Abstract
Keratan sulfate (KS) was isolated from chicken egg white in amounts corresponding to ∼0.06 wt% (dry weight). This KS had a weight-average molecular weight of ∼36–41 kDa with a polydispersity of ∼1.3. The primary repeating unit present in chicken egg white KS was →4) β-N-acetyl-6-O-sulfo-d-glucosamine (1 → 3) β-d-galactose (1→ with some 6-O-sulfo galactose residues present. This KS was somewhat resistant to depolymerization using keratanase 1 but could be depolymerized efficiently through the use of reactive oxygen species generated using copper (II) and hydrogen peroxide. Of particular interest was the presence of substantial amounts of 2,8- and 2,9-linked N-acetylneuraminic acid residues in the form of oligosialic acid terminating the non-reducing ends of the KS chains. Most of the KS appears to be N-linked to a protein core as evidenced by its sensitivity to PNGase F.
Keywords: chicken egg white, glycosaminoglycan, keratan sulfate
Introduction
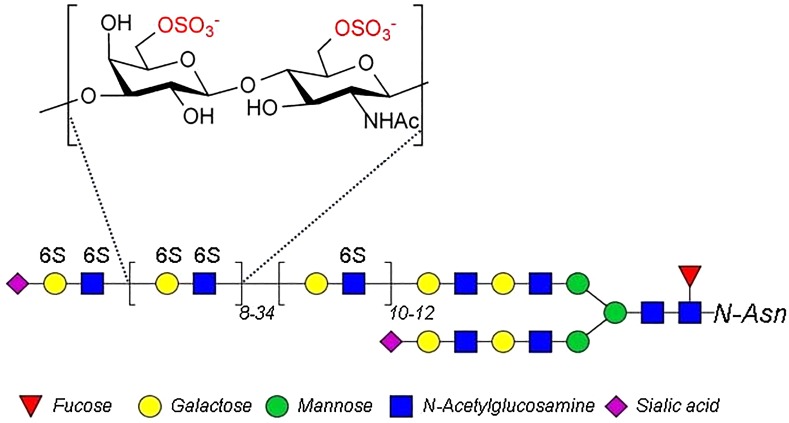
Keratan sulfate (KS) is a 6-O-sulfo group-containing glycosaminoglycan (GAG) having a backbone structure of →4) β-GlcNAc (1 → 3) β-Gal (1→, where GlcNAc is d-N-acetyl glucosamine and Gal is d-galactose (Greiling and Scott 1989; Schaefer and Schaefer 2010). The KS GAG is linked as either N- or O-linked polysaccharide chains to various core proteins of proteoglycans (Greiling and Scott 1989). KS has been identified in a wide range of tissues, such as animal cornea, cartilage, bones, neural cells, dermis, ovarian zona pellucida and even mammal uterine lining (Funderburgh 2000). Cornea stands out, among these tissues, with a KS (Figure 1) content of >10-fold that of cartilage and is 2–4 orders of magnitude more than the KS contents of other tissues (Funderburgh 2000).
Fig. 1.
Structure of bovine corneal KS.
As with other GAGs, KS is distributed on various animal cell surfaces and in the extracellular matrix (Schaefer and Schaefer 2010; Weyers et al. 2013). In cornea, the high abundance of KS is involved with maintaining the proper levels of tissue hydration, which is critical for corneal transparency (Quantock et al. 2010). KS also plays a fundamental role in ocular inflammation, corneal injury, keratitis and uveitis (Amjadi et al. 2013). KS is also an active ingredient in eye drops to help restore ocular health (Pomin 2015).
As with corneal KS, cartilage KS also plays a role in keeping balanced hydration properties of this tissue, and as a component of aggrecan it efficiently confers resistance to physical stress and loads on these tissues (Sommarin et al. 1998). Mice with homozygotic mutation in N-acetylglucosamine 6-O-sulfotransferase (GlcNAc6ST) isoform 1 (GlcNAc6ST-1−/−) show cartilage that damages easier than normal mice and this damage can be significantly ameliorated through the intraperitoneal injection of KS. This suggests the utility of exogenous KS as a therapeutic molecule for combating cartilage degradation (Hayashi et al. 2011). Furthermore, a fully sulfated KS disaccharide, β-GlcNAc6S (1 → 3) β-Gal6S or β-Gal6S (1 → 4) β-GlcNAc6S, where S is sulfo, has been proposed as a potent new drug for the prevention and treatment of chronic obstructive pulmonary disease, the third leading cause for death in the World by 2020 (Shirato et al. 2013). The lack of KS is associated with the early-phase pathogenesis of amyotrophic lateral sclerosis (ALS), suggesting that KS might be a therapeutic candidate for treatment of ALS (Hirano et al. 2013). KS is now being explored by the pharmaceutical industry for its various potential therapeutic effects (Pomin et al. 2012; Pomin 2015).
Commercial KS (Figure 1) is primarily produced through its extraction from bovine corneas (Weyers et al. 2013). Since corneas are a central nervous system tissue their availability has been severely limited since the outbreak of bovine spongiform encephalopathy or mad-cow disease in the 1990s. Thus, the increasing demand for research and commercial sources of KS has resulted in shortages. Therefore, we under took to broaden the tissue sources from which KS could be conveniently and successfully prepared. A previous study in our lab suggested that eggs might represent a potential new source of GAGs, including hyaluronan, chondroitin sulfates, heparan sulfate and KS (Liu et al. 2014). In particular, KS was shown to be abundant in egg white. In present study, we purify KS from chicken egg white and characterize its structure to help broaden access for the GAG research community and pharmaceutical industry for this valuable and important polysaccharide.
