Abstract
Background:
Tumor development is angiogenesis dependent. There is evidence that leptin contributes to tumor growth. However, all the mechanisms by which leptin does this has not been clearly established. The objective of the present study was to test the hypothesis that leptin enhances melanoma tumor growth through inducing angiogenesis and cell proliferation.
Materials and Methods:
We injected 2 × 106 B16F10 melanoma cells subcutaneously to 32 C57BL6 mice. The mice were randomly divided into four groups of eight animals, on day 8. Two groups received twice daily intraperitoneal (i.p.) injections of either phosphate buffered saline or recombinant murine leptin (1 μg/g initial body weight). Two groups received i.p. injections of either 9F8 an anti leptin receptor antibody or the control mouse IgG at 50 μg/injection every 3 consecutive days. By the end of the 2nd week, the animals were euthanized and blood samples and tumors were analyzed. Angiogenesis and proliferation were assessed by immunohistochemical staining for CD31 and Ki-67 respectively.
Results:
Tumors size, capillary density, plasma levels of vascular endothelial growth factor, and the number of Ki-67-positive stained cells were significantly more in the leptin than 9F8 and both control groups (P < 0.05).
Conclusion:
Taken together, our findings reinforce the idea that leptin acts as an angiogenic and mitogenic factor to promote melanoma growth.
Keywords: Angiogenesis, Ki-67, leptin, melanoma, vascular endothelial growth factor
INTRODUCTION
From an epidemiological viewpoint, obesity, a growing health problem worldwide, has been associated with increased risk of several types of cancers including colon, breast, gastric, prostate, and melanoma and so on.[1] However, exact mechanisms underlying the association between obesity and cancer are not well clear. Key candidate molecule that linking obesity and cancer is leptin. Leptin is a multifunctional adipokine which exhibits a wide range of physiological roles in energy balance, inflammation, and reproduction through its receptors.[2,3] Leptin has been also shown to have pro-angiogenic activity and endothelial cells express functionally active leptin receptors.[4,5,6] Recent studies indicated that leptin and its receptor, OB-R, are overexpressed in several cancer types, where they induce tumor growth by stimulating proliferation, inflammation, and angiogenesis.[7,8,9,10] Angiogenesis is critical to ensure the supply of oxygen and nutrient for tumor’s growth, invasion, and metastasis.[11] Tumor progression is associated with a switch in the balance between angiogenesis inducers and inhibitors.[12,13] Vascular endothelial growth factor (VEGF) as a major angiogenic inducer stimulates endothelial cell survival, proliferation, migration, and finally capillary-like tube formation.[14] The level of VEGF expression appears to have a strong correlation with the blood vessel density and grade of tumors.[15] Leptin has been found to exert angiogenic effects mediated by enhancement of VEGF and VEGF-receptor 2 (VEGFR2) and activation of endogenous fibroblastic growth factor-2.[7,16]
Data from one study suggested that melanoma cells express leptin and its receptor, creating a potential autocrine growth pathway which is absent in normal cells.[17] We have previously shown that leptin promotes tumor growth through mechanisms involving nitric oxide (NO) production, increasing of the endothelial progenitor cell numbers in peripheral blood and consequently vasculogenesis.[18]
In spite of the increasing data supporting leptin’s role in some plemalignancyies including breast and prostate cancer there is a lack of information on relationship between leptin and melanoma, as a most aggressive form of skin cancer.[7,10,19,20,21] To this purpose, we have investigated whether leptin may induce angiogenesis and proliferation of melanoma tumor in an animal model.
MATERIALS AND METHODS
Cell culture
B16-F10 melanoma cell line which is syngenic with the C57BL/6 strain mouse was purchased from the National Cell Bank of Iran (NCBI, Pasteur institute of Iran). Cells were cultured at 37°C in Dulbecco’s modified Eagle’s medium supplemented with 4 mM L-glutamine, 4.5 g/L glucose, 10% fetal bovine serum, and antibiotics (100 μg/ml streptomycin, 100 μg/ml penicillin) in 5% CO2 atmosphere until confluent. When the monolayer reached about 80% confluence, the cells were washed with phosphate buffered saline (PBS) and harvested using 0.25% trypsin and 0.03% ethylenediaminetetraacetic acid, thereafter, pelleted by brief centrifugation at 100 g. The supernatant was discarded, cell pellets were resuspended in PBS, and the cell number was counted.
