Abstract
In 2002, 80 isolates of Enterobacteriaceae producing extended-spectrum β-lactamases (ESBLs) were collected from infected patients in our hospital. Enterobacter aerogenes was the most common bacterium isolated from all specimens (36.5%). The ESBLs were predominantly (90%) TEM derivatives (TEM-24, TEM-3). Pulsed-field gel electrophoresis highlighted that E. aerogenes, Klebsiella pneumoniae, and Citrobacter koseri had a clonal propagation.
Over the last 20 years, there has been an increased resistance to β-lactams because of the secretion of extended-spectrum β-lactamases (ESBLs) mediated by plasmids. This type of resistance is now observed in all species of Enterobacteriaceae and is currently disseminated throughout the world (22, 29, 35). From 1991 to 1993, we described the first ESBL-producing Enterobacteriaceae strains isolated in our hospital, a 1,588-bed university hospital in southern France.
To evaluate the epidemiological evolution of Enterobacteriaceae producing ESBL in our hospital from 1993 onward, a prospective study was conducted from April 2002 to March 2003 (20). We screened 3,063 nonrepetitive clinical isolates of Enterobacteriaceae recovered consecutively from infection sites of hospital patients. Antibiotic susceptibility testing was performed on Muller-Hinton agar with antibiotic disks from Pasteur Diagnostics (Marne-la-Coquette, France), placed at defined points, with the Vitek 2 GNS-F7 card (bioMérieux, Marcy-l'Etoile, France). ESBL production was tested with the double-disk synergy test (31). Strains were studied whenever the synergy test was positive. Duplicates isolated from the same patient were excluded. Isolates from superficial wounds, those from stool, ear, nose, and throat specimens, and those not involved in infections as defined by the Centers for Disease Control and Prevention criteria were excluded (17).
The β-lactamases were characterized by isoelectric focusing, performed with polyacrylamide gels as previously described. Standard enzymes (including TEM-1, TEM-3, TEM-24, SHV-5, and CTX-M-1) were used as pI markers (6). The ESBL that was neither a TEM nor an SHV derivative was identified by direct sequencing of the PCR product obtained with specific primers CTX-MF (5′-GCGATGTGCAGCACCAGTAA-3′) and CTX-MR (5′-GGTTGAGGCTGGGTGAAGTA-3′), which were previously described (19). DNA sequencing of both strands of the PCR products was performed with an ABI 1377 automated sequencer with the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit with AmpliTaq DNA polymerase FS (Perkin-Elmer/Applied Biosystems, Foster City, Calif.) at C. Chanal's laboratory.
The clonality of the strains was examined by pulsed-field gel electrophoresis (PFGE) with a CHEF DRII system (Bio-Rad SA, Ivry-sur-Seine, France) as previously described (20). The Enterobacter aerogenes, Escherichia coli, Klebsiella pneumoniae, and Citrobacter koseri chromosomal DNAs were digested overnight with the restriction enzyme XbaI (Promega, Madison, Wis.), whereas the Proteus mirabilis and Providencia stuartii DNAs were digested with SmaI (Promega). Electrophoresis was performed at 6 V/cm for 30 h; the pulse time ranged from 40 to 5 s for E. aerogenes, K. pneumoniae, C. koseri, and E. coli strains and from 25 to 5 s for P. mirabilis and P. stuartii. Because a single base mutation in the chromosomal DNA of an isolate is sufficient to introduce differences in three fragments of its restriction pattern, isolates with restriction patterns showing the same differences in one to three fragments were considered to belong to the same genotype (32). The PFGE patterns were analyzed with the GelCompar computer software for Windows, version 3.5 (Applied Maths, Kortrijk, Belgium), and compared by the algorithmic clustering method known as the unweighted pair group method using arithmetic averages with the Dice coefficient of similarity. Isolates were considered to be within a cluster if the coefficient of similarity was >80%.