Results
KS purification from chicken egg whites
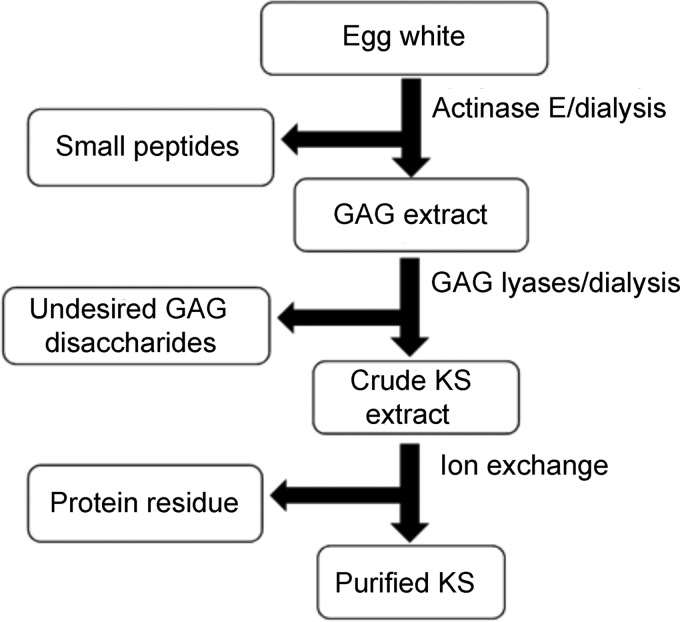
Commercial egg white and freshly prepared thick and thin egg white were treated with a non-specific protease, actinase E, to remove egg white proteins and to release KS GAG chains from their protein core. After thermal inactivation of the protease, small peptides were removed using a 3-kDa molecular weight cutoff (MWCO) spin column. The resulting GAG mixture was treated with heparin lyases and chondroitin lyases to remove most of the undesired GAGs. Egg white KS was then enriched in the 1.1 M aq. NaCl eluent from Vivapure Q maxi H column (Figure 2). Nearly all of the egg white GAGs, including heparan sulfate, chondroitin sulfates and hyaluronan were successfully removed using polysaccharide lyase treatment followed by this purification step (Figure 2). The KS could be quantitatively recovered from the 1.1 M NaCl wash fractions. The recovery of KS from a commercial egg white preparation was 5.8 mg KS per 10 g of wet egg white (Table I). The recovery of KS from thick egg white and thin egg white of fresh eggs were 7.6 and 5.6 mg per 10 mg of freeze-dried matter, respectively (Table I). The KS recovery of thick egg white was 20% higher than that from thin egg white.
Fig. 2.
Purification and analytical flow chart used for chicken egg white KS.
Table I.
Egg white KS recovery and molecular weights
| KS (mg) recovered from 10 g of egg white (freeze-dried weight) | MN a | MW a | Polydispersitya | |
|---|---|---|---|---|
| Commercial egg white | 5.80 | 30,800 | 40,600 | 1.32 |
| Thin egg white | 5.60 | 29,700 | 37,700 | 1.27 |
| Thick egg white | 7.60 | 27,200 | 36,800 | 1.36 |
| Desialylated thick egg white KS | – | 24,300 | 31,200 | 1.28 |
aFor details, please refer to Materials and methods.
Mass spectral analysis of depolymerized KS
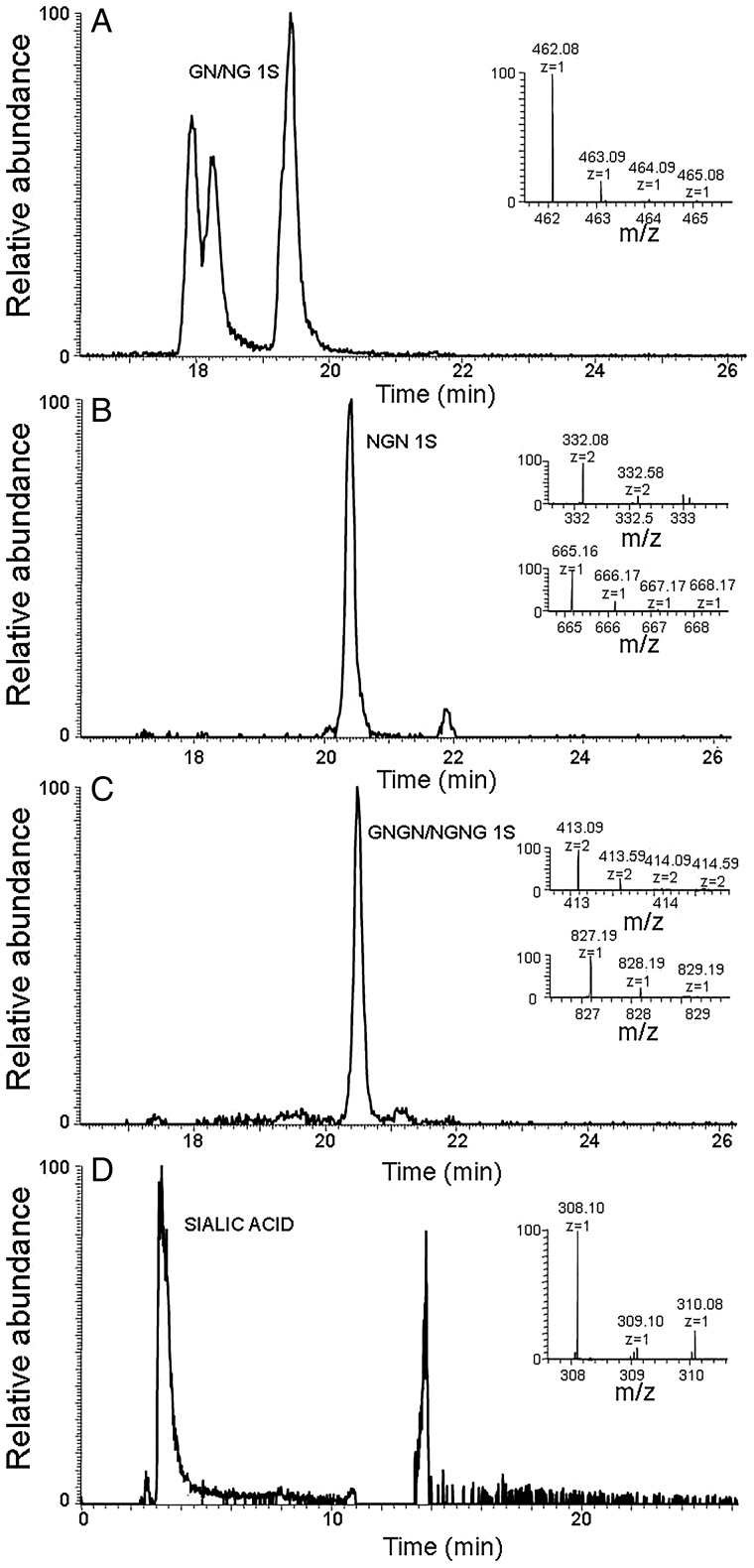
There are two keratanases that are commonly used to perform disaccharide analysis of KS. Keratanase 1, an endo-β-galactosidase from Pseudomonas sp., can only act at non-sulfated galactose residues and keratanase 2, an endo-β-galactosidase, (EC 3.2.1.103) can also act on 6-O-sulfo galactose residues (Nakazawa et al. 1989; Fukuda 1995; Weyers et al. 2013). Unfortunately, keratanase 2 is no longer commercially available and as expected, treatment with keratanase 1 only affords a single disaccharide NSKS (GlcN6S-Gal) with no 2SKS (GlcN6S-Gal6S), which had been observed in the keratanase 2 treatment of bovine corneal KS (Weyers et al. 2013). Small amounts of a tetrasaccharide with 3 sulfo groups and a hexasaccharide with 5 sulfo groups were also observed on keratanase 1 treatment of egg white KS. No oligosaccharides having an odd number sugar residues and no linkage region oligosaccharide were observed. An alternative method of KS depolymerization was undertaken using reactive oxygen species (Li et al. 2014). Using this method and FT-MS detection, KS di-, tri-, tetra- and pentasaccharides could be detected (Table II, Figure 3; Supplementary data, Figures S2–S6). These include oligosaccharides with both GlcNAc and Gal at their non-reducing and reducing ends and a sulfation level ranging from 0 sulfo groups per saccharide residue to 1.0 group per saccharide residue (fully sulfated). The oligosaccharides with a GlcNAc residue at their non-reducing end appeared to elute later that those with a Gal residue at their non-reducing end. Finally, the oxidative depolymerization of chicken egg white KS affords a single identifiable monosaccharide, prominently eluting early from the HILIC column, corresponding in mass to N-acetylneuraminic acid (Figure 3D).