Animal experiments
Six to eight-week-old male C57BL/6 mice (Pasteur Institute of Iran) served as recipient mice for tumor inoculation. The animals were housed in microisolator cages and maintained in a 12 h light/dark cycle at room temperature between 20°C and 25°C and given food and water ad libitum. All experimental procedures were performed in accordance with the guideline of Animal Care and Ethical Committee of XXXX.
C57BL/6 mice were injected with 2 × 106 B16-F10 melanoma cells subcutaneously in the right flank using a disposable tuberculin syringe. The day of inoculation was defined as day 0. Animals developed tumors (one tumor per mouse) within 6–7 days. On day 8, the tumor bearing mice were randomly divided into four groups of eight animals. Two groups received twice daily intraperitoneal (i.p.) injections of either PBS or recombinant murine leptin (R and D, 1 μg/g initial body weight). Two groups received i.p. injections of either 9F8 monoclonal antibody or the control mouse IgG at 50 μg/injection every 3 consecutive days on days 8, 11 after tumor induction. 9F8 is a monoclonal antibody to the human leptin receptor (ObR) which has been developed by Fazeli and Zarkesh-Esfahani and tested for antagonist activity using a leptin signaling bioassay.[19] The mouse IgG was generous gift of Dr. Ali Mostafaei (Medical Biology Research Center, Kermanshah University of Medical Sciences). Upon appearance, tumor size was measured daily with calipers.[18] Tumor volumes were calculated as prolate spheroid: V = (4/3 × π × [a] 2 × [b]), were “a” is half of the minor axis and “b” is half of the major axis of the prolate spheroid. On the day 14, mice were sacrificed by pentobarbital overdose. Tumors were then carefully removed and fixed in 10% buffered formalin.
Vascular endothelial growth factor measurement
Mice were fasted for 14 h prior to sacrifice in order to obtain fasted blood samples. Serum VEGF concentration was measured using sandwich enzyme immunoassay kits and reagents (R and D systems, USA) according to the manufacturer’s protocol.
Ki-67 evaluation
Paraffin-embedded samples were cut at 5-μm-thickness, dewaxed with xylene before rehydration through graded alcohol. For antigen retrieval, the samples were boiled for 10 min in a microwave oven in 10 mmol/L sodium citrate buffer (pH 6.0).
Mouse monoclonal antibody Ki-67 (RTU-MMI, NovoCastra-Germany) was diluted to a concentration of 1:60, applied to the sections, and incubated for 30 min at room temperature. This antibody was detected using a proprietary horseradish peroxidase enzyme labeled polymer (DAKO Envision_HRP) conjugated to mouse secondary antibody. Staining was developed with 3,3'-diaminobenzidine. Sections were counterstained with hematoxylin. In the negative controls, the primary antibody was omitted. The number of mitosis was counted in five high-power fields (×400) in the areas with highest mitotic activity. In order to control for inter-observer variation, randomly selected cases were evaluated by two observers.
Capillary density assessment
For endothelial cells immunostaining, tumor samples were fixed in 10% neutral buffered formalin overnight, embedded in paraffin, and cut in 5 μm. Then, the sections were deparaffinized in xylol and followed by rehydration in graded alcohol series. Endogenous peroxidase activity was blocked with 3% H2O2 in methanol. Antigen retrieval on these sections was performed by microwave irradiation for 15 min in citrate buffer 10 mM, pH 6.0. Sections were allowed to cool for 20 min and then washed in PBS. To avoid nonspecific staining, protein block (RE7120) was used. The slides were then incubated for 60 min with monoclonal antibodies (dilution 1/100) directed against mouse CD31 (NovoCastra). The bound antibody was detected by horseradish peroxidase enzyme labeled polymer conjugated to mouse secondary antibody and visualized by diaminobenzidine as chromogen. Counterstaining was performed with hematoxylin. Paraffin – embedded sections of normal samples were included as positive control. Negative control had the primary antibody replaced by PBS. Capillary density was measured at ×400 in three separate fields from each tissue preparation, final vessel count was expressed as the mean number of vessels in these three fields.
