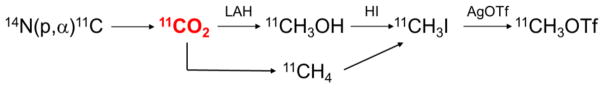Abstract
The logic of total synthesis transformed a stagnant state of medicinal and synthetic organic chemistry when there was a paucity of methods and reagents to synthesize drug molecules and/or natural products. Molecular imaging by positron emission tomography (PET) is now experiencing a renaissance in the way radiopharmaceuticals for molecular imaging are synthesized, however, a paradigm shift is desperately needed in the discovery pipeline to accelerate in vivo imaging studies. A significant challenge in radiochemistry is the limited choice of labeled reagents (or building blocks) available for the synthesis of novel radiopharmaceuticals with the most commonly used short-lived radionuclides carbon-11 (11C; half-life ~20 minutes) and fluorine-18 (18F; half-life ~2 hours). In fact, most drugs cannot be labeled with 11C or 18F due to a lack of efficient and diverse radiosynthetic methods. In general, routine radiopharmaceutical production relies on the incorporation of the isotope at the last or penultimate step of synthesis, ideally within one half-life of the radionuclide, to maximize radiochemical yields and specific activities thereby reducing losses due to radioactive decay. Reliance on radiochemistry conducted within the constraints of an automated synthesis unit (“box”) has stifled the exploration of multi-step reactions with short-lived radionuclides. Radiopharmaceutical synthesis can be transformed by considering logic of total synthesis to develop novel approaches for 11C- and 18F-radiolabeling complex molecules via retrosynthetic analysis and multi-step reactions. As a result of such exploration, new methods, reagents and radiopharmaceuticals for in vivo imaging studies are discovered. A new avenue to develop radiotracers that were previously unattainable due to the lack of efficient radiosynthetic methods is necessary to work towards our ultimate, albeit impossible goal – the concept we term total radiosynthesis - to radiolabel virtually any molecule.
As with the vast majority of drugs, most radiotracers also fail, therefore expeditious evaluation of tracers in preclinical models prior to optimization or derivatization of the lead molecules/drugs is necessary. Furthermore the exact position of the 11C and 18F radionuclide in tracers is often critical for metabolic considerations, and flexible methodologies to introduce the radiolabel are needed. Using the principles of total synthesis our laboratory and others have shown that multi-step radiochemical reactions are indeed suitable for preclinical and even clinical use. As the goal of total synthesis is to be concise, we have also simplified the syntheses of radiopharmaceuticals. We are presently developing new strategies via [11C]CO2 fixation which has enabled library radiosynthesis as well as labeling non-activated arenes using [18F]fluoride via iodonium ylides. Both of which have proven to be suitable for human PET imaging. We concurrently utilize state-of-the-art automation technologies including microfluidic flow chemistry and rapid purification strategies for radiopharmaceutical production. In this account we highlight how total radiosynthesis has impacted our radiochemistry program, with prominent examples from others, focusing on its impact towards preclinical and clinical research studies.
Introduction
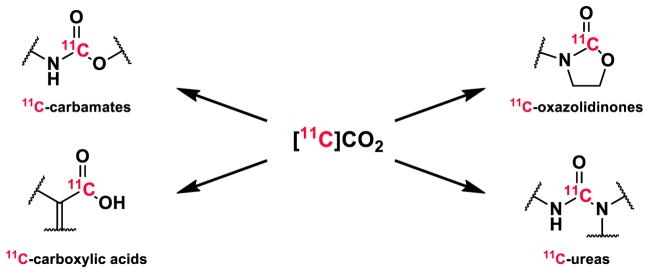
Radiochemistry discovery is hindered by the lack of efficient methods to enable strategic introduction of radionuclides into biological molecules. Fluorine-18 (18F; t1/2 = 109.7 min) and carbon-11 (11C; t1/2 = 20.4 min) are the dominant radionuclides for molecular imaging with positron emission tomography (PET). The vast majority of PET radiochemical syntheses incorporate the radioactive atom in the final or penultimate reaction step. This standard approach generally has the advantage that the number of radiochemical (“hot”) reactions are kept to a minimum which reduces activity losses due to radioactive decay over time and during transfer steps. However, most drugs and bioactive molecules cannot be radiolabeled in the final steps with 18F or 11C without derivatizing the chemical structure. In practice, routine radiofluorine labeling is almost entirely limited to nucleophilic displacement reactions with [18F]fluoride ([18F]F −), while radiocarbon labeling is generally restricted to 1-step nucleophilic 11C-methyl substitutions (via [11C]CH3I or [11C]CH3OTf, prepared from [11C]CO2.; Figure 1) with phenolic, amino or thiol precursors.
Figure 1.
Production of 11C-methylating agents from [11C]CO2
Although early radiotracer discovery employed manual and semi-automated reactions, this approach has become less common as radiopharmaceutical production is usually carried out within the restraints of an automated synthesis unit (“box”) and is further restricted by its remote operation within a lead shielded fume hood (hot cell). It is important to remember that the earliest radiochemistry strategies for some of the original PET radiopharmaceuticals for clinical use including [18F]2-fluoro-2-deoxy-D-glucose (FDG)[1] and [18F]fluoro-L-dihydroxyphenylalanine (FDOPA) derivatives[2] were multi-step manual procedures that were simplified only after their clinical utility was proven.
Whereas total synthesis has had a dramatic impact on fundamental chemistry and drug discovery by establishing a logical method to prepare virtually any chemical compound by retrosynthetic analysis and efficient multi-step reactions, which concurrently unveils new methods, reaction and reagents,[3] it has had a minimal impact on radiopharmaceutical discovery. A paradigm shift to access previously unattainable labeled compounds and to overcome the lack of efficient radiosynthetic methods is necessary to work towards our ultimate, goal to radiolabeling virtually any molecule, the concept we term total radiosynthesis. This concept offers the following advantages:
Discovery of new building blocks. The incorporation of the radioisotope into a building block at an early step facilitates the use of a more diverse range of chemical reactions beyond routine 11C- and 18F-nucleophilic substitution. Such reactions can be fast and efficient, offering stable and pure intermediates.
Labeling new functional groups. Since the isotope incorporation step – the key step – is accomplished early, reaction conditions can be varied and optimized to favour the formation of new functional groups that would be unstable under standard radiolabeling conditions.
New radiotracers. Multi-step radiosynthesis enables access to novel 11C- and 18F-labeled drug scaffolds and isotopologues of currently inaccessible pharmaceuticals.
Assess attrition early. Just as most drugs fail, most radiotracers also fail. Expediting the time to radiolabel lead molecules of interest (in spite of efficiency) for in vivo imaging studies is critical to assess attrition rates, thereby reducing capital expenditures and timelines in drug development.