Table II.
Mass spectral analysis is oligosaccharides derived from chicken egg white KS
| Structure | Retention Time (min) | |
|---|---|---|
| dp2 | Gal-GlcNAc (0S) | 16.93 |
| Gal-GlcNAc (1S) | 17.94 | |
| Gal-GlcNAc (2S) | 18.85 | |
| dp3 | Gal-GlcNAc-Gal (2S) | 17.99 |
| dp4 | Gal-GlcNAc-Gal-GlcNAc (1S) | 20.49 |
| Gal-GlcNAc-Gal-GlcNAc (2S) | 20.95 | |
| Gal-GlcNAc-Gal-GlcNAc (3S) | 22.16 | |
| Gal-GlcNAc-Gal-GlcNAc (4S) | 23.11 | |
| dp5 | Gal-GlcNAc-Gal-GlcNAc-Gal (1S) | 21.22 |
| Gal-GlcNAc-Gal-GlcNAc-Gal (4S) | 24.70 | |
| dp2 | GlcNAc-Gal (1S) | 19.42 |
| GlcNAc-Gal (2S) | 19.40 | |
| dp3 | GlcNAc-Gal-GlcNAc (0S) | 18.88 |
| GlcNAc-Gal-GlcNAc (1S) | 20.43 | |
| GlcNAc-Gal-GlcNAc (2S) | 21.29 | |
| GlcNAc-Gal-GlcNAc (3S) | 21.23 | |
| dp4 | GlcNAc-Gal-GlcNAc-Gal (0S) | 20.69 |
| GlcNAc-Gal-GlcNAc-Gal (1S) | 21.09 | |
| GlcNAc-Gal-GlcNAc-Gal (2S) | 22.45 | |
| GlcNAc-Gal-GlcNAc-Gal (3S) | 22.52 | |
| GlcNAc-Gal-GlcNAc-Gal (4S) | 23.26 | |
| dp5 | GlcNAc-Gal-GlcNAc-Gal-GlcNAc (3S) | 24.78 |
| GlcNAc-Gal-GlcNAc-Gal-GlcNAc (4S) | 24.81 |
Gal, galactose; GlcNAc, N-acetylglucosamine; S, sulfo group; dp, degree of polymerization.
This table is based on the extracted ion chromatography of the oligosaccharide listed in Supplementary data, Figures S2–S6.
Fig. 3.
HILIC–HPLC-MS analysis of chicken egg white KS. Extracted ion chromatography and mass spectrum of (A) major disaccharide. (B) Major trisaccharide. (C) Major tetrasaccharide. (D) Sialic acid. (G: galactose; N: N-acetylgucosamine; S: sulfo group.).
Molecular weights of chicken egg white KS
The weight average molecular weight (MW) of a commercial egg white (containing both thick and thin white) KS was 40.6 kDa while freshly prepared thick egg white KS had an MW of 36.8 kDa, and thin egg white KS had a of MW 37.7 kDa (Table I; Supplementary data, Figure S1). These results suggest that there may be subtle differences in the KS was obtained in thick egg white and thin egg white KS. In addition, the results clearly show that the molecular weight of egg white KS was much larger than that of bovine corneal KS, which ranged from 11.6 to 14.3 kDa (Weyers et al. 2013). The polydispersity of egg white KS was also much larger than that of bovine corneal KS, which ranged from 1.23 to 1.32 (Weyers et al. 2013). KS samples prepared from egg white showed similar polydispersity of molecular weight properties (Table I; Supplementary data, Figure S1).