Statistical analysis
Data are presented as mean ± standard deviation and were tested for normal distribution with the Kolmogorov–Smirnov test. Comparisons between groups were analyzed by one-way ANOVA followed by the Bonferroni method as post-hoc test. P < 0.05 was considered significant. All statistical analysis was performed with SPSS 16 (SPSS Inc.).
RESULTS
Serum biomarker of angiogenesis
Plasma VEGF concentrations for each study group are shown in Figure 1. In plasma taken from mice at the time of sacrifice, VEGF level was significantly higher in mice receiving leptin compared to all other groups while no significant difference was found between other groups (P < 0.05).
Figure 1.
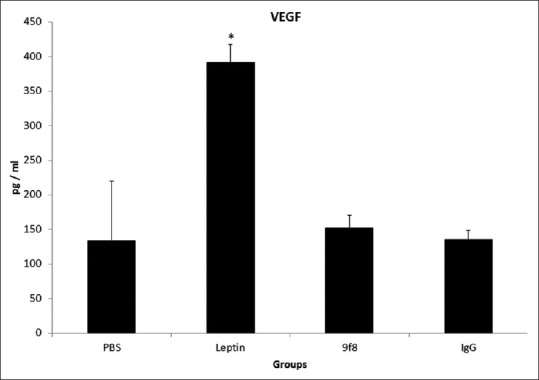
The plasma concentration of vascular endothelial growth factor. The plasma levels of vascular endothelial growth factor were significantly higher in leptin group compared to all other groups of mice while there was no significant difference between other groups (*P < 0.05)
Capillary density
Samples of immunohistochemical staining in all experimental groups are presented in Figure 2. Blood vessels formed in the tumors from all groups of mice, but leptin treated animals have significantly higher percentage of CD31 staining in their tumors (P < 0.05). Differences in vessel counts between other groups were not statistically significant [Figure 3].
Figure 2.
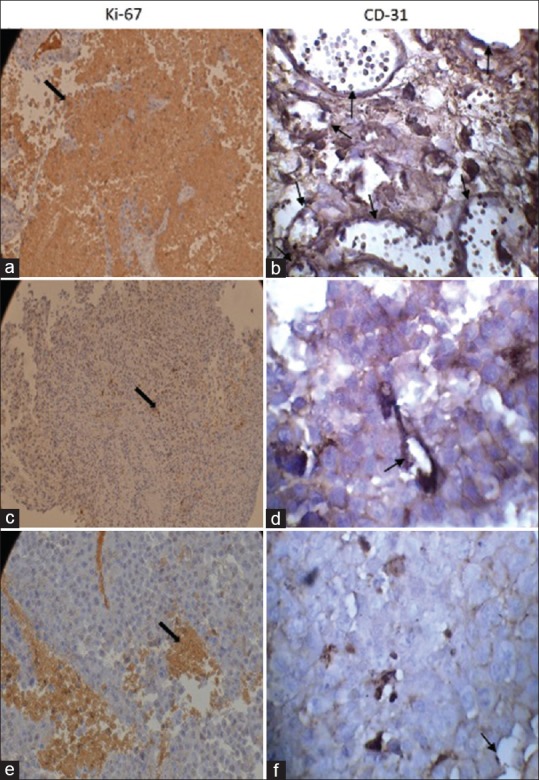
Immunostaining of CD31 and Ki-67 in melanoma tumor, (a and b) leptin, (c and d) 9F8, (e and f) control groups. Leptin treated animals appeared to have significantly higher percentage of CD31 and Ki-67 staining in their tumors while no significant difference was found between other groups (*P <0.05). Original magnification ×400. Note the strong brown staining in tumors excised from leptin treated mice. Arrows depict cells in tumor with intensive staining
Figure 3.