Metabolic considerations. Flexible radiochemistry methods enable molecules to be labeled in different positions and has been critical for metabolic considerations in PET radiopharmaceutical design.
Concise new methods. Once a lead radiotracer is identified efforts are made to simplify and automate the radiochemistry for routine use and multi-center clinical trials. Our vision of total radiosynthesis is to be concise and to develop rapid, efficient and broadly useful methodologies.
Despite the advantages outlined above, multi-step radiochemistry reactions are not often pursued because of the notion that the isotope must be introduced at the last or penultimate step and are rarely considered to be viable strategies for producing radiotracers with short-lived isotopes. In this account we highlight a series of examples for how total radiosynthesis has impacted our radiochemistry program, with selected prominent examples from other groups, to expedite preclinical and clinical PET research.
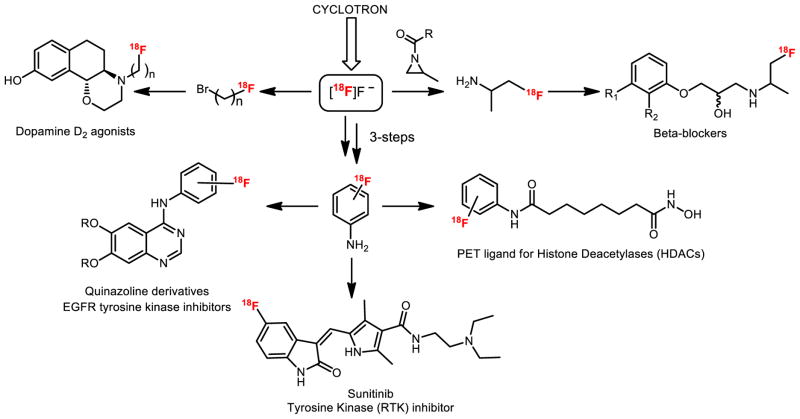
Analogous to combinatorial chemistry approaches, we and others have exploited the power of using versatile 11C- and 18F-labeled building blocks, a strategy we term as library radiosynthesis. Multi-step reactions have been useful to synthesize reagents from [11C]CO2 such as 11C-acid chlorides[4] or 11C-isocyanates.[5] as well as reactive intermediates from [18F]fluoride, for example, via ring-opening of aziridines[6] or from [18F]fluoroanilines[7] (Figure 2). Herein we highlight selected examples of multi-step 11C- and 18F-radiochemistry for preparing complex scaffolds, including strategies for labeling different positions within the same molecule for metabolic reasons. Simplification of radiochemistry reactions by reducing steps and for widespread use via concise reactions are also discussed, along with new automated technologies including microfluidic flow chemistry and our first-in-human experience. Finally, we will showcase our recent work in [11C]CO2 fixation to label diverse carbonyl groups as well as iodonium ylide-based radiofluorination to label non-activated arenes, not only to make building blocks and new radiotracers, but to also demonstrate that these methods are suitable for preparing radiopharmaceuticals for clinical research.
Figure 2.
Multi-step radiochemistry using small 18F-labeled building blocks
Multi-step 11C and 18F reactions to prepare radiotracers for EGFR
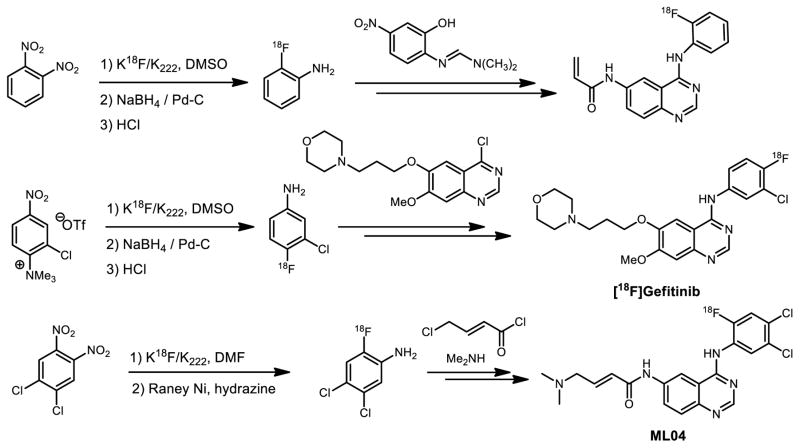
Upregulation of epidermal growth factor receptor (EGFR) has been associated with several cancers, including non-small cell lung carcinomas. Specifically several 4-aminoquinazoline-based inhibitors bind to the intracellular ATP domain of EGFR. Several FDA approved drugs, for example Gefitinib and Erlotinib, were discovered based on these findings. In parallel. several 11C or 18F PET radioligands for imaging EGFR were also derived from [18F]fluoroanilinoquinazolines.[8] A lead drug, Gefitinib, contains fluorine nested on the aniline motif with strong electron donating character as well as in the presence of other sensitive functional groups. A classical aromatic nucleophilic substitution was not suitable for the incorporation of fluorine-18 due to complex electronic and steric factors, therefore a multi-step approach was necessary for labeling this drug (Figure 3). Other efforts to achieve the same goal utilized the coupling reaction between [18F]fluoroaniline and quinazoline precursor in the presence of isopropanol to generate the [18F]Gefitinib.[9] In the radiosynthesis of ML04 (Figure 3), 4,5-dichloro-2-[18F]fluoroaniline was prepared via a two-step sequence of 18F addition and diimide reduction by Raney Nickel. After the condensation with 4-chloro-6-nitroquinazoline and subsequent reduction, the ensuing product was further functionalized via coupling with chlorocrotonylchloride and then dimethylamine, to yield [18F]ML04 in six steps.[10]
Figure 3.