Neuraminic acid attached to chicken egg white KS
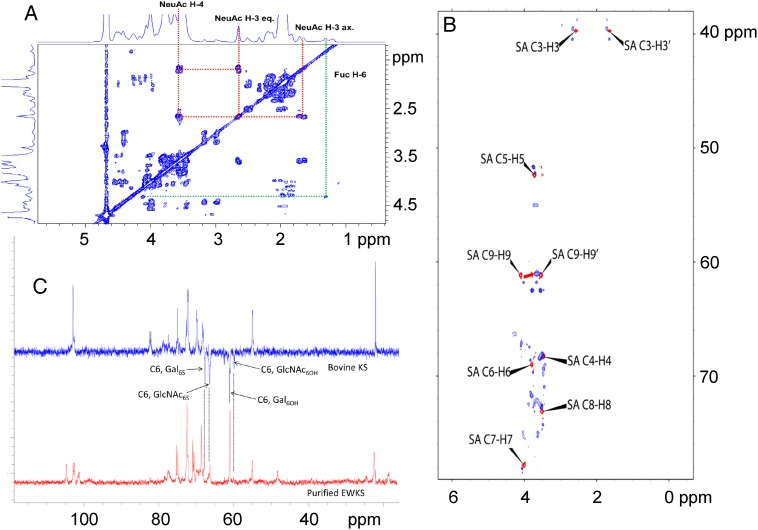
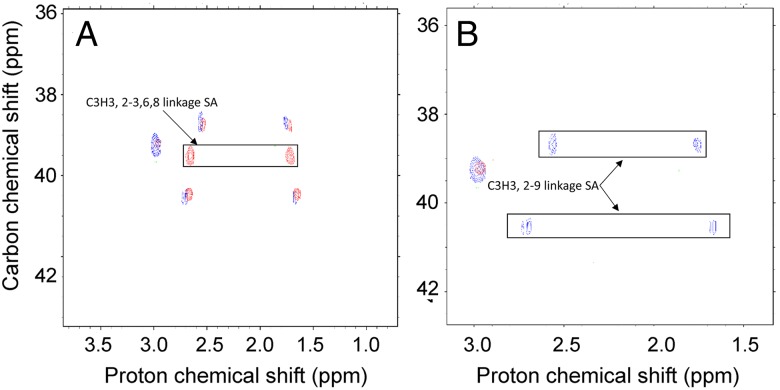
One dimensional (1D) 1H NMR analysis showed that the spectra of various egg white KS preparations were very similar; however, the spectrum of egg white KS (Figure 4) showed marked differences to the previously determined spectrum of bovine corneal KS (Weyers et al. 2013). The 1H NMR spectra of KS derived from corneal KS has a crowded region between 3.4 and 4.8 ppm, as the result of a severe signal overlap for the majority of the resonances (Huckerby and Lauder 2000). The 1H NMR spectra of egg white KS shows a similarly crowded region between 3.4 and 4.6 ppm (Figure 4). The 1D 1H NMR and 2D 1H–1H COSY analysis showed that egg white KS contained significant amounts of neuraminic acid (Figures 4 and 5A). The 1H–13C cross-peaks corresponding to C3-H3, C5-H5, C6-H6 and C9-H9 have splittings and shifts comparable with a standard polysialic acid, indicating multiple linkages corresponding to oligosialic acid structures (Figure 5B). Only a portion of oligosialic acid was removed from the egg white KS chain after α2-3,6,8 neuraminidase treatment (Figure 6A) leaving a portion of oligosialic acid that could only be removed using a α2–3,6,8,9 neuraminidase (Figure 6B). Comparison of these HSQC spectra (Figures 5B and 6A, B) suggested that the structures of oligosialic acid in chicken egg white KS not only contains the typical 2,8-linkages but also contains 2,9-linkages. Comparison of the 13C DEP-135 spectrum of bovine cornea KS spectrum with the 13C DEP-135 spectrum of KS isolated from chicken egg white indicates that most of the GlcNAc residues in chicken egg white KS are substituted with 6-O-sulfo groups while less than half of the Gal residues contain 6-O-sulfo groups. Gel permeation chromatography (GPC) analysis shows that neuraminidase treatment of egg white KS results in a substantial decrease in MW, corresponding to 7 kDa, further suggesting that the attachment of significant amounts of neuraminic acid is part of the molecular structure of egg white KS (Table I).
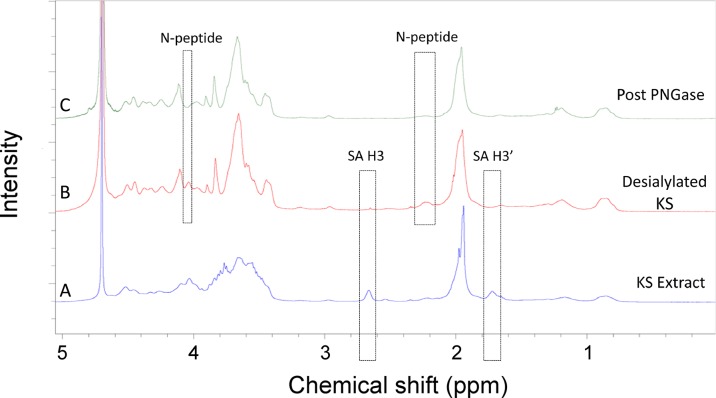
Fig. 4.
1D 1H NMR spectra of chicken egg white KS. (A) Chicken egg white KS as isolated. (B) Chicken egg white KS following neuraminidase treatment where the H3 and H3′ peaks of sialic acid peaks are observed at 2.7 and 1.7 ppm. (C). Chicken egg white KS following treatment with neuraminidase, fucosidase and PNGase where N-peptide peaks are observed at 4.1 and 2.2 ppm. This figure is available in black and white in print and in color at Glycobiology online.
Fig. 5.
NMR analysis of chicken egg white KS. (A) 1H–1H COSY. (B) Overlapped 1H–13C HSQC NMR spectra of chicken egg white KS as isolated (blue) and poly[2,8-(N-acetylneuraminic acid)] from E. coli (red). (C) Comparison of the 13C DEP-135 spectrum of bovine cornea KS spectrum with the 1D 13C NMR spectrum of chicken egg white KS as isolated.
Fig. 6.
Overlapped 1H–13C HSQC NMR spectra of chicken extracted egg white KS as isolated and desialylated KS samples. (A) Overlapped 1H–13C HSQC NMR spectra of chicken egg white KS as isolated (red) and chicken egg white KS following α2–3,6,8 neuraminidase treatment (blue). (B) Overlapped 1H–13C HSQC NMR spectra of chicken egg white KS following α2–3,6,8 neuraminidase treatment (blue) and α2–3,6,8,9 neuraminidase treatment (red).
Examination of chicken egg white KS linkage
Since KS is known to be synthesized as an O-linked proteoglycan in cartilage and an N-linked proteoglycan in cornea, we next examined the predominant linkage type present in chicken egg white KS. Since small amounts of fucose were detected in the 1D 1H NMR and 2D 1H–1H NMR spectra by peaks at 1.3 ppm, it was necessary to first treat chicken egg white KS with fucosidase to ensure its sensitivity to PNGase F should it be an N-linked proteoglycan. Following fucosidase and PNGase F treatment 1H NMR showed the loss of N-peptide signal suggesting that chicken egg white KS was an N-linked proteoglycan.
Discussion
Chicken egg contains various important nutrients, such as proteins, monounsaturated fatty acids, polyunsaturated fatty acids, minerals, vitamins and peptides of unique biological activities, making eggs an important human food and nutraceutical source (Abdou et al. 2013). An estimated 60 million tons of commercial eggs were produced worldwide in 2013, with China being the largest egg producer, at ∼27 million tons (Liu et al. 2014). From a single chicken egg of 71 g of weight (comprising ∼2.5 g of thick egg white and 1.65 g of thin egg white (freeze-dried weight)), 2.85 mg of KS was recovered using the purification method described in this paper, suggesting that chicken eggs might represent an excellent source of KS.