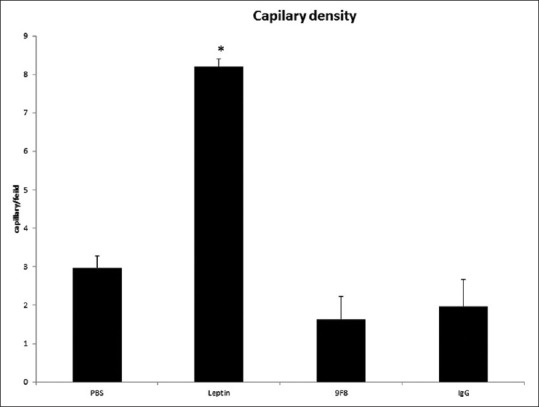
The vascular density was measured in CD31-labeled vessels. CD31 staining was significantly higher in leptin group compared to all other groups of mice. Results are presented as mean ± standard deviation
Ki-67 expression in melanoma tumor
Figure 2 shows representative photograph of Ki 67-stained tumor section. We examined proliferation in the mouse melanomas using immunohistochemistry for proliferative marker Ki-67. Leptin administration resulted in significant more Ki-67+ cells in tumor bearing mice whereas there was no significant difference between other groups [Figure 4] (P < 0.05).
Figure 4.
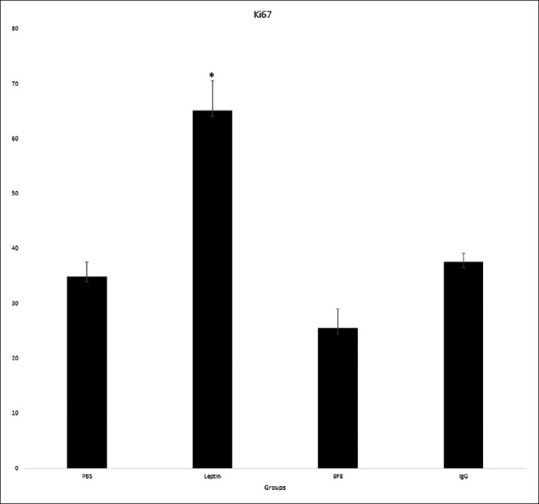
Tumor section stained for Ki-67. Leptin administration resulted in significant more Ki-67+ cells in tumor bearing mice whereas there was no significant difference between other groups (*P < 0.05)
Tumor size
The results of tumor size measurement are shown in Figure 5. The melanoma tumor size differs between leptin group and others (P < 0.05). It is significantly higher in leptin treated mice than those in other groups.
Figure 5.
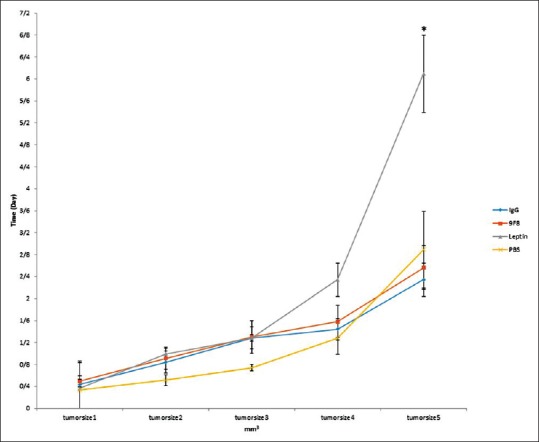
Tumor size: The volume of melanoma tumors excised from leptin treated mice was significantly larger than tumors from other groups of mice. There was no significant difference between three other study groups (*P < 0.05)
DISCUSSION
In the present study, we found that leptin promotes angiogenesis in melanoma tumor cells, which are mediated, at least in part, by angiogenic factor VEGF. In addition, we observed proliferative responses of melanoma cells to leptin.
Tumor angiogenesis is an essential process that provides blood supply for tumor growth and their subsequent metastasis. To date, several reports have suggested a positive effect of leptin on tumor growth via angiogenesis.[7,10] Although, direct cause and mechanism of leptin-mediated tumor angiogenesis has not been extensively investigated.
