palmer, Kou, Yu, Kattan.
Study supervision: Ganly, Shah, Kattan, Patel.
Conflict of Interest Disclosures: None reported.
Previous Presentations: This study was presented at The New York Head and Neck Society 2014 Annual Proffered Paper Session; March 12, 2014; New York, New York; and at the International Federation of Head and Neck Oncologic Societies Fifth World Congress and American Head & Neck Society 2014 Annual Meeting; July 27, 2014; New York, New York.
References
- 1.Bhattacharyya N, Fried MP. Benchmarks for mortality, morbidity, and length of stay for head and neck surgical procedures. Arch Otolaryngol Head Neck Surg. 2001;127(2):127–132. doi: 10.1001/archotol.127.2.127. [DOI] [PubMed] [Google Scholar]
- 2.Ch’ng S, Choi V, Elliott M, Clark JR. Relationship between postoperative complications and survival after free flap reconstruction for oral cavity squamous cell carcinoma. Head Neck. 2014;36(1):55–59. doi: 10.1002/hed.23266. [DOI] [PubMed] [Google Scholar]
- 3.Ojo B, Genden EM, Teng MS, Milbury K, Misiukiewicz KJ, Badr H. A systematic review of head and neck cancer quality of life assessment instruments. Oral Oncol. 2012;48(10):923–937. doi: 10.1016/j.oraloncology.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weymuller EA, Yueh B, Deleyiannis FW, Kuntz AL, Alsarraf R, Coltrera MD. Quality of life in patients with head and neck cancer: lessons learned from 549 prospectively evaluated patients. Arch Otolaryngol Head Neck Surg. 2000;126(3):329–335. doi: 10.1001/archotol.126.3.329. [DOI] [PubMed] [Google Scholar]
- 5.Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110(4):593–602. doi: 10.1097/00005537-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Weber RS, Lewis CM, Eastman SD, et al. Quality and performance indicators in an academic department of head and neck surgery. Arch Otolaryngol Head Neck Surg. 2010;136(12):1212–1218. doi: 10.1001/archoto.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361(14):1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 8.Patel SG, Lydiatt WM. Staging of head and neck cancers: is it time to change the balance between the ideal and the practical? J Surg Oncol. 2008;97(8):653–657. doi: 10.1002/jso.21021. [DOI] [PubMed] [Google Scholar]
- 9.Awad MI, Shuman AG, Montero PH, Palmer FL, Shah JP, Patel SG. Accuracy of administrative and clinical registry data in reporting postoperative complications after surgery for oral cavity squamous cell carcinoma. Head Neck. 2015;37(6):851–861. doi: 10.1002/hed.23682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA. 1961;178:261–266. doi: 10.1001/jama.1961.03040420001001. [DOI] [PubMed] [Google Scholar]
- 11.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- 12.Piccirillo JF, Lacy PD, Basu A, Spitznagel EL. Development of a new head and neck cancer–specific comorbidity index. Arch Otolaryngol Head Neck Surg. 2002;128(10):1172–1179. doi: 10.1001/archotol.128.10.1172. [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer (AJCC) Cancer Staging Manual. 7th. New York, NY: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 14.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 15.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. [Google Scholar]
- 17.Santoro L, Tagliabue M, Massaro MA, et al. An algorithm to predict postoperative complications in oropharyngeal and oral cavity carcinoma. Head Neck. 2015;37(4):548–556. doi: 10.1002/hed.23637. [DOI] [PubMed] [Google Scholar]
- 18.Roman BR, Awad MI, Patel SG. Defining value-driven care in head and neck oncology. Curr Oncol Rep. 2015;17(1):424–431. doi: 10.1007/s11912-014-0424-y. [DOI] [PubMed] [Google Scholar]
- 19.Thomas M, George NA, Gowri BP, George PS, Sebastian P. A novel morbidity prediction model for head and neck oncosurgery. Indian J Surg. 2010;72(6):463–469. doi: 10.1007/s12262-010-0161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Cássia Braga Ribeiro K, Kowalski LP, Dias de Oliveira Latorre MdoR Perioperative complications, comorbidities, and survival in oral or oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2003;129(2):219–228. doi: 10.1001/archotol.129.2.219. [DOI] [PubMed] [Google Scholar]