Out of the 3,063 Enterobacteriaceae strains isolated, 80 produced an ESBL, i.e., 2.62%, in accordance with other French publications (1, 2, 11, 15), and corresponded to: E. aerogenes (n = 29 [36.3%]), K. pneumoniae (n = 15 [18.8%]), E. coli (n = 13 [16.2%]), C. koseri (n = 12 [15%]), P. mirabilis (n = 6 [7.5%]), P. stuartii (n = 4 [5%]), and K. oxytoca (n = 1 [1.2%]). No epidemic was reported during the surveillance period. The prevalence of the ESBL production in the various species was 20.34% (12 of 59) for C. koseri, 17.9% (29 of 162) for E. aerogenes, 8.24% (15 of 182) for K. pneumoniae, 7.55% (4 of 53) for P. stuartii, 2.33% (6 of 258) for P. mirabilis, 1.22% (1 of 82) for K. oxytoca, and 0.71% (13 of 1,827) for E. coli. These results were close to those found in other French hospitals, except for E. aerogenes (17.9% in our hospital compared to high levels ranging from 31.9 to 53.5% in other hospitals) (5, 11, 25). The majority of strains were isolated from urinary specimens (n = 51 [63.8%]) (Table 1). Out of the 80 ESBL-producing strains isolated, 11.25% were found in the recovery unit, 20% were found in the intensive care unit (ICU), 20% were found in the geriatric unit, and 36.25% were found in the medicine unit (Table 1). However, among the Enterobacteriaceae strains isolated in each unit, the proportion of ESBL-producing strains was 7.3% (9 of 123) in the geriatric unit and 6.75% (16 of 237) in the recovery unit. This rate was only 1.8% in the medicine unit (29 of 1,596). Indeed, 32 (41.6%) out of 77 patients had stayed in an ICU in the 6 months prior to isolation of the ESBL-producing bacteria. The propagation of the ESBL-producing strain could be correlated to time spent in an ICU, as already described by others (12, 36). Contrary to previous studies, ESBL strains were not detected in pediatric patients (21, 28). E. aerogenes was the predominant bacterium, and this has been the trend in France since 1993, while ESBL-producing K. pneumoniae isolates are decreasing (3, 5, 20). This phenomenon has also been observed in other countries such as the United States and Spain, although not in Italy (10, 29, 30). Furthermore, we isolated few strains of K. pneumoniae in geriatric wards (13.3%, 2 out of 15), where the first ESBL-producing Enterobacteriaceae strains were described in our hospital (20).
TABLE 1.
Characteristics of ESBL-producing Enterobacteriaceae strains isolated in a French university hospital in 2002
| Species (total no. of isolates) | Total no. (%) of ESBL-producing isolates | PFGE profilea | Specimen(s)b | Unit or ward (no.) | β-Lactamase contentc | Antibiotyped |
|---|---|---|---|---|---|---|
| E. aerogenes (162) | 29 (36.3) | EAIA (9) | Pus (5), urine (3), respiratory tract (1) | Medicine (4), ICU (2), surgery (2), geriatric | TEM-24, AmpC | KTNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL (6) |
| KTGNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL (3) | ||||||
| EAIB (6) | Pus (3), urine (3) | Medicine (5), geriatric | TEM-24, AmpC | KTNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL (5) | ||
| KTGNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL (1) | ||||||
| EAIC (4) | Urine (2), cutaneous (1), respiratory tract (1) | ICU (4) | TEM-24, AmpC | KTNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL (1) | ||
| EAIE (4) | Urine (3), pus (1) | Medicine, surgery, geriatric, recovery | TEM-24, AmpC | KTNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| EAIG (3) | Urine (2), pus (1) | Medicine, geriatric, recovery | TEM-24, AmpC | KTNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| EAIF | Pus | Surgery | TEM-24, AmpC | KTNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| EAID | Urine | Recovery | TEM-24, AmpC | K-NAL, OFX, NOR, CIP, PEF-TET CHL | ||