KS has been characterized as a linear polymer of lactosamine, 3Galβ1 → 4GlcNAcβ1, having O-sulfate groups at the C6 of both hexose moieties (Meyer et al. 1953). In our previous research on bovine corneal KS, using keratanase 2, two disaccharides, 2SKS (Gal6S(1 → 4)GlcNAc6S) and NSKS (Gal(1 → 4)GlcNAc6S), could be detected by LC–MS (Weyers et al. 2013; Ucakturk et al. 2014). Unfortunately, keratanase 2 is no longer commercially available. On treating egg white KS with commercially available keratanase 1, only resulted in a single disaccharide product, NSKS (Gal(1 → 4)GlcNAc6S) (Liu et al. 2014). These results suggest that either egg white KS is only the GlcNAc residues contain 6-O-sulfo groups or that if 6-O-sulfo Gal residues are present they reside in keratanase 1-resistant domains to and, thus, are undetected by LC–MS. Oxidative depolymerization of chicken egg white KS, however, afforded a range of oligosaccharides having 6-O-sulfo on both GlcNAc and Gal residues. DEP-135 NMR spectroscopy confirms the presence of 6-O-sulfo on both GlcNAc and Gal residues and shows that most of the GlcNAc residues and less than half of the Gal residues contain 6-O-sulfo groups. GPC analysis showed that molecular weight of egg white KS (Table I) was much larger than that of bovine corneal KS (Weyers et al. 2013) and that egg white KS also contained significant amounts of neuraminic acid. It is known that the non-reducing terminus of KS chain is generally capped with a variety of structures, especially neuraminic acid (Funderburgh 2002). In bovine cornea, up to 70% of the KS chains terminate with neuraminic acid (Tai et al. 1997). However, the neuraminic acid content in egg white KS is much higher than can be accommodated by simple chain terminating sialic acid residues. We are confident that our KS recovery and purification method, involving protease treatment and strong anion exchange would eliminate either protein linked or non-covalently bound polysialic acid. The 2D 1H–1H NMR analysis showed that, based on the integration of each peak, the molar ratio of NeuAc: GlcNAc was 3:2 and GPC results also showed that the molecular weight of egg white KS decreased by 7 kDa after neuraminidase treatment. It is reported that, in ascites fluid from patients with ovarian cancer, the O-linked oligosaccharides (including KS) were shown to consist of mainly highly sialylated structures and a smaller amount of sulfated structures; and the structure of di-neuraminic acid NeuAc-NeuAc was frequently found as the terminating structure of the O-linked oligosaccharides (Karlsson and McGuckin 2012). Chicken egg white KS is different structure from bovine cornea KS, as it is rich in neuramininic acid in the form of both 2,8- and 2,9-linked oligosialic acid. This oligosialic acid present may not completely be the non-reducing terminal end type and could contain branched side chains. However, the lack of more specific enzymes currently prevents the determination of the exact structure of these oligosialic acid residues. While is noteworthy that there is reportedly no sialyltransferase activity in the chicken oviduct that can use Galβ1,4GlcNAc as an acceptor (ref), KS is a complex structure that might represent an acceptor for an, as yet, unidentified sialyltransferase.
Chicken egg white KS proteoglycan appears to be primarily N-linked based on its observed sensitivity to PNGase F. Based on the current study the structure of chicken egg white KS it is clearly different from bovine cornea KS (Figure 1). Chicken egg white offers a rich new source of KS and eliminates some of the concerns associated with bovine corneal KS.
Materials and methods
Materials
Eggs, used in these experiments, were laid by Hy-line brown free-range chickens from Homestead Farms (Troy, NY). Commercial egg white (i.e., Egg Beaters™) was purchased locally. Unsaturated disaccharide standards of CS/DS (0SCS-0: ΔUA-GalNAc; 4SCS-A: ΔUA-GalNAc4S; 6SCS-C: ΔUA-GalNAc6S; 2SCS: ΔUA2S-GalNAc; 2S4SCS-B: ΔUA2S-Gal-NAc4S; 2S6SCS-D: ΔUA2S-GalNAc6S; 4S6SCS-E: ΔUA-GalNAc4S6S; TriSCS: ΔUA2S-GalNAc4S6S), unsaturated disaccharide standards of HS/HP (0SHS: ΔUA-GlcNAc; NSHS: ΔUA-GlcNS; 6SHS: ΔUA-GlcNAc6S; 2SHS: ΔUA2S-GlcNAc; 2SNSHS: ΔUA2S-GlcNS; NS6SHS: ΔUA-GlcNS6S; 2S6SHS: ΔUA2S-GlcNAc6S; TriSHS: ΔUA2S-GlcNS6S) and unsaturated disaccharide standard of HA (0SHA: ΔUA-GlcNAc) were purchased from Seikagaku (Japan). The saturated KS disaccharide standard (NSKS: GlcN6S-Gal) was prepared in our laboratory by keratanase 1 treatment of bovine corneal KS. Actinase E was obtained from Kaken Biochemicals (Japan). α-(2-3,6,8) Neuraminidase and α-(2-3,6,8,9) neuraminidase were from New England Biolab (Ipswich, MA). α-l-Fucosidase (bovine kidney) was from Sigma-Aldrich (St. Louis, MO). PNGase F was a generous gift from Professor Lai-Xi Wang (Department of Chemistry and Biochemistry University of Maryland, Baltimore). Chondroitin lyase ABC from Proteus vulgaris and chondroitin lyase ACII from Arthrobacteraurescens; keratanase 1 (KS endo-β-galactosidase) from Pseudomonas sp. was obtained from Seikagaku (Japan). Recombinant Flavobacterial heparin lyases I, II and III were expressed in our laboratory using Escherichia coli strains provided by Professor Jian Liu (College of Pharmacy, University of North Carolina). The 2-amino acridone (AMAC), E. coli poly[2,8-(N-acetylneuraminic acid)] (colominic acid) and sodium cyanoborohydride were obtained from Sigma-Aldrich (St. Louis, MO). Acetic acid and copper (II) acetate were purchased form Sigma Aldrich, St. Louis, Missouri. Hydrogen peroxide acetonitrile (HPLC Grade) and ammonium acetate (HPLC Grade) were purchased from Fisher Scientific, Springfield, New Jersey. Vivapure® Q Maxi H strong anion exchange spin columns were from Sartorius Stedim Biotech (Bohemia, NY).