Selected syntheses of 18F-labeled EGFR inhibitors
New reactions were developed to discover radiotracers based on irreversible EGFR inhibitors that bear a Michael acceptor in the acrylamide position. Initial work to achieve this goal employed a multi-step carbon-11 strategy via a Grignard reaction to prepare 11C-acryloyl chloride for acylation.[11] Among the most complex reactions, we have demonstrated that a 7-step synthesis of 6-acylamido-4-(2-[18F]fluoroanilino)quinazoline (Figure 3) could be achieved where the acrylamide was incorporated in the final step, 7-steps after introduction of the 18F-label.[7a] The synthesis commenced with 1,2-dinitrobenzene, an activated precursor, for fluorine-18 incorporation, followed by NaBH4/ Pd-C reduction to form the synthetically versatile building block 2-[18F]fluoroaniline. Condensation with imine yielded the corresponding quinazoline core, which was reduced and subsequently coupled with acrylic acid to produce the radiotracer. An analogous chemistry approach was applied to the synthesis of 3-chloro-4-[18F]fluoroaniline. In all, multi-step strategies played a critical role in the preparation of 11C- and 18F- labeled quinazoline based EGFR inhibitors and supported in vivo imaging studies.[10b, 12]
Flexible chemistry is key to label radiotracers at different positions for metabolic considerations
Within the realms of medicinal chemistry, the “fluorine-effect”[13] (i.e. the influence of positional fluoro-isomers in on the biological properties of drug molecules) is well-known to alter the behavior and suitability of drug candidates. For example, an evaluation of aromatic ring-substituted 2-, 5- or 6-[18F]FDOPA revealed dramatically different biological properties in human imaging studies.[14] We also found that 2-, 3- and 4-substituted [18F]fluoroanilino-quinazolines demonstrated different biological properties in vivo, specifically that 4-[18F]fluoroanilinoquinazolines readily defluorinate and are not stable in vivo. These studies further illustrate the importance of investigating different istopologues to identify the optimal compounds in radiopharmaceutical design and the need for radiochemistry methods to enable strategic incorporation of the radionuclides.
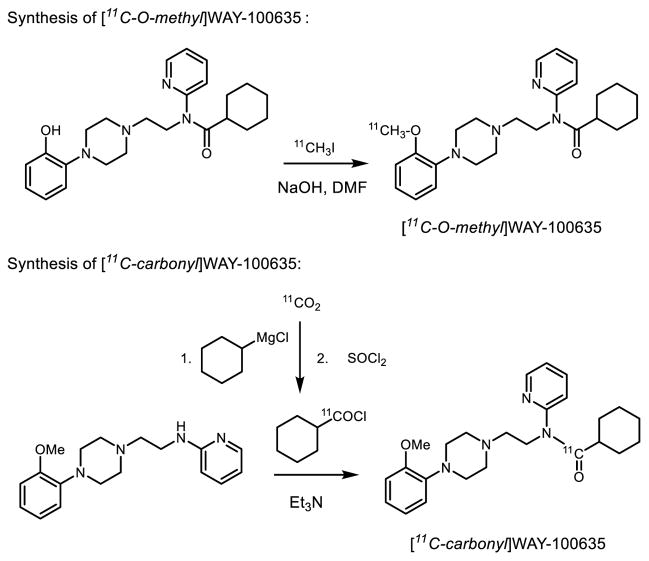
Different positional isomers can also be critical in radiotracer design with 11C. A prominent example is with carbon-11 labeled WAY-100635 (Figure 4), an antagonist radiotracer that selectively interacts with 5-hydroxytrptamine (serotonin, 5-HT) subtype 1A receptors, which regulate inhibition of serotonin release and play a central role in anxiety, depression and schizophrenia.[15] Carbon-11 labeled WAY-100635 was first prepared at the most accessible O-methyl position using [11C]CH3I in a one-step fashion.[16] Unfortunately, a major radioactive brain-penetrating metabolite, [11C-O-methyl]WAY-100634 has contributed significantly to non-specific and some specific binding since it has high affinity to 5HT1A receptors. Other possible labeling strategies proved more fruitful when using a multi-step approach to incorporate 11C at the carbonyl position via the condensation between amine and [11C]cyclohexoyl chloride from the corresponding Grignard reagent [17]. PET imaging of [11C-carbonyl]WAY-100635 demonstrated superior PET imaging of 5HT1A neuroreceptors in human brain by overcoming the troublesome brain penetrating metabolite when labeled at the O-11C-methyl position [18].
Figure 4.
Multi-step radiosynthesis of 11C-labeled WAY-100635
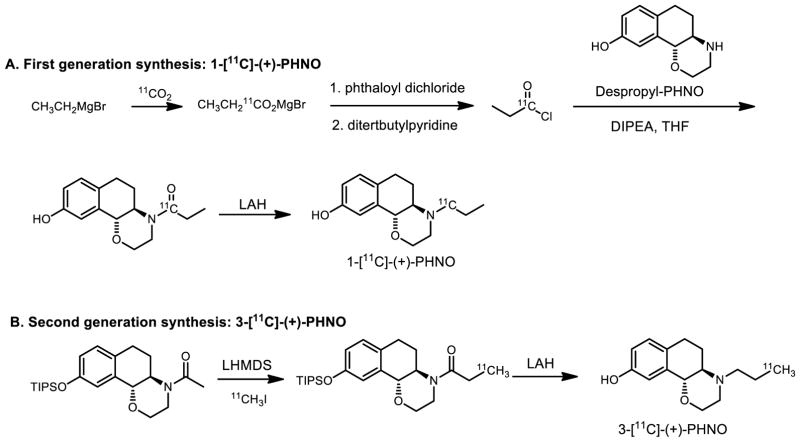
Carbon-11 labeled, (+)-PHNO, was introduced and evaluated in vivo first in preclinical studies [19] and translated to healthy volunteers [20] originally to study high affinity state of dopamine receptor subtype 2 (D2) but resulted in the serendipitous discovery of a D3-preferring agonist radiotracer. As shown in Figure 5A, the first generation of the [11C]-(+)-PHNO radiosynthesis utilized a five-step route to support the initial imaging studies.[19, 21] Starting with a Grignard reaction with [11C]CO2, intermediate [11C]propionyl chloride was prepared and distilled. The coupling reaction between the despropyl precursor and the acid chloride yielded the amide intermediate which was reduced with LiAlH4 to afford 1-[11C]-(+)-PHNO. [19, 22] Due to the importance of the PET ligand and the need of extensive imaging studies in multiple centers, it is necessary to simply the radiochemistry to reduce the number of reaction steps to facilitate automation on commercial synthesis modules. Our laboratory and GE Healthcare have worked towards simplifying the process to prepare 11C-PHNO from commonly used [11C]CH3I with a new silyl-protected acetamide precursor (Figure 5B).[23] The enolate of the precursor was generated in the reaction with strong base (LHMDS) followed by alkylation with [11C]CH3I, to give 11C-labeled propionamide. 3-[11C]-(+)-PHNO, was synthesized via LiAlH4 reduction of the amide and deprotection of silyl group in one pot.[23a] The improved radiosynthesis of 11C-PHNO reduced the reaction from five- to three steps and eliminates the technically challenging isolation of 11C-propionyl chloride by distillation. We have also explored, 11C-Preclamol[4] as a partial D2-agonist using the same radiochemical approach and 18F-labeled PHNO derivatives.[24]
Figure 5.