| EAIH | Urine | Geriatric | TEM-24, AmpC | KTNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| K. pneumoniae (182) | 15 (18.8) | KPI | Urine | Recovery | TEM-24, TEM-1 | KTNt(A)-NAL-SXT TET CHL |
| KPII | Urine | Medicine | TEM-3 | KTNt(A)-NAL-SXT TET CHL | ||
| KPIIIA (8) | Urine (5), cutaneous (1), pus (1), respiratory tract (1) | ICU (4), geriatric (2), surgery, recovery | TEM-3, TEM-1, SHV-1 | KTGNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| KPIIIC (2) | Pus, respiratory tract | Surgery, medicine | TEM-3, TEM-1 | KTGNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| KPIIIB | Urine | Medicine | TEM-3 | KTGNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| KPIIID | Urine | Medicine | TEM-3, TEM-1, SHV-1 | KTGNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| KPIIIE | Urine | Recovery | TEM-3, TEM-24 | KTNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| E. coli (1,827) | 13 (16.2) | ECIA (2) | Pus, urine | Medicine, geriatric | TEM-24, TEM-1 | KTNt(A)-NAL, OFX, NOR, PEF-SXT TET CHL |
| ECIIA (2) | Pus, urine | Medicine, surgery | CTX-M-3, TEM-1 | KTGNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| EC1 | Respiratory tract | Medicine | TEM-24 | KTNt(A)-NAL-SXT TET CHL | ||
| EC2 | Urine | Medicine | CTX-M-15, TEM-1/OXA-1 | KTGNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| EC3 | Urine | Medicine | CTX-M-15, OXA-1 | KTGNt(A)-NAL, OFX, NOR, PEF-SXT TET CHL | ||
| EC4 | Urine | Medicine | CTX-M-15, OXA-1 | KTGNt(A)-NAL, OFX, NOR, PEF-SXT TET CHL | ||
| EC5 | Pus | Surgery | CTX-M-14, TEM-1 | K(A)-NAL-SXT TET CHL | ||
| EC6 | Cutaneous | ICU | CTX-M-14, TEM-1 | K(A)-NAL-SXT TET CHL | ||
| EC7 | Venereal | ICU | CTX-M-15, TEM-1/OXA-1 | KTGNt(A)-NAL, OFX, NOR, PEF-TET CHL | ||
| EC8 | Urine | Geriatric | TEM-24 | KTNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| EC9 | Urine | Surgery | TEM-3 | KTNt(A)-NAL-TET CHL | ||
| C. koseri (59) | 12 (15) | CKIA (4) | Urine | Medicine (2), geriatric, ICU | TEM-24 | KTNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL |
| CKID (4) | Urine | Geriatric (2), medicine (2) | TEM-3 | KTNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| CKIE (2) | Urine | Recovery, ICU | TEM-3 | K(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| CKIB (1) | Urine | Surgery | TEM-3 | KTNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| CKIC (1) | Pus | Medicine | TEM-3 | K(A)-NAL, OFX, NOR, PEF-SXT TET CHL | ||
| P. mirabilis (258) | 6 (7.5) | PM1 | Urine | Medicine | TEM-3 | K(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL |
| PM2 | Urine | Medicine | TEM-3, TEM-1 | K(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| PM3 | Urine | ICU | TEM-3 | K(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| PM4 | Urine | Geriatric | TEM-3 | KTGNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| PM5 | Cutaneous | Geriatric | TEM-3 | KTNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| PM6 | Cutaneous | Geriatric | TEM-3 | KTNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| P. stuartii (53) | 4 (5) | PS1 | Urine | Geriatric | TEM-24 | KTGNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL |
| PS2 | Pus | Recovery | TEM-24 | KTGNt(A)-NAL, OFX, NOR, PEF-SXT TET CHL | ||
| PS3 | Urine | ICU | TEM-24 | KTGNt(A)-NAL, OFX, NOR, CIP, PEF-SXT TET CHL | ||
| PS4 | Urine | Recovery | TEM-24 | KTGNt(A)-NAL, OFX, NOR, CIP, PEF-TET CHL | ||
| K. oxytoca (82) | 1 (1.2) | KO1 | Urine | Medicine | TEM-3 | KTNt(A)-NAL-SXT TET CHL |
PFGE profiles were generated after restriction digestion of chromosomal DNA with the restriction enzyme XbaI.
The number of isolates is given in parentheses if more than one isolate was recovered.
AmpC and SHV-1, species-specific cephalosporinase and SHV-1 like chromosomal penicillinase, respectively.