Preparation of thick and thin egg white
The thick and thin egg whites were isolated as previously reported and all eggs collected had the same age (Liu et al. 2014). Briefly, after breaking an egg into a plate, the egg white embracing the yolk was torn from the yolk and the yolk was carefully removed out from egg white. The thick egg white was next successively picked up and transferred to a clean plate and this transfer was repeated three more times to remove the thin white. The thick white in the final plate was freeze-dried. The residual thin white in all except the final plate were combined and freeze-dried to obtain the thin white.
GAG recovery from thick egg white
Protein digestion
Approximately 7 g of freeze-dried thick egg white and thin egg white were individually dissolved in 210 mL of water, and 1 g of actinase E was added to digest the protein. The mixture was reacted in water bath of 54°C for 44 h. Commercial egg white, 200 g of fresh mixture, was digested with 3.6 g of actinase E at 54°C for 44 h.
Peptide removal
After digestion, sample was boiled 6 min, and centrifuged at 4500 × g for 28 min. The peptides were removed from supernatant using spin column (MWCO 3 kDa). After washing with water seven times the solution retained in the spin column was recovered and freeze-dried.
HS/HP and CS/DS digestion
Freeze-dried sample was dissolved into water, and heparin lyase I (120 mU), II (720 mU) and III (3300 mU) in 25 mM Tris, 500 mM NaCl, 300 mM imidazole buffer (pH 7.4) were added to each sample (final volume 6 mL), the reacting mixtures were incubated at 35°C for 8 h. Then 3 mL of 3× chondroitin digestion buffer (300 mM NH4Ac containing 30 mM CaCl2, pH 7.5) was added, and chondroitin lyase ABC (50 000 mU) and chondroitin lyase ACII (20 mU) in 0.1% BSA were added, the reaction mixtures were continuously incubated at 35°C for an additional 16 h.
Purification of KS
After digestion of both HS/HP and CS/DS, 33 mL urea (8 M) and CHAPS (2 wt%) and 52 mL of water were added into each reaction mixture. KS was purified by binding it to a Vivapure® Q Maxi H spin column and washing four times with 8 mL of 200 mM aq. NaCl. The bound KS was eluted by washing with 8 mL each of 550 mM aq. NaCl, 1.1 M aq. NaCl, 2.2 M aq. NaCl and 3.3 M aq. NaCl. The salt was removed from each wash using a spin column (MWCO 3 kDa, Millipore). Each desalted KS sample was collected and freeze-dried for further disaccharide analysis using LC–MS.
Enzymatic depolymerization of KS
Each purified KS eluent was again individually treated using polysaccharide lyases to remove residual CS/DS and HS/HP. Chondroitin lyase ABC (150 mU) and chondroitin lyase ACII (10 mU) in 0.1% BSA were added to 8 μL eluent in buffer of 100 mM NH4Ac containing 10 mM CaCl2 (pH 7.5; final volume 100 μL). Keratanase 1 (5 mU) in 0.1% BSA was added to 8 μL eluent in 50 mM Tris–HCl buffer (pH 7.5; final volume 100 μL). Heparin lyase I (20 mU), II (120 mU) and III (550 mU) in 25 mM Tris, 500 mM NaCl and 300 mM imidazole buffer (pH 7.4) were added to 8 μL eluent (final volume 100 μL). Reaction mixtures were incubated at 36°C for 16 h. After boiling to inactivation the enzymes at 100°C for 2 min, the mixtures were centrifuged with 14,500 × g for 15 min, and the supernatants were freeze-dried.
The freeze-dried disaccharide samples or a mixture of unsaturated disaccharide standards (500 ng/per each disaccharide) was added 10 μL 0.1 M AMAC solution in acetic acid/dimethyl sulfoxide (3/17, v/v) and periodically vortex mixed and at room temperature for 30 min. Next, 10 μL of 1 M NaBH3CN was added in the reaction mixture and incubated at 45°C for 4.5 h. Finally, the AMAC-tagged disaccharide mixtures were centrifuged with 14,500 × g for 15 min, and the supernatants were used for LC–MS analysis.
LC–MS for disaccharide analysis
LC–MS analyses were performed on an Agilent 1200 LC/MSD Instrument (Agilent Technologies, Inc., Wilmington, DE) equipped with a 6300 ion-trap and a binary pump equipped with a high-pressure cell. The column used was an Agilent Poroshell 120 EC-C18 column (3.0 mm × 150 mm, 2.7 μm, Agilent Technologies, Inc.) at 45°C.
For dual ammonium acetate and methanol gradient, eluent A was 50 mM ammonium acetate solution and eluent B was methanol. Solution A and 5% solution B was flowed (150 μL/min) through the column followed by linear gradients 5–17% solution B from 0 to 20 min, 17–40% solution B from 20 to 30 min and 40–100% solution B from 30 to 31 min.
The column effluent entered the ESI-MS source for continuous detection by MS. The electrospray interface was set in negative ionization mode with a skimmer potential of −40.0 V, a capillary exit of −40.0 V and a source temperature of 350°C, to obtain the maximum abundance of the ions in a full-scan spectrum (350–900 Da). Nitrogen (8 L/min, 40 psi) was used as a drying and nebulizing gas.
Oxidative depolymerization of KS
KS (250 μg) was dissolved in a 100-μL volume of ammonium acetate (0.1 M) containing copper (II) acetate (0.2 mM) at pH 7. Then 5 μL of 30% hydrogen peroxide was added and an oxidative depolymerization reaction was allowed to take place at 45°C for 3 h after which the reaction was terminated by lyophilization. The resulting KS oligosaccharides were dissolved 20 μL of 50 vol% aqueous acetonitrile.