Multi-step radiosynthesis of 11C-labeled PHNO
Radiolabeling drug molecules by multi-step 11C-chemistry is certainly not a thing of the past. Two recent and noteworthy examples including a 5-step synthesis of the myeloperioxidase inhibitor, [11C]AZD3241, that took advantage of a 14C-labeling strategy via a [11C]benzoylisothiocyanate intermediate (prepared via [11C]KCN), which enabled microdosing to support clinical translation, as well as [11C]lapatinib, prepared in 4-steps by reaction of a [11C]fluorobenzylazide building block, that was used in human PET imaging studies.[25]
New concise 11C- and 18F-methods for human PET imaging
Although many new radiochemical methods are often reported in the literature, in order to have clinical impact such procedures should be easily automated and validated for routine radiopharmaceutical production. Examples of multi-step reactions with 11C- and 18F- that we have simplified for routine use include [11C]Ritalin,[26] and [18F]-AV1451 (T807)[27] which are now efficiently carried out in less steps, via automated and simplified processes. Two areas of active research in our laboratory are: 1) exploring are 11C-CO2 fixation to label a diverse array of carbonyl groups, and 2) 18F-iodonium ylide based chemistry for labeling of non-activated aromatic rings (vide infra). Both of these methods have proved to be suitable for automated radiopharmaceutical production and validated for human use.
11C-carbonylation via [11C]CO2 fixation: 11C=O bonds made easily!
Recent advances with [11C]CO2-fixation have been applied to prepare previously inaccessible functional groups and the operational simplicity of this method has opened the doors to a diverse array of radiotracers bearing 11C-carbonyl groups.[5, 28] [11C]Carbon monoxide, [11C]cyanide, and [11C]phosgene, also typically prepared from [11C]CO2, represent alternative carbon-11 reactants to enable 11C-carbonylation, but have yet to see widespread adoption due to the specialized apparatus required for their production. In contrast, [11C]CO2 is routinely handled by nearly all medical and research cyclotrons and can be used directly. This methodology incorporates [11C]CO2 directly into a molecule in one-pot and at room temperature, thereby eliminating the need to use esoteric and inconvenient radioactive precursors such as [11C]COCl2 and [11C]CO.
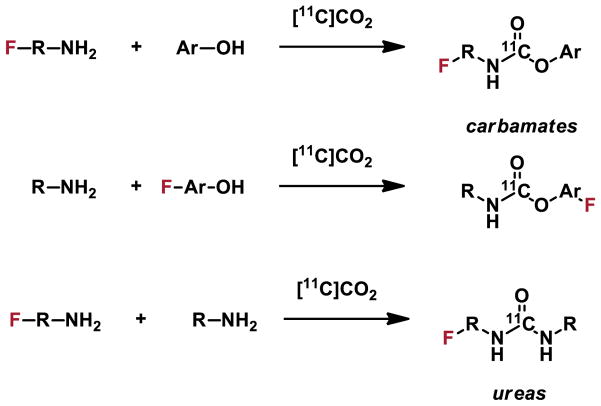
[11C]CO2 fixation is a viable strategy for non-conventional radiochemistry to prepare [11C]carbamates and [11C]ureas via [11C]isocyates generated in situ[5, 29] and intramolecular cyclization reactions that lead to [11C]oxazolidinones.[30] Synthetic strategies demonstrate the application of [11C]CO2 fixation in four different classes of compounds, three of which have been recently used in first-in-human studies and/or are advancing for clinical translation. (Figure 6). Given that 11C-methylation is considered to be a 1-step reaction from CH3I is prepared from CO2, perhaps we should consider direct [11C]CO2 fixation is 1-step removed (with this nomenclature these would be technically “-1 step reactions”!) and represents the minimal number of steps for 11C-reactions.
Figure 6.
11C-carbonylation via [11C]CO2 fixation
Clinical translation has already been seen with these reactions. A reversible, MAO-B selective radiotracer, [11C]SL25.1188, was initially developed for preclinical use via [11C]COCl2 to form the 11C-labeled oxazolidinone.[31] To translate [11C]SL25.1188 for widespread clinical research, we designed the radiosynthesis to avoid [11C]COCl2. Our synthesis using [11C]CO2-fixation for intramolecular cyclization to prepare the 11C-oxazolidinone that proved to be superior, both operationally and in terms of radiochemical yield, to the previous synthesis, and was recently advanced to human PET imaging studies.[28c, 32]
Another noteworthy target is fatty acid amide hydrolase (FAAH), an enzyme that regulates levels of endocannabinoids, with numerous FAAH inhibitors in clinical trials. Having identified carbamates as potent inhibitors of FAAH, [11C]CURB was prepared using [11C]CO2-fixation to link an amine and a phenol.[29] In this example, [11C]CO2 was trapped in a solution of cyclohexylamine by an organic base (BEMP), followed by dehydration with phosphoryl chloride and addition of the required phenol and was recently advanced to human use.[33] The mechanism of FAAH inhibition by carbamate hydrolysis drove the design of [11C]CURB and required 11C-carbonyl labeling, since a labeled O-aryl fragment would be cleaved by the enzyme. Noteworthy preclinical studies with other classes of compounds prepared by [11C]CO2 fixation that we are planning for clinical translation include [11C-carbonyl]PF-04457845, a FAAH inhibitor in clinical trials, which represents a class of unsymmetrical [11C-carbonyl]ureas. We also we prepared [11C]bexarotene by a copper-catalyzed [11C]CO2-fixation reaction to generate the carbon-11 labeled carboxylic acid and promising PET imaging studies were carried out.[34] We are presently assessing this tracer as a candidate for clinical research studies.
Library radiosynthesis by [11C]CO2-fixation
Library radiosynthesis is achievable by [11C]CO2-fixation and shares attributes of combinatorial chemistry as conventional amines and/or phenols can be used to synthesize limitless arrays of 11C-carbamates and symmetrical or unsymmetrical 11C-ureas (Figure 6). Development of next-generation FAAH imaging agents is facilitated by the robust and selective [11C]carbamate labeling strategy. In vitro FAAH inhibition assays were used to identify a small collection of highly potent O-aryl carbamates and lead radiotracers were labeled by a general [11C]CO2-fixation protocol and evaluated in preclinical models. This methodology can be easily envisioned for labeling fluorinated compounds (Figure 7) in a high throughput fashion. We exploit this high-throughput [11C]CO2-fixation strategy for radiolabeling lead fluorinated radiotracers with 11C at the carbonyl position for in vivo imaging thereby obviating time-intensive optimization of 18F-labeling. We have already employed this strategy to prepare 18F-labeled urea and carbamate radiotracers for FAAH[29, 35] and oxazolidinones for MAO-B.[36]
Figure 7.
Radiotracer development by combinatorial [11C]CO2-fixation
18F-labeling of non-activated aromatics via iodonium ylides: Suitable for human use!