- 21.de Melo GM, Ribeiro KC, Kowalski LP, Deheinzelin D. Risk factors for postoperative complications in oral cancer and their prognostic implications. Arch Otolaryngol Head Neck Surg. 2001;127(7):828–833. [PubMed] [Google Scholar]
- 22.Shah MD, Goldstein DP, McCluskey SA, et al. Blood transfusion prediction in patients undergoing major head and neck surgery with free-flap reconstruction. Arch Otolaryngol Head Neck Surg. 2010;136(12):1199–1204. doi: 10.1001/archoto.2010.202. [DOI] [PubMed] [Google Scholar]
- 23.Wu CC, Lin PY, Chew KY, Kuo YR. Free tissue transfers in head and neck reconstruction: complications, outcomes and strategies for management of flap failure: analysis of 2019 flaps in single institute. Microsurgery. 2014;34(5):339–344. doi: 10.1002/micr.22212. [DOI] [PubMed] [Google Scholar]
- 24.McGurk MG, Fan KF, MacBean AD, Putcha V. Complications encountered in a prospective series of 182 patients treated surgically for mouth cancer. Oral Oncol. 2007;43(5):471–476. doi: 10.1016/j.oraloncology.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Patel RS, McCluskey SA, Goldstein DP, et al. Clinicopathologic and therapeutic risk factors for perioperative complications and prolonged hospital stay in free flap reconstruction of the head and neck. Head Neck. 2010;32(10):1345–1353. doi: 10.1002/hed.21331. [DOI] [PubMed] [Google Scholar]
- 26.Farwell DG, Reilly DF, Weymuller EA, Jr, Greenberg DL, Staiger TO, Futran NA. Predictors of perioperative complications in head and neck patients. Arch Otolaryngol Head Neck Surg. 2002;128(5):505–511. doi: 10.1001/archotol.128.5.505. [DOI] [PubMed] [Google Scholar]
- 27.Ferrier MB, Spuesens EB, Le Cessie S, Baatenburg de Jong RJ. Comorbidity as a major risk factor for mortality and complications in head and neck surgery. Arch Otolaryngol Head Neck Surg. 2005;131(1):27–32. doi: 10.1001/archotol.131.1.27. [DOI] [PubMed] [Google Scholar]
- 28.Singh B, Cordeiro PG, Santamaria E, Shaha AR, Pfister DG, Shah JP. Factors associated with complications in microvascular reconstruction of head and neck defects. Plast Reconstr Surg. 1999;103(2):403–411. doi: 10.1097/00006534-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Haughey BH, Wilson E, Kluwe L, et al. Free flap reconstruction of the head and neck: analysis of 241 cases. Otolaryngol Head Neck Surg. 2001;125(1):10–17. doi: 10.1067/mhn.2001.116788. [DOI] [PubMed] [Google Scholar]
- 30.Borggreven PA, Kuik DJ, Quak JJ, de Bree R, Snow GB, Leemans CR. Comorbid condition as a prognostic factor for complications in major surgery of the oral cavity and oropharynx with microvascular soft tissue reconstruction. Head Neck. 2003;25(10):808–815. doi: 10.1002/hed.10291. [DOI] [PubMed] [Google Scholar]
- 31.Suh JD, Sercarz JA, Abemayor E, et al. Analysis of outcome and complications in 400 cases of microvascular head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 2004;130(8):962–966. doi: 10.1001/archotol.130.8.962. [DOI] [PubMed] [Google Scholar]
- 32.Loupatatzi A, Stavrianos SD, Karantonis FF, et al. Are females predisposed to complications in head and neck cancer free flap reconstruction? J Oral Maxillofac Surg. 2014;72(1):178–185. doi: 10.1016/j.joms.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 33.le Nobel GJ, Higgins KM, Enepekides DJ. Predictors of complications of free flap reconstruction in head and neck surgery: analysis of 304 free flap reconstruction procedures. Laryngoscope. 2012;122(5):1014–1019. doi: 10.1002/lary.22454. [DOI] [PubMed] [Google Scholar]
- 34.Perisanidis C, Herberger B, Papadogeorgakis N, et al. Complications after free flap surgery: do we need a standardized classification of surgical complications? Br J Oral Maxillofac Surg. 2012;50(2):113–118. doi: 10.1016/j.bjoms.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Mulvey CL, Pronovost PJ, Gourin CG. Hospital volume and failure to rescue after head and neck cancer surgery. Otolaryngol Head Neck Surg. 2015;152(5):783–789. doi: 10.1177/0194599815570026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