Leptin and its receptors are present in several types of tumor cells including breast, prostate, colon where they induce tumor growth.[22,23,24,25,26] Melanoma cells do express these receptors providing a hypothesis that leptin is a growth factor for these cells.[17] Gogas et al., in a case control study has indicated elevated leptin levels is a risk factor for melanoma cancer.[27]
In keeping with our previous investigation showing potential role of leptin in vasculogenesis, herein we demonstrate the possibility of leptin as an inducer of angiogenesis in melanoma accounting in part for tumor growth effect. Similar to our findings, an in vivo study revealed that leptin dose- and time-dependently stimulated angiogenesis which was equivalent to that obtained with angiogenic cytokine VEGF.[5] Furthermore, investigational study on tumor development have explored that leptin induced angiogenesis in human hepatocellular carcinoma.[28] Others reported that neutralizing of leptin activity can suppress leukemia through inhibiting angiogenesis.[29]
The mechanisms for leptin governing angiogenesis and supporting the growth of tumors are not well clarified and literature data are controversial. Result from our study suggests that a mechanism by which leptin promotes angiogenesis in melanoma is upregulation of VEGF. In agree to our results, Gonzalez et al. showed that leptin signaling enhanced the development of mammary tumors and upregulated the VEGF and VEGFR2.[7] However, another study in the chick embryo chorioallantoic membrane (CAM) illustrated that leptin-mediated angiogenesis is in part via fibroblast growth factor-2.[16] Previous study from our laboratory have reported that leptin treatment increases NO, a potent angiogenic mediator.[18] In a clinical study of gastric cancer, leptin expression was positively correlated with VEGF expression.[30] In contrast, vona-davis et al., showed that leptin decreased VEGF production in prostate cancer cells in vitro.[31] This little discrepancy may possibly relate to different model of angiogenesis and leptin doses used in this work.
Beyond the angiogenic effect of leptin in development of tumors, there are numerous in vivo and in vitro studies that have implicated this adipokine promotes the proliferation of different types of tumor cells.[7,19,21,32,33,34,35,36] Here, proliferation was evidenced by Ki-67 immunostaining, a nuclear protein present in all proliferating cells.[37] We found a significant correlation between leptin and the Ki-67 mitogenic labeling index in melanoma suggesting another important role for leptin in tumor growth. Proliferative activity of leptin in prostate cancer cells are related to Akt and STAT3 activation.[38] In addition, leptin promotes endometrial cancer growth and invasiveness by activation of extracellular signal-regulated kinase 2 signaling pathway.[39] In investigation of the effect of leptin on melanoma promotion, Ellerhorst et al. showed that melanoma cells themselves also secret leptin that may be responsible for uncontrolled proliferation of tumor cells.[17] Brandon et al. have illustrated that leptin deficiency diminishes but does not abolish melanoma tumor growth.[1] Surprisingly, antagonism of leptin by 9F8 antibody have nonsignificant effect on plasma VEGF concentration, angiogenesis and proliferation of melanoma. We conclude pharmacological concentration of leptin may be related to these effects. This finding is consisted with a previous report where high concentrations of leptin have been shown to effectively stimulate angiogenesis in the chick CAM.[16]
CONCLUSION
In summary, leptin enhanced melanoma tumor growth in vivo. Promoting angiogenesis as well as proliferation may confer a benefit to growth of melanoma. However, further research should be made to clarify the exact role of leptin and its signaling pathway responsible for these leptin induced actions.
Financial support and sponsorship
This study was supported by Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Zandifar for skillful technical assistance.