Labeling of non-activated aromatics with [18F]fluoride has been a longstanding goal in the field of PET radiochemistry. One of the most prominent motivations was to apply such reactions to the synthesis of 6-[18F]FDOPA which has been extensively employed in the diagnosis of neurodegenerative diseases [37] and has shown recent applications in cancer imaging studies.[37b, 38] This radiopharmaceutical was first synthesized for human imaging studies in the early 1980’s using unprotected L-DOPA in anhydrous HF solvent and fluorinated with electrophilic carrier-added [18F]F2 gas.[39] Isotopic 18F-exchange to prepare [18F]XeF2 [40] were also explored at that time and used for the preparation of [18F]FDG[41] and [18F]FDOPA,[42]. This method has since been revisited, but not proven to be practical for routine use.[43] Significant research efforts have since been made in the radiosynthesis using more readily accessible nucleophilic [18F]fluoride in a non-carrier-added fashion and has been recently reviewed.[2c]
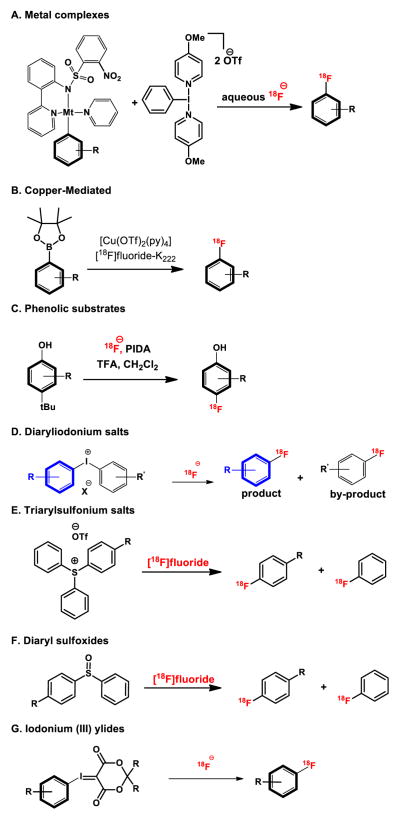
With the commercial availability of high specific activity [18F]fluoride ion, several new strategies for reactions of [18F]fluoride with electron-rich aromatics have emerged (Figure 8), and have been recently reviewed.[44] Such methods include the development of transition metal-mediated reactions with isolable aryl palladium or nickel complexes, copper-mediated fluorinations with aryl borate esters , oxidative fluorination with phenolic substrates and fluorination of diaryliodonium salts. These newer methods appear promising for preparing radiolabeled aromatics, however, drawbacks include the use of air-sensitive and/or toxic transition metals, limited substrate scope, or poor regioselectivity, and/or low isolated radiochemical yields. A newer approach employs sulfonium salts and diarylsulfoxides[45] . To date, these newer methods have had limited success for clinical translation.
Figure 8.
Strategies to radiolabel non-activated aromatics with [18F]fluoride
The ideal criteria for radiofluorination of non-activated arenes with [18F]fluoride are outlined below:
❑ One-step labeling
❑ Stable precursor
❑ Metal-free and no fluorinated counterion
❑ Regioselective
❑ Compatible with a wide variety of functional groups
❑ Easily automated
❑ Readily translatable to routine clinical use
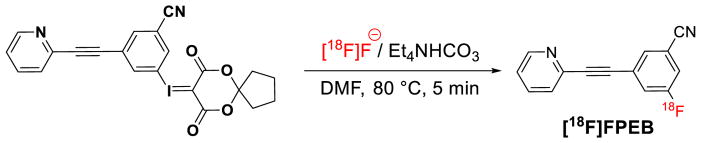
Our radiochemistry program has satisfied the above-mentioned criteria by discovering the synthesis of novel hypervalent iodine(III) ylides as precursors for regiospecific radiofluorination of non-activated and sterically-demanding (hetero)aromatic rings using [18F]fluoride.[46] This methodology takes advantage of spirocyclic iodonium ylide precursors and allows for the design of radiotracers without the need for electron withdrawing arenes or prosthetic groups. Over 30 18F-labeled compounds including heterocycles, amino acids, steroids, azide building blocks and radiotracers such as [18F]5-fluorouracil have been synthesized by this technology. A radiopharmaceutical, [18F]FPEB, has now been validated for human use by this methodology and resulted in a 10-fold increase in radiochemical yield (20% vs. 2%, uncorrected; relative to [18F]fluoride) and high specific activity (18 Ci/μmol at end of synthesis), ready for injection (Figure 9).[47]
Figure 9.
Radiosynthesis of [18F]FPEB based on iodonium ylide strategy suitable for human use
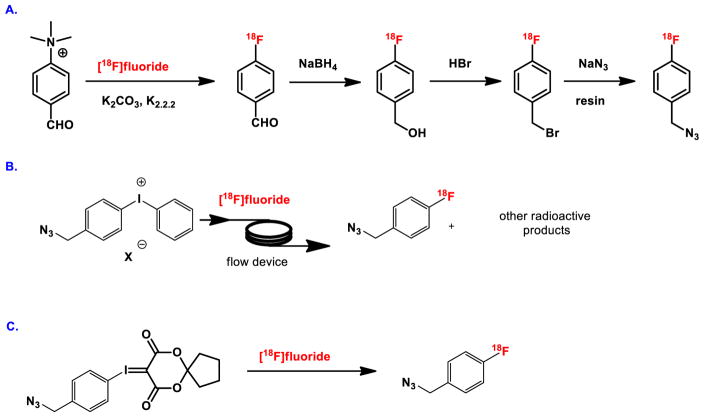
Prosthetic groups are widely utilized in the labeling of biomolecules such as proteins, peptides and antibodies during the PET imaging studies. 4-[18F]Fluorobenzyl azide, also known as 1-(azidomethyl)-4-[18F]fluorobenzene, is an important and versatile building block for the radiolabeling of biomolecules via [3+2] cycloaddition (‘click’ chemistry) in PET. Routine production of this agent is challenged by inefficient multistep and/or esoteric radiochemical approaches (Figure 10A and 10B). We have developed a novel one-step regioselective labeling method using our novel spirocyclic iodonium ylide precursor, which reacts with the readily available nucleophilic [18F]fluoride (Figure 10C). The precursor for radiolabeling is easily accessible from the corresponding iodo compound in one-step, for direct reaction with tetraethylammonium [18F]fluoride in DMF at 120°C for 10 min. The reaction mixture is purified by solid phase extraction to provide 4-[18F]fluorobenzyl azide in 65 ± 2% isolated radiochemical yield (non decay-corrected) with >95% radiochemical purity, or by an automated synthesis with HPLC purification in 35 ± 2% isolated radiochemical yield (non-decay corrected) and >99% radiochemical purity with a specific activity greater than 1.5 Ci · μmol−1.