Resistance to different antimicrobial agents. Abbreviations: T, tobramycin; A, amikacin; G, gentamicin; K, kanamycin; Nt, netilmicin; SXT, cotrimoxazole; NAL, nalidixic acid; OFX, ofloxacin; NOR, norfloxacin; CIP, ciprofloxacin; IPM, imipenem; TET, tetracycline; CHL, chloramphenicol. Parentheses indicate a low level of resistance.
The following five different ESBLs were characterized: TEM-24 (n = 38 [47.5%]), TEM-3 (n = 34 [42.5%]), CTX-M-15 (n = 4 [5%]), and CTX-M-3 and CTX-M-14 (n = 2 [2.5%] each) (Table 1). E. aerogenes and P. stuartii secreted exclusively TEM-24, and P. mirabilis, C. koseri, and K. oxytoca, secreted exclusively TEM-3. K. pneumoniae mainly produced TEM-3 (n = 14, [93.3%]). Lastly, E. coli produced the greatest range of ESBLs, especially the CTX-M type. Since 1988, members of the family Enterobacteriaceae producing TEM-24, particularly E. aerogenes, have spread massively throughout several European countries such as France, Belgium, Italy, and Spain (7, 9, 13, 14, 16, 18, 23, 25, 27). TEM-24 has been found in other strains that produced ESBL (E. coli and P. stuartii) and confirmed plasmidic diffusion of this β-lactamase, without providing evidence of epidemic outbreaks. However, the production of TEM-24 in K. pneumoniae only concerned 13.3% of the K. pneumoniae isolates producing ESBLs, while in a neighboring geographic region these bacteria remained at epidemic proportions (18). In France, TEM-3 is secreted largely by Klebsiella spp., P. mirabilis, and C. koseri, while in other countries, other ESBLs are in the majority (4, 8, 11, 24, 26, 30, 34). ESBLs in the CTX-M group (CTX-M-3, CTX-M-14, and CTX-M-15) were only observed in E. coli strains. The majority of these enzymes have been found in South America, Australia, Japan, South Africa, Israel, and Eastern Europe, while a recent study confirmed their absence in the United States (11, 26, 33). It could therefore be concluded that these enzymes are responsible for an increased role in resistance mechanisms especially for E. coli.
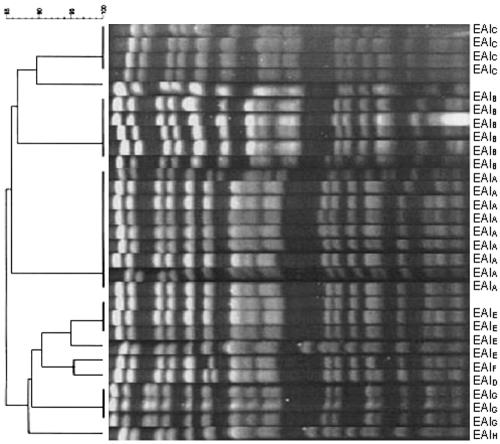
In our study, PFGE analysis showed that E. aerogenes, K. pneumoniae, and C. koseri had a clonal propagation. All of the results are summarized in Table 1. Eight clusters, each containing isolates with coefficients of similarity of more than 80%, were identified among E. aerogenes isolates. An example of patterns obtained with XbaI are shown in Fig. 1. Four clusters were identified among K. pneumoniae isolates. However, a high level of genetic heterogeneity was found in two isolates. Thus, five clusters were identified among C. koseri strains.
FIG. 1.
Dendrogram and PFGE of XbaI-digested genomic DNAs from ESBL-producing E. aerogenes from our university hospital. Strains were clustered by the unweighted pair group method using arithmetic averages (UPGMA). The scale indicates the percentage of genetic similarity. Max. tol., maximum tolerance in percentage of the curve to match bands; Min. surf., minimum surface area of a band.