REFERENCES
- 1.Brandon EL, Gu JW, Cantwell L, He Z, Wallace G, Hall JE. Obesity promotes melanoma tumor growth: Role of leptin. Cancer Biol Ther. 2009;8:1871–9. doi: 10.4161/cbt.8.19.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carino C, Olawaiye AB, Cherfils S, Serikawa T, Lynch MP, Rueda BR, et al. Leptin regulation of proangiogenic molecules in benign and cancerous endometrial cells. Int J Cancer. 2008;123:2782–90. doi: 10.1002/ijc.23887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Münzberg H, Björnholm M, Bates SH, Myers MG., Jr Leptin receptor action and mechanisms of leptin resistance. Cell Mol Life Sci. 2005;62:642–52. doi: 10.1007/s00018-004-4432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin X, Fukuda N, Su J, Takagi H, Lai Y, Lin Z, et al. Effects of leptin on endothelial function with OB-Rb gene transfer in Zucker fatty rats. Atherosclerosis. 2003;169:225–33. doi: 10.1016/s0021-9150(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 5.Anagnostoulis S, Karayiannakis AJ, Lambropoulou M, Efthimiadou A, Polychronidis A, Simopoulos C. Human leptin induces angiogenesis in vivo. Cytokine. 2008;42:353–7. doi: 10.1016/j.cyto.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A. 2001;98:6390–5. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, et al. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J Biol Chem. 2006;281:26320–8. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Perez RR, Xu Y, Guo S, Watters A, Zhou W, Leibovich SJ. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFkappaB/HIF-1alpha activation. Cell Signal. 2010;22:1350–62. doi: 10.1016/j.cellsig.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rene Gonzalez R, Watters A, Xu Y, Singh UP, Mann DR, Rueda BR, et al. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36. doi: 10.1186/bcr2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W, Guo S, Gonzalez-Perez RR. Leptin pro-angiogenic signature in breast cancer is linked to IL-1 signalling. Br J Cancer. 2011;104:128–37. doi: 10.1038/sj.bjc.6606013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun B, Zhang S, Ni C, Zhang D, Liu Y, Zhang W, et al. Correlation between melanoma angiogenesis and the mesenchymal stem cells and endothelial progenitor cells derived from bone marrow. Stem Cells Dev. 2005;14:292–8. doi: 10.1089/scd.2005.14.292. [DOI] [PubMed] [Google Scholar]
- 12.Ribatti D, Nico B, Crivellato E, Roccaro AM, Vacca A. The history of the angiogenic switch concept. Leukemia. 2007;21:44–52. doi: 10.1038/sj.leu.2404402. [DOI] [PubMed] [Google Scholar]
- 13.Dudek AZ, Gupta K, Ramakrishnan S, Mukhopadhyay D. Tumor angiogenesis 2012. J Oncol 2012. 2012:857383. doi: 10.1155/2012/857383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vona-Davis L, Rose DP. Angiogenesis, adipokines and breast cancer. Cytokine Growth Factor Rev. 2009;20:193–201. doi: 10.1016/j.cytogfr.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Patel D, Bassi R, Hooper AT, Sun H, Huber J, Hicklin DJ, et al. Enhanced suppression of melanoma tumor growth and metastasis by combined therapy with anti-VEGF receptor and anti-TYRP-1/gp75 monoclonal antibodies. Anticancer Res. 2008;28:2679–86. [PubMed] [Google Scholar]
- 16.Ribatti D, Nico B, Belloni AS, Vacca A, Roncali L, Nussdorfer GG. Angiogenic activity of leptin in the chick embryo chorioallantoic membrane is in part mediated by endogenous fibroblast growth factor-2. Int J Mol Med. 2001;8:265–8. doi: 10.3892/ijmm.8.3.265. [DOI] [PubMed] [Google Scholar]
- 17.Ellerhorst JA, Diwan AH, Dang SM, Uffort DG, Johnson MK, Cooke CP, et al. Promotion of melanoma growth by the metabolic hormone leptin. Oncol Rep. 2010;23:901–7. doi: 10.3892/or_00000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amjadi F, Javanmard SH, Zarkesh-Esfahani H, Khazaei M, Narimani M. Leptin promotes melanoma tumor growth in mice related to increasing circulating endothelial progenitor cells numbers and plasma NO production. J Exp Clin Cancer Res. 2011;30:21. doi: 10.1186/1756-9966-30-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Somasundar P, McFadden DW, Hileman SM, Vona-Davis L. Leptin is a growth factor in cancer. J Surg Res. 2004;116:337–49. doi: 10.1016/j.jss.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Mistry T, Digby JE, Desai KM, Randeva HS. Obesity and prostate cancer: A role for adipokines. Eur Urol. 2007;52:46–53. doi: 10.1016/j.eururo.