LC–MS for oligosaccharide analysis
The KS oligosaccharides prepared through oxidative depolymerization were analyzed by HPLC-FTMS (Fu, Li, et al. 2014). LC relied on a Luna® 3 µm HILIC 200 Å column (150 × 2 mm) attached to an Agilent 1200 HPLC coupled with on-line detection using an LTQ-Orbitrap XL FT MS (Thermo Fisher Scientific) was equipped with ESI source with a spray voltage of 4.2 kV, a capillary voltage of −40 V, a tube lens voltage of −50 V, a capillary temperature of 275°C, a sheath flow rate of 30 and an auxiliary gas flow rate of 6. Mobile phase A was 5 mM ammonium acetate aqueous solution and mobile phase B was 5 mM ammonium acetate 98% vol% acetonitrile in water. A linear gradient, at a flow rate of 200 μL/min, began with 92% B to 70% B from 0 to 35 min and then to 60% B from 35 to 45 min, then to 30% B from 45 to 49 min to elute all analytes.
Glycosidase digestions
α2–3,6,8 Neuraminidase (200 units) was incubated with 8 mg of purified egg white KS in 50 mM sodium acetate buffer containing 5 mM CaCl2 (pH 5.5), in water bath of 37°C for 20 h to remove the neuraminic acid (sialic acid) residues from thick egg white KS. The digestion mixture was purified using Vivapure Q Maxi H. Half of the resulting KS sample was incubated with α2–3,6,8,9 neuraminidase and recovered using the same protocol described above. Both purified de-sialylated KS samples were analyzed by NMR spectroscopy or determined molecular weight using GPC.
Before removal of linkage peptide from purified thick egg white KS, above KS without neuraminic acid was treated with α-l-fucosidase. Briefly, α-l-fucosidase was desalted prior to use and re-suspended in 50 mM of sodium acetate (pH 5.75) and 0.6 units of enzyme was incubated neuraminidase-treated KS in water bath of 37°C for 20 h. After recovering the enzymatically treated KS using a Vivapure Q Maxi H spin column, the sample was further digestion with four units of PNGase F in 50 mM ammonium bicarbonate (pH 7.86) to remove N-linked peptide from KS. After each digestion, the sample was analyzed by NMR spectroscopy and its molecular weight was determined using GPC.
NMR spectroscopy
Egg white KS samples were analyzed by 1D 1H, 13C DEP-135, 2D 1H–1H COSY and 2D HSQC NMR spectroscopy. All NMR experiments were performed at 298 K on Bruker 800 MHz spectrometer with Topsin 2.1 software. Samples (1.0–2.0 mg) were each dissolved in 0.5 mL D2O (99.996%, Sigma-Aldrich) and lyophilized one time to remove the exchangeable protons. The samples were re-dissolved in 0.4 mL D2O and transferred to NMR microtubes (OD 5 mm, Norrell). 1H NMR experiments were performed with 32 scans, an acquisition time of 2.66 s and relaxation delay of 8 s. (Fu et al. 2013; He et al. 2015) 1D 13C NMR experiments were performed with 5000 scans, acquisition time of 228 ms and relaxation delay of 1.5 s. 1D 13C DEP-135 experiments were performed with 4000 scans, acquisition time of 350 ms and relaxation delay of 1.5 s. 2D 1H–1H COSY experiments were performed with 16 scans, 1.5 s relaxation delay, and 280 ms acquisition time (Xiong et al. 2013; Fu, Zhang, et al. 2014). 2D HSQC experiments were performed with 16 scans, an acquisition time of 280 ms and relaxation delay of 2 s. (Fu et al. 2013; Fu, Zhang, et al. 2014).
Gel permeation chromatography
GPC was performed according to the official monograph for heparin (USP37 Official Monograph) with minor modifications (Bhaskar et al. 2015). A guard column TSK SWXL 6-mm × 4-cm, 7-µm diameter was used in series with two analytical columns: TSK G4000 SWXL 7.8-mm × 30 cm, 8-µm in series with TSK G3000 SWXL 7.8-mm × 30-cm, 5-µm (Tosoh Corporation, Minato-Ku, Tokyo, Japan). These columns were connected to an HPLC system consisting of Shimadzu LC-10Ai pump, a Shimadzu CBM-20A controller and a Shimadzu RID-10A refractive index detector (Shimadzu, Kyoto, Japan). The mobile phase was 0.1 M ammonium acetate with 0.02% sodium azide. Columns and refractive index detector were maintained at 30°C using Eppendorf column heater (Eppendorf, Hamburg, Germany). A sample injection volume was 20 μL (sample concentration was ∼5 mg/mL) and a flow rate was 0.6 mL/min. The chromatograms were recorded with the LC solution version 1.25 software (Shimadzu, Kyoto, Japan), and analyzed with its “GPC Postrun” function. For molecular weight determination, USP Heparin Sodium Molecular Weight Calibrant RS (The United States Pharmacopeial Convention, Inc., MD) was used as a calibrant and USP Heparin Sodium Identification RS (The United States Pharmacopeial Convention, Inc.) was used to confirm system suitability. For calculation, third-order polynominal equation was used.
The number-average molecular weight (MN) was determined by summing the molecular weights of n polymer molecules, and dividing by sum of polymers.
where Mi was special molecular weight, Ni was the number of molecules of molecular weight Mi.
The weight-average molecular weight (MW) was calculated by where Ni was the number of molecules of molecular weight Mi.
The polydispersity was MW divided by MN.
Supplementary data
Supplementary data for this article are available online at http://glycob.oxfordjournals.org/.
Funding
This work was supported by NIH grants HL62244 and HL125371 to R.J.L.
Conflict of interest statement
None declared.
Abbreviations
AMAC, 2-amino acridone; CS, chondroitin sulfate; DEP, distortionless enhancement by polarization; ESI: electrospray ionization; FT, Fourier transform; GAG, glycosaminoglycan; HHCOSY, proton–proton correlation spectroscopy; HILIC, hydrophilic interaction liquid chromatography; HMQC, heteronuclear multiple-quantum coherence; HS/HP, heparan sulfate/heparin; KS, keratan sulfate; LC–MS, liquid chromatography mass spectrometry; NMR, nuclear magnetic resonance
Supplementary Material
Acknowledgements
The authors thank Professor Jian Liu (College of Pharmacy, University of North Carolina) for providing recombinant Escherichia coli strains for expressing heparin lyases I, II and III; Professor Lai-Xi Wang (Department of Chemistry and Biochemistry University of Maryland, Baltimore) for the gift of PNGase F.