Figure 10.
Syntheses of 4-[18F]fluorobenzyl azide
C. Flow Radiosynthesis: First in human imaging by microfluidic flow chemistry
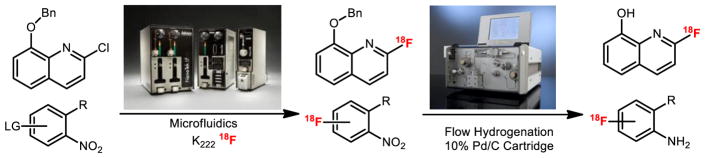
Microfluidic technology (a.k.a. lab-on-a-chip) involves the miniaturization of components and equipment, and has recently emerged as a promising tool in the field of PET radiopharmaceutical chemistry.[48] Specifically, the main advantages of using flow chemistry include: 1) low sample and reagent consumption (as sub-milligram quantities of starting materials are used); 2) faster reaction kinetics derived from efficient mixing and steady-state reagent concentrations; 3) enhanced product yields with rapid sample analysis; 4) high reproducibility; and 5) facile automation for good manufacturing practice (GMP) standards. Our preliminary data shows that 18F-fluoroaniline building blocks can be prepared efficiently via hydrogenation despite the multi-step reaction sequences (Figures 2 and 3). These reactions reliably and routinely provide quantitative conversion for hydrogenation for deprotection or reduction reactions under 5 minutes. We have demonstrated that the classic hydrogenation reaction is feasible for routine clinical production and was previously too difficult and time consuming for PET radiochemistry.[49] (Figure 11)
Figure 11.
Microfluidic hydrogenation for the preparation of 18F-labeled compounds
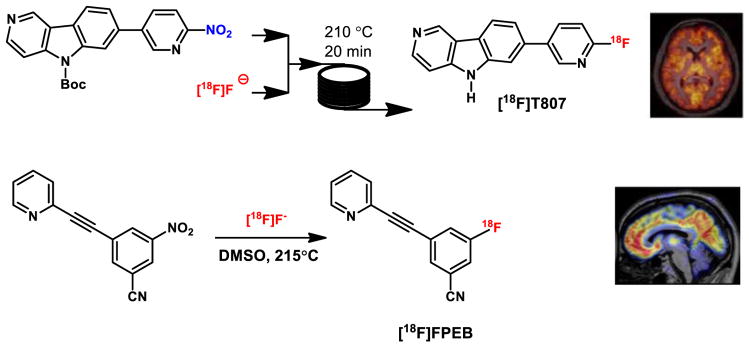
A prototypic microreactor device has been used to prepare [18F]fallypride for human use. We validated the synthesis of [18F]FPEB using continuous flow chemistry as a proof of concept and recently carried out a clinical research study with [18F]T807 representing the first reported human imaging study with a radiopharmaceutical prepared by this technology (Figure 12).[50]
Figure 12.
Microfluidics device in radiopharmaceutical production for human use
Conclusion
PET radiopharmaceutical chemistry will always benefit from more innovative approaches to radiolabel different functional groups, and consequently new classes of molecules that have the potential to explore pathologic changes in various biological processes, and thus improve detection accuracy and assist in evaluating patient response to therapeutic intervention. We have demonstrated that over 5-step reactions can be achieved using 11C within 40 minutes or with 18F within 2 hours. Through these processes new 11C- and 18F-labeled building blocks were discovered and applied to label numerous radiotracers. All of the aforementioned examples showcase the importance of multi-step radiosynthesis and demonstrate the advantage of such a strategy in the discovery and development of PET radiotracers. Total radiosynthesis is a vision that we hope will be actively pursued to not only to create new radiopharmaceuticals but to also expand the toolbox of radiochemical building blocks and offer new methods and reagents to radiolabel virtually any molecule.
Acknowledgments
This article is dedicated to Prof. Thomas J. Brady for his support of basic and applied radiochemistry research. We thank Dr. Lee Collier, Dr. Benjamin Rotstein and Dr. Nicky Stephenson for helpful discussions and for striving to radiolabel unprecedented compounds.
References
- 1.(a) Ido T, Wan CN, Casella V, Fowler JS, Wolf AP, Reivich M, Kuhl DE. J Labelled Compd Radiopharm. 1978;14:175–183. [Google Scholar]; (b) Fowler JS, Ido T. Semin Nucl Med. 2002;32:6–12. doi: 10.1053/snuc.2002.29270. [DOI] [PubMed] [Google Scholar]
- 2.(a) Firnau G, Nahmias C, Garnett S. Int J Appl Radiat Istopes. 1973;24:182–184. doi: 10.1016/0020-708x(73)90008-2. [DOI] [PubMed] [Google Scholar]; (b) Chirakal R, Firnau G, Garnett ES. J Nucl Med. 1986;27:417–421. [PubMed] [Google Scholar]; (c) Edwards R, Wirth T. J Labelled Compd Radiopharm. 2015;58:183–187. doi: 10.1002/jlcr.3285. [DOI] [PubMed] [Google Scholar]
- 3.(a) Corey EJ, Cheng X-m. The logic of chemical synthesis. John Wiley; New York: 1989. [Google Scholar]; (b) Corey EJ, Czakó B, Kürti L. Molecules and medicine. John Wiley & Sons; Hoboken, N.J: 2007. [Google Scholar]
- 4.Vasdev N, Natesan S, Galineau L, Garcia A, Stableford WT, McCormick P, Seeman P, Houle S, Wilson AA. Synapse. 2006;60:314–318. doi: 10.1002/syn.20304. [DOI] [PubMed] [Google Scholar]
- 5.Wilson AA, Garcia A, Houle S, Sadovski O, Vasdev N. Chem-Eur J. 2011;17:259–264. doi: 10.1002/chem.201002345. [DOI] [PubMed] [Google Scholar]
- 6.(a) Vasdev N, van Oosten EM, Stephenson KA, Zadikian N, Yudin AK, Lough AJ, Houle S, Wilson AA. Tetrahedron Lett. 2009;50:544–547. [Google Scholar]; (b) van Oosten EM, Gerken M, Hazendonk P, Shank R, Houle S, Wilson AA, Vasdev N. Tetrahedron Lett. 2011;52:4114–4116. [Google Scholar]