Ten years after our principal study, six new varieties of Enterobacteriaceae were identified as producing ESBLs. We noted a complex evolution: the persistence of TEM-3 as the major ESBL secreted by K. pneumoniae, dissemination of clonal strains of E. aerogenes producing TEM-24, diffusion of these resistance mechanisms to other microorganisms such as E. coli and P. stuartii, isolation of E. coli producing CTX-M, and dissemination of ESBL-producing strains throughout the hospital. This type of propagation in the hospital environment is rapid and alarming, despite the introduction of procedures aimed at limiting patient-to-patient diffusion of multiresistant bacteria, as well as a concerted policy regarding the use of extended-spectrum β-lactams (1, 5, 11). These surveillance measures, combined with effective screening, should assist in the fight against the worrying propagation of these microorganisms.
REFERENCES
- 1.Albertini, M. T., C. Benoit, L. Berardi, Y. Berrouane, A. Boisivon, P. Cahen, C. Cattoen, Y. Costa, P. Darchis, E. Deliere, D. Demontrond, F. Eb, F. Golliot, G. Grise, A. Harel, J. L. Koeck, M. P. Lepennec, C. Malbrunot, M. Marcollin, S. Maugat, M. Nouvellon, B. Pangon, S. Ricouart, M. Roussel-Delvallez, A. Vachee, A. Carbonne, L. Marty, V. Jarlier, and the Microbiology Surveillance Network of Northern France. 2002. Surveillance of methicillin-resistant Staphylococcus aureus (MRSA) and Enterobacteriaceae producing extended-spectrum beta-lactamase (ESBLE) in northern France: a five-year multicentre incidence study. J. Hosp. Infect. 52:107-113. [DOI] [PubMed] [Google Scholar]
- 2.Arpin, C., V. Dubois, L. Coulange, C. Andre, I. Fischer, P. Noury, F. Grobost, J. P. Brochet, J. Jullin, B. Dutilh, G. Larribet, I. Lagrange, and C. Quentin. 2003. Extended-spectrum β-lactamase-producing Enterobacteriaceae in community and private health care centers. Antimicrob. Agents Chemother. 47:3506-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arpin, C., C. Coze, A. M. Rogues, J. P. Gachie, C. Bebear, and C. Quentin. 1996. Epidemiological study of an outbreak due to multidrug-resistant Enterobacter aerogenes in a medical intensive care unit. J. Clin. Microbiol. 34:2163-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barroso, H., A. Freitas-Vieira, L. M. Lito, J. M. Cristino, M. J. Salgado, H. F. Neto, J. C. Sousa, G. Soveral, T. Moura, and A. Duarte. 2000. Survey of Klebsiella pneumoniae producing extended-spectrum beta-lactamases at a Portuguese hospital: TEM-10 as the endemic enzyme. J. Antimicrob. Chemother. 45:611-616. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand, X., D. Hocquet, K. Boisson, E. Siebor, P. Plesiat, and D. Talon. 2003. Molecular epidemiology of Enterobacteriaceae producing extended-spectrum beta-lactamase in a French university-affiliated hospital. Int. J. Antimicrob. Agents 22:128-133. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet, R., J. L. M. Sampaio, C. Chanal, D. Sirot, C. De Champs, J. L. Viallard, R. Labia, and J. Sirot. 2000. A novel class A extended-spectrum β-lactamase (BES-1) in Serratia marcescens isolated in Brazil. Antimicrob. Agents Chemother. 44:3061-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosi, C., A. Davin-Regli, C. Bornet, M. Mallea, J. M. Pages, and C. Bollet. 1999. Most Enterobacter aerogenes strains in France belong to a prevalent clone. J. Clin. Microbiol. 37:2165-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford, P. A., C. E. Cherubin, V. Idemyor, B. A. Rasmussen, and K. Bush. 1994. Multiply resistant Klebsiella pneumoniae strains from two Chicago hospitals: identification of the extended-spectrum TEM-12 and TEM-10 ceftazidime-hydrolyzing β-lactamases in a single isolate. Antimicrob. Agents Chemother. 38:761-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canton, R., A. Oliver, T. M. Coque, M. C. Varela, J. C. Perez-Diaz, and F. Baquero. 