References
- Abdou AM, Kim M, Sato K. 2013. Functional proteins and peptides of hen's egg origin. In: Hernández-Ledesma B, Hsieh CC, editors. Bioactive Food Peptides in Health and Disease. Rijeka, Croatia: InTech; p. 115–144. [Google Scholar]
- Amjadi S, Mai K, McCluskey P, Wakefield D. 2013. The role of lumican in ocular disease. ISRN Ophthalmol. 2013:632302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar U, Li G, Fu L, Onishi A, Suflita M, Dordick JS, Linhardt RJ. 2015. Combinatorial one-pot chemoenzymatic synthesis of heparin. Carbohydr Polym. 122:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Li L, Cai C, Li G, Zhang F, Linhardt RJ. 2014. Heparin stability by determining unsubstituted amino groups using HILIC-MS. Anal Biochem. 461:46–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Li G, Yang B, Onishi A, Li L, Sun P, Zhang F, Linhardt RJ. 2013. Structural characterization of pharmaceutical heparins prepared from different animal tissues. J Pharm Sci. 102:1447–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Zhang F, Li G, Onishi A, Bhaskar U, Sun P, Linhardt RJ. 2014. Structure and activity of a new low-molecular-weight heparin produced by enzymatic ultrafiltration. J Pharm Sci. 103:1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda MN. 1995. Endo-β-galactosidases and keratanase. In: Current Protocols in Molecular Biology. A. Varki (ed.). John Wiley & Sons, Inc. 17.17.6–17.17.13. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL. 2000. Mini review keratan sulfate: Structure, biosynthesis, and function. Glycobiology. 10:951–958. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL. 2002. Keratan sulfate biosynthesis. IUBMB Life. 54:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiling H, Scott JE, editors. 1989. Keratan Sulfate: Chemistry, Biology, Chemical Biology. London: The Biochemical Society. [Google Scholar]
- Hayashi M, Kadomatsu K, Kojima T, Ishiguro N. 2011. Keratan sulfate and related murine glycosylation can suppress murine cartilage damage in vitro and in vivo. Biochem Biophys Res Commun. 409:732–737. [DOI] [PubMed] [Google Scholar]
- He W, Fu L, Li G, Jones JA, Linhardt RJ, Koffas M. 2015. Production of chondroitin in metabolically engineered E. coli. Metab Eng. 27:92–100. [DOI] [PubMed] [Google Scholar]
- Hirano K, Ohgomori T, Kobayashi K, Tanaka F, Matsumoto T, Natori T, Matsuyama Y, Uchimura K, Sakamoto K, Takeuchi H et al. 2013. Ablation of keratan sulfate accelerates early phase pathogenesis of ALS. PLoS ONE. 8:e66969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckerby TN, Lauder RM. 2000. Keratan sulfates from bovine tracheal cartilage Structural studies of intact polymer chains using 1H and 13C NMR spectroscopy. Eur J Biochem. 267:3360–3369. [DOI] [PubMed] [Google Scholar]
- Karlsson NG, McGuckin MA. 2012. O-Linked glycome and proteome of high-molecular-mass proteins in human ovarian cancer ascites: Identification of sulfation, disialic acid and O-linked fucose. Glycobiology. 22:918–929. [DOI] [PubMed] [Google Scholar]
- Li G, Cai C, Li L, Fu L, Chang Y, Zhang F, Toida T, Xue C, Linhardt RJ. 2014. Method to detect contaminants in heparin using radical depolymerization and liquid chromatography-mass spectrometry. Anal Chem. 86:326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhang F, Li L, Li G, He W, Linhardt RJ. 2014. Compositional analysis and structural elucidation of glycosaminoglycans in chicken eggs. Glycoconj J. 31:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Linker A, Davidson EA, Weissmann B. 1953. The mucopolysaccharides of bovine cornea. J Biol Chem. 205:611–616. [PubMed] [Google Scholar]
- Nakazawa K, Makoto I, Yamagata T, Suzuki S. 1989. In: Greiling H, Scott J, editors. Keratan Sulphate: Chemistry, Biology, Chemical Pathology. London: The Biochemical Society; p. 99–110. [Google Scholar]
- Pomin VH. 2015. Keratan sulfate: An up-to-date review. Int J Biol Macromol. 72:282–289. [DOI] [PubMed] [Google Scholar]
- Pomin VH, Piquet AA, Pereira MS, Mourão PA. 2012. Residual keratan sulfate in chondroitin sulfate formulations for oral administration. Carbohydr Polym. 90:839–846. [DOI] [PubMed] [Google Scholar]
- Quantock AJ, Young RD, Akama TO. 2010. Structural and biochemical aspects of keratan sulphate in the cornea. Cell Mol Life Sci. 67:891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Schaefer RM. 2010. Proteoglycans: From structural compounds to signaling molecules. Cell Tissue Res. 339:237–246. [DOI] [PubMed] [Google Scholar]
- Shirato K, Gao C, Ota F, Angata T, Shogomori H, Ohtsubo K, Yoshida K, Lepenies B, Taniguchi N. 2013. Flagellin/Toll-like receptor 5 response was specifically attenuated by keratan sulfate disaccharide via decreased EGFR phosphorylation in normal human bronchial epithelial cells. Biochem Biophys Res Commun. 435:460–465. [DOI] [PubMed] [Google Scholar]
- Sommarin Y, Wendel M, Shen Z, Hellman U, Heinegârd D. 1998. Osteoadherin, a cell-binding keratan sulfate proteoglycan in bone, belongs to the family of leucine-rich repeat proteins of the extracellular matrix. J Biol Chem. 273:16723–16729. [DOI] [PubMed] [Google Scholar]
- Tai GH, Nieduszynski IA, Fullwood NJ, Huckerby TN. 1997. Human corneal keratan sulfates. J Biol Chem. 272:28227–28231. [DOI] [PubMed] [Google Scholar]
- Ucakturk E, Cai C, Li L, Li G, Zhang F, Linhardt RJ. 2014. Capillary electrophoresis for total glycosaminoglycan analysis. Anal Bioanal Chem. 406:4617–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USP37 Official Monograph, Heparin Sodium, D. Molecular Weight Determinations. p. 3224 (United States Pharmacopeial Convention, Rockville, MD, 2014). [Google Scholar]
- Weyers A, Yang B, Solakyildirim K, Yee V, Li L, Zhang F, Linhardt RJ. 2013. Isolation of bovine corneal keratan sulfate and its growth factor and morphogen binding. FEBS J. 280:2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Bhaskar U, Li G, Fu L, Li L, Zhang F, Dordick JS, Linhardt RJ. 2013. Immobilized enzymes to convert N-sulfo, N-acetyl heparosan to a critical intermediate in the production of bioengineered heparin. J Biotechnol. 167:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.