- 7.(a) Vasdev N, Dorff PN, Gibbs AR, Nandanan E, Reid LM, O'Neil JP, VanBrocklin HF. J Labelled Compd Radiopharm. 2005;48:109–115. [Google Scholar]; (b) Vasdev N, Dorff PN, O’Neil JP, Chin FT, Hanrahan S, VanBrocklin HF. Biorg Med Chem. 2011;19:2959–2965. doi: 10.1016/j.bmc.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hicks JW, VanBrocklin HF, Wilson AA, Houle S, Vasdev N. Molecules. 2010;15:8260–8278. doi: 10.3390/molecules15118260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seimbille Y, Phelps ME, Czernin J, Silverman DHS. J Labelled Compd Radiopharm. 2005;48:829–843. [Google Scholar]
- 10.(a) Dissoki S, Laky D, Mishani E. J Labelled Compd Radiopharm. 2006;49:533–543. [Google Scholar]; (b) Abourbeh G, Dissoki S, Jacobson O, Litchi A, Daniel RB, Laki D, Levitzki A, Mishani E. Nucl Med Biol. 2007;34:55–70. doi: 10.1016/j.nucmedbio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Ben-David I, Rozen Y, Ortu G, Mishani E. Appl Radiat Isot. 2003;58:209–217. doi: 10.1016/s0969-8043(02)00301-9. [DOI] [PubMed] [Google Scholar]
- 12.Su H, Seimbille Y, Ferl G, Bodenstein C, Fueger B, Kim K, Hsu YT, Dubinett S, Phelps M, Czernin J, Weber W. Eur J Nucl Med Mol Imaging. 2008;35:1089–1099. doi: 10.1007/s00259-007-0636-6. [DOI] [PubMed] [Google Scholar]
- 13.Cantacuzene D, Kirk KL, McCulloh DH, Creveling CR. Science. 1979;204:1217–1219. doi: 10.1126/science.221978. [DOI] [PubMed] [Google Scholar]
- 14.Chirakal R, Vasdev N, Asselin MC, Schrobilgen GJ, Nahmias C. J Fluorine Chem. 2002;115:33–39. [Google Scholar]
- 15.Lacivita E, Di Pilato P, De Giorgio P, Colabufo NA, Berardi F, Perrone RLM. Expert Opinion on Therapeutic Patents. 2012;22:887–902. doi: 10.1517/13543776.2012.703654. [DOI] [PubMed] [Google Scholar]
- 16.(a) Mathis CA, Simpson NR, Mahmood K, Kinahan PE, Mintun MA. Life Sci. 1994;55:403–407. doi: 10.1016/0024-3205(94)00324-6. [DOI] [PubMed] [Google Scholar]; (b) Pike VW, McCarron JA, Lammerstma AA, Hume SP, Poole K, Grasby PM, Malizia A, Cliffe IA, Fletcher A, Bench CJ. Eur J Pharmacol. 1995;283:R1–R3. doi: 10.1016/0014-2999(95)00438-q. [DOI] [PubMed] [Google Scholar]
- 17.(a) McCarron JA, Turton DR, Pike VW, Poole KG. J Labelled Compd Radiopharm. 1996;38:941–953. [Google Scholar]; (b) Pike V, Cliffe I, Fletcher A, Hume S, McCarron J, Ashworth S, Opacka-Juffry J, Osman S, Lammertsma A, Poole K, White A, Bench C, Grasby P. In: PET for Drug Development and Evaluation. Comar D, editor. Vol. 26. Springer; Netherlands: 1995. pp. 93–108. [Google Scholar]
- 18.Pike VW, McCarron JA, Lammertsma AA, Osman S, Hume SP, Sargent PA, Bench CJ, Cliffe IA, Fletcher A, Grasby PM. Eur J Pharmacol. 1996;301:R5–R7. doi: 10.1016/0014-2999(96)00079-9. [DOI] [PubMed] [Google Scholar]
- 19.Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, Houle S, Seeman P, Ginovart N. J Med Chem. 2005;48:4153–4160. doi: 10.1021/jm050155n. [DOI] [PubMed] [Google Scholar]
- 20.Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, Wilson AA. Biol Psychiatry. 2006;59:389–394. doi: 10.1016/j.biopsych.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Brown LSDJ, Brady F, Prenant C, Dijkstra D, Wikstrom H. Proceedings of the XIIth International Symposium on Radiopharmaceutical Chemistry; Uppsala, Sweden. Chichester, UK: Wiley; 1997. pp. 565–566. [Google Scholar]
- 22.Plisson C, Huiban M, Pampols-Maso S, Singleton G, Hill SP, Passchier J. Appl Radiat Isot. 2012;70:380–387. doi: 10.1016/j.apradiso.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 23.(a) Francisco Garcia-Arguello S, Fortt R, Steel CJ, Brickute D, Glaser M, Turton DR, Robins EG, Årstad E, Luthra SK. Appl Radiat Isot. 2013;73:79–83. doi: 10.1016/j.apradiso.2012.11.009. [DOI] [PubMed] [Google Scholar]; (b) Shoup TM, McCauley JP, Lee DF, Jr, Chen R, Normandin MD, Bonab AA, El Fakhri G, Vasdev N. Tetrahedron Lett. 2014;55:682–685. [Google Scholar]
- 24.Vasdev N, Seeman P, Garcia A, Stableford WT, Nobrega JN, Houle S, Wilson AA. Nucl Med Biol. 2007;34:195–203. doi: 10.1016/j.nucmedbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 25.(a) Johnström P, Bergman L, Varnäs K, Malmquist J, Halldin C, Farde L. Nucl Med Biol. 2015;42:555–560. doi: 10.1016/j.nucmedbio.2015.02.001. [DOI] [PubMed] [Google Scholar]; (b) Saleem A, Searle G, Kenny L, Huiban M, Kozlowski K, Waldman A, Woodley L, Palmieri C, Lowdell C, Kaneko T, Murphy P, Lau M, Aboagye E, Coombes R. EJNMMI Research. 2015;5:30. doi: 10.1186/s13550-015-0103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moran MD, Wilson AA, Stableford WT, Wong M, Garcia A, Houle S, Vasdev N. J Labelled Compd Radiopharm. 2011;54:168–170. [Google Scholar]
- 27.Shoup TM, Yokell DL, Rice PA, Jackson RN, Livni E, Johnson KA, Brady TJ, Vasdev N. J Labelled Compd Radiopharm. 2013;56:736–740. doi: 10.1002/jlcr.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.(a) Hooker JM, Reibel AT, Hill SM, Schueller MJ, Fowler JS. Angew Chem Int Ed. 2009;48:3482–3485. doi: 10.1002/anie.200900112. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wilson AA, Garcia A, Houle S, Vasdev N. Org Biomol Chem. 2010;8:428–432. doi: 10.1039/b916419g. [DOI] [PubMed] [Google Scholar]; (c) Rotstein BH, Liang SH, Holland JP, Collier TL, Hooker JM, Wilson AA, Vasdev N. Chem Commun. 2013;49:5621–5629. doi: 10.1039/c3cc42236d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson AA, Garcia A, Parkes J, Houle S, Tong J, Vasdev N. Nucl Med Biol. 2011;38:247–253. doi: 10.1016/j.nucmedbio.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Vasdev N, Sadovski O, Garcia A, Dollé F, Meyer JH, Houle S, Wilson AA. J Labelled Compd Radiopharm. 2011;54:678–680. [Google Scholar]