2002. Epidemiology of extended-spectrum β-lactamase-producing Enterobacter isolates in a Spanish hospital during a 12-year period. J. Clin. Microbiol. 40:1237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coque, T. M., A. Oliver, J. C. Perez-Diaz, F. Baquero, and R. Canton. 2002. Genes encoding TEM-4, SHV-2, and CTX-M-10 extended-spectrum β-lactamases are carried by multiple Klebsiella pneumoniae clones in a single hospital (Madrid, 1989 to 2000). Antimicrob. Agents Chemother. 46:500-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Champs, C., D. Sirot, C. Chanal, R. Bonnet, and J. Sirot. 2000. A 1998 survey of extended-spectrum β-lactamases in Enterobacteriaceae in France. Antimicrob. Agents Chemother. 44:3177-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decre, D., B. Gachot, J. C. Lucet, G. Arlet, E. Bergogne-Berezin, and B. Regnier. 1998. Clinical and bacteriologic epidemiology of extended-spectrum beta-lactamase-producing strains of Klebsiella pneumoniae in a medical intensive care unit. Clin. Infect. Dis. 27:834-844. [DOI] [PubMed] [Google Scholar]
- 13.De Gheldre, Y., M. J. Struelens, Y. Glupczynski, P. De Mol, N. Maes, C. Nonhoff, H. Chetoui, C. Sion, O. Ronveaux, M. Vaneechoutte, and Groupement pour le Dépistage, l'Etude et la Prévention des Infections Hospitalières (GDEPIH-GOSPIZ). 2001. National epidemiologic surveys of Enterobacter aerogenes in Belgian hospitals from 1996 to 1998. J. Clin. Microbiol. 39:889-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumarche, P., C. De Champs, D. Sirot, C. Chanal, R. Bonnet, and J. Sirot. 2002. TEM derivative-producing Enterobacter aerogenes strains: dissemination of a prevalent clone. Antimicrob. Agents Chemother. 46:1128-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eveillard, M., M. Biendo, B. Canarelli, F. Daoudi, G. Laurans, F. Rousseau, and D. Thomas. 2001. Spread of Enterobacteriaceae producing broad-spectrum beta-lactamase and the development of their incidence over a 16-month period in a university hospital center. Pathol. Biol. 49:515-521. [DOI] [PubMed] [Google Scholar]
- 16.Galdbart, J. O., F. Lemann, D. Ainouz, P. Feron, N. Lambert-Zechovsky, and C. Branger. 2000. TEM-24 extended-spectrum beta-lactamase-producing Enterobacter aerogenes: long-term clonal dissemination in French hospitals. Clin. Microbiol. Infect. 6:316-323. [DOI] [PubMed] [Google Scholar]
- 17.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control 16:128-139. [DOI] [PubMed] [Google Scholar]
- 18.Giraud-Morin, C., and T. Fosse. 2003. A seven-year survey of Klebsiella pneumoniae producing TEM-24 extended-spectrum beta-lactamase in Nice University Hospital (1994-2000). J. Hosp. Infect. 54:25-31. [DOI] [PubMed] [Google Scholar]
- 19.Gniadkowski, M., I. Schneider, A. Palucha, R. Jungwirth, B. Mikiewicz, and A. Bauernfeind. 1998. Cefotaxime-resistant Enterobacteriaceae isolates from a hospital in Warsaw, Poland: identification of a new CTX-M-3 cefotaxime-hydrolyzing β-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob. Agents Chemother. 42:827-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouby, A., C. Neuwirth, G. Bourg, N. Bouziges, M. J. Carles-Nurit, E. Despaux, and M. Ramuz. 1994. Epidemiological study by pulsed-field gel electrophoresis of an outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a geriatric hospital. J. Clin. Microbiol. 32:301-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammami, A., G. Arlet, S. Ben Redjeb, F. Grimont, A. Ben Hassen, A. Rekik, and A. Philippon. 1991. Nosocomial outbreak of acute gastroenteritis in a neonatal intensive care unit in Tunisia caused by multiply drug resistant Salmonella wien producing SHV-2 beta-lactamase. Eur. J. Clin. Microbiol. Infect. Dis. 10:641-646. [DOI] [PubMed] [Google Scholar]
- 22.Jacoby, G. A. 1997. Extended-spectrum beta-lactamases and other enzymes providing resistance to oxyimino-beta-lactams. Infect. Dis. Clin. N. Am. 11:875-887. [DOI] [PubMed] [Google Scholar]