- 31.Vasdev N, Sadovski O, Moran MD, Parkes J, Meyer JH, Houle S, Wilson AA. Nucl Med Biol. 2011;38:933–943. doi: 10.1016/j.nucmedbio.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Rusjan PM, Wilson AA, Miler L, Fan I, Mizrahi R, Houle S, Vasdev N, Meyer JH. J Cereb Blood Flow Metab. 2014;34:883–889. doi: 10.1038/jcbfm.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rusjan PM, Wilson AA, Mizrahi R, Boileau I, Chavez SE, Lobaugh NJ, Kish SJ, Houle S, Tong J. J Cereb Blood Flow Metab. 2013;33:407–414. doi: 10.1038/jcbfm.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rotstein BH, Hooker JM, Woo J, Collier TL, Brady TJ, Liang SH, Vasdev N. ACS Med Chem Lett. 2014;5:668–672. doi: 10.1021/ml500065q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson AA, Hicks JW, Sadovski O, Parkes J, Tong J, Houle S, Fowler CJ, Vasdev N. J Med Chem. 2013;56:201–209. doi: 10.1021/jm301492y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.(a) Vasdev N, Sadovski O, Moran MD, Parkes J, Meyer JH, Houle S, Wilson AA. Nucl Med Biol. 2011;38:933–943. doi: 10.1016/j.nucmedbio.2011.03.003. [DOI] [PubMed] [Google Scholar]; (b) Rusjan PM, Wilson AA, Miler L, Fan I, Mizrahi R, Houle S, Vasdev N, Meyer JH. J Cereb Blood Flow Metab. 2014;34:883–889. doi: 10.1038/jcbfm.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.(a) Fischman AJ. Radiol Clin North Am. 2005;43:93–106. doi: 10.1016/j.rcl.2004.08.002. [DOI] [PubMed] [Google Scholar]; (b) Jager PL, Chirakal R, Marriott CJ, Brouwers AH, Koopmans KP, Gulenchyn KY. J Nucl Med. 2008;49:573–586. doi: 10.2967/jnumed.107.045708. [DOI] [PubMed] [Google Scholar]
- 38.(a) Chen W, Silverman DHS, Delaloye S, Czernin J, Kamdar N, Pope W, Satyamurthy N, Schiepers C, Cloughesy T. J Nucl Med. 2006;47:904–911. [PubMed] [Google Scholar]; (b) Weisbrod AB, Kitano M, Gesuwan K, Millo C, Herscovitch P, Nilubol N, Linehan WM, Kebebew E. J Clin Endocrinol Metab. 2012;97:E613–E617. doi: 10.1210/jc.2011-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.(a) Garnett ES, Firnau G, Nahmias C. Nature. 1983;305:137–138. doi: 10.1038/305137a0. [DOI] [PubMed] [Google Scholar]; (b) Firnau G, Chirakal R, Garnett ES. J Nucl Med. 1984;25:1228–1233. [PubMed] [Google Scholar]
- 40.Firnau G, Schrobilgen G, Chirakal R, Garnett ES. J Chem Soc, Chem Commun. 1981:198–199. [Google Scholar]
- 41.Sood S, Firnau G, Garnett ES. Int J Appl Radiat Isotopes. 1983;34:743–745. doi: 10.1016/0020-708x(83)90254-5. [DOI] [PubMed] [Google Scholar]
- 42.Firnau GC, Sood RS, Garnett ES. J Labelled Compd Radiopharm. 1981;18:S3–4. [Google Scholar]
- 43.Vasdev N, Pointner BE, Chirakal R, Schrobilgen GJ. J Am Chem Soc. 2002;124:12863–12868. doi: 10.1021/ja020604y. [DOI] [PubMed] [Google Scholar]
- 44.(a) Brooks AF, Topczewski JJ, Ichiishi N, Sanford MS, Scott PJ. Chem Sci. 2014;5:4545–4553. doi: 10.1039/C4SC02099E. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Campbell MG, Ritter T. Chem Rev. 2015;115:612–633. doi: 10.1021/cr500366b. [DOI] [PubMed] [Google Scholar]
- 45.(a) Mu L, Fischer CR, Holland JP, Becaud J, Schubiger PA, Schibli R, Ametamey SM, Graham K, Stellfeld T, Dinkelborg LM, Lehmann L. Eur J Org Chem. 2012;2012:889–892. [Google Scholar]; (b) Chun JH, Morse CL, Chin FT, Pike VW. Chem Commun. 2013;49:2151–2153. doi: 10.1039/c3cc37795d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sander K, Gendron T, Yiannaki E, Cybulska K, Kalber TL, Lythgoe MF, Arstad E. Sci Rep. 2015;5 doi: 10.1038/srep09941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rotstein BH, Stephenson NA, Vasdev N, Liang SH. Nat Commun. 2014;5 doi: 10.1038/ncomms5365. [DOI] [PubMed] [Google Scholar]
- 47.Stephenson NA, Holland JP, Kassenbrock A, Yokell DL, Livni E, Liang SH, Vasdev N. J Nucl Med. 2015;56:489–492. doi: 10.2967/jnumed.114.151332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pascali G, Matesic L, Collier TL, Wyatt N, Fraser BH, Pham TQ, Salvadori PA, Greguric I. Nat Protocols. 2014;9:2017–2029. doi: 10.1038/nprot.2014.137. [DOI] [PubMed] [Google Scholar]
- 49.Liang SH, Collier TL, Rotstein BH, Lewis R, Steck M, Vasdev N. Chem Commun. 2013;49:8755–8757. doi: 10.1039/c3cc45166f. [DOI] [PubMed] [Google Scholar]
- 50.(a) Liang SH, Yokell DL, Jackson RN, Rice PA, Callahan R, Johnson KA, Alagille D, Tamagnan G, Collier TL, Vasdev N. Medchemcomm. 2014;5:432–435. doi: 10.1039/C3MD00335C. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liang SH, Yokell DL, Normandin MD, Rice PA, Jackson RN, Shoup TM, Brady TJ, El Fakhri G, Collier TL, Vasdev N. Mol Imaging. 2014;13 doi: 10.2310/7290.2014.00025. [DOI] [PubMed] [Google Scholar]