- 23.Jalaluddin, S., J. M. Devaster, R. Scheen, M. Gerard, and J. P. Butzler. 1998. Molecular epidemiological study of nosocomial Enterobacter aerogenes isolates in a Belgian hospital. J. Clin. Microbiol. 36:1846-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, P. Y., J. C. Tung, S. C. Ke, and S. L. Chen. 1998. Molecular epidemiology of extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolates in a district hospital in Taiwan. J. Clin. Microbiol. 36:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mammeri, H., G. Laurans, M. Eveillard, S. Castelain, and F. Eb. 2001. Coexistence of SHV-4- and TEM-24-producing Enterobacter aerogenes strains before a large outbreak of TEM-24-producing strains in a French hospital. J. Clin. Microbiol. 39:2184-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paterson, D. L., K. M. Hujer, A. M. Hujer, B. Yeiser, M. D. Bonomo, L. B. Rice, R. A. Bonomo, and the International Klebsiella Study Group. 2003. Extended-spectrum β-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: dominance and widespread prevalence of SHV- and CTX-M-type β-lactamases. Antimicrob. Agents Chemother. 47:3554-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perilli, M., E. Dell'Amico, B. Segatore, M. R. de Massis, C. Bianchi, F. Luzzaro, G. M. Rossolini, A. Toniolo, G. Nicoletti, and G. Amicosante. 2002. Molecular characterization of extended-spectrum β-lactamases produced by nosocomial isolates of Enterobacteriaceae from an Italian nationwide survey. J. Clin. Microbiol. 40:611-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poilane, I., P. Cruaud, E. Lachassinne, F. Grimont, P. A. Grimont, M. Collin, J. Gaudelus, J. C. Torlotin, and A. Collignon. 1993. Enterobacter cloacae cross-colonization in neonates demonstrated by ribotyping. Eur. J. Clin. Microbiol. Infect. Dis. 12:820-826. [DOI] [PubMed] [Google Scholar]
- 29.Rice, L. B., S. H. Willey, G. A. Papanicolaou, A. A. Medeiros, G. M. Eliopoulos, R. C. Moellering, Jr., and G. A. Jacoby. 1990. Outbreak of ceftazidime resistance caused by extended-spectrum β-lactamases at a Massachusetts chronic-care facility. Antimicrob. Agents Chemother. 34:2193-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanguinetti, M., B. Posteraro, T. Spanu, D. Ciccaglione, L. Romano, B. Fiori, G. Nicoletti, S. Zanetti, and G. Fadda. 2003. Characterization of clinical isolates of Enterobacteriaceae from Italy by the BD Phoenix extended-spectrum β-lactamase detection method. J. Clin. Microbiol. 41:1463-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirot, J. 1996. Detection of extended-spectrum plasmid-mediated beta-lactamases by disk diffusion. Clin. Microbiol. Infect. 2(Suppl. 1):S35-S39. [DOI] [PubMed] [Google Scholar]
- 32.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tenover, F. C., P. M. Raney, P. P. Williams, J. K. Rasheed, J. W. Biddle, A. Oliver, S. K. Fridkin, L. Jevitt, and J. E. McGowan, Jr. 2003. Evaluation of the NCCLS extended-spectrum β-lactamase confirmation methods for Escherichia coli with isolates collected during Project ICARE. J. Clin. Microbiol. 41:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urban, C., K. S. Meyer, N. Mariano, J. J. Rahal, R. Flamm, B. A. Rasmussen, and K. Bush. 1994. Identification of TEM-26 β-lactamase responsible for a major outbreak of ceftazidime-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 38:392-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winokur, P. L., R. Canton, J. M. Casellas, and N. Legakis. 2001. Variations in the prevalence of strains expressing an extended-spectrum beta-lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clin. Infect. Dis. 15:S94-S103. [DOI] [PubMed] [Google Scholar]
- 36.Yuan, M., H. Aucken, L. M. Hall, T. L. Pitt, and D. M. Livermore. 1998. Epidemiological typing of klebsiellae with extended-spectrum beta-lactamases from European intensive care units. J. Antimicrob. Chemother. 41:527-539. [DOI] [PubMed] [Google Scholar]