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 21.Kim HS. Leptin and leptin receptor expression in breast cancer. Cancer Res Treat. 2009;41:155–63. doi: 10.4143/crt.2009.41.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao J, Tian J, Lv Y, Shi F, Kong F, Shi H, et al. Leptin induces functional activation of cyclooxygenase-2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Sci. 2009;100:389–95. doi: 10.1111/j.1349-7006.2008.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cirillo D, Rachiglio AM, La Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: An overview. J Cell Biochem. 2008;105:956–64. doi: 10.1002/jcb.21911. [DOI] [PubMed] [Google Scholar]
- 24.Hoon Kim J, Lee SY, Myung SC, Kim YS, Kim TH, Kim MK. Clinical significance of the leptin and leptin receptor expressions in prostate tissues. Asian J Androl. 2008;10:923–8. doi: 10.1111/j.1745-7262.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- 25.Dieudonne MN, Machinal-Quelin F, Serazin-Leroy V, Leneveu MC, Pecquery R, Giudicelli Y. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2002;293:622–8. doi: 10.1016/S0006-291X(02)00205-X. [DOI] [PubMed] [Google Scholar]
- 26.Hardwick JC, Van Den Brink GR, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001;121:79–90. doi: 10.1053/gast.2001.25490. [DOI] [PubMed] [Google Scholar]
- 27.Gogas H, Trakatelli M, Dessypris N, Terzidis A, Katsambas A, Chrousos GP, et al. Melanoma risk in association with serum leptin levels and lifestyle parameters: A case-control study. Ann Oncol. 2008;19:384–9. doi: 10.1093/annonc/mdm464. [DOI] [PubMed] [Google Scholar]
- 28.Ribatti D, Belloni AS, Nico B, Di Comite M, Crivellato E, Vacca A. Leptin-leptin receptor are involved in angiogenesis in human hepatocellular carcinoma. Peptides. 2008;29:1596–602. doi: 10.1016/j.peptides.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Iversen PO, Drevon CA, Reseland JE. Prevention of leptin binding to its receptor suppresses rat leukemic cell growth by inhibiting angiogenesis. Blood. 2002;100:4123–8. doi: 10.1182/blood-2001-11-0134. [DOI] [PubMed] [Google Scholar]
- 30.Zhao X, Huang K, Zhu Z, Chen S, Hu R. Correlation between expression of leptin and clinicopathological features and prognosis in patients with gastric cancer. J Gastroenterol Hepatol. 2007;22:1317–21. doi: 10.1111/j.1440-1746.2007.04941.x. [DOI] [PubMed] [Google Scholar]
- 31.Vona-Davis L, Tighe G, Vedula G, Somasundar P, Mcfadden W. Leptin decreases VEGF production in vitro. Gastroenterology. 2003;124:A464. [Google Scholar]
- 32.Tessitore L, Vizio B, Jenkins O, De Stefano I, Ritossa C, Argiles JM, et al. Leptin expression in colorectal and breast cancer patients. Int J Mol Med. 2000;5:421–6. doi: 10.3892/ijmm.5.4.421. [DOI] [PubMed] [Google Scholar]
- 33.Somasundar P, Frankenberry KA, Skinner H, Vedula G, McFadden DW, Riggs D, et al. Prostate cancer cell proliferation is influenced by leptin. J Surg Res. 2004;118:71–82. doi: 10.1016/j.jss.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 34.Okumura M, Yamamoto M, Sakuma H, Kojima T, Maruyama T, Jamali M, et al. Leptin and high glucose stimulate cell proliferation in MCF-7 human breast cancer cells: Reciprocal involvement of PKC-alpha and PPAR expression. Biochim Biophys Acta. 2002;1592:107–16. doi: 10.1016/s0167-4889(02)00276-8. [DOI] [PubMed] [Google Scholar]
- 35.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin – A growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94:1704–11. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 36.Sierra-Honigmann MR, Nath AK, Murakami C, García-Cardeña G, Papapetropoulos A, Sessa WC, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–6. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 37.Romero Q, Bendahl PO, Klintman M, Loman N, Ingvar C, Rydén L, et al. Ki67 proliferation in core biopsies versus surgical samples – A model for neo-adjuvant breast cancer studies. BMC Cancer. 2011;11:341. doi: 10.1186/1471-2407-11-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyazaki T, Bub JD, Iwamoto Y. c-Jun NH(2)-terminal kinase mediates leptin-stimulated androgen-independent prostate cancer cell proliferation via signal transducer and activator of transcription 3 and Akt. Biochim Biophys Acta. 2008;1782:593–604. doi: 10.1016/j.bbadis.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer. 2006;13:629–40. doi: 10.1677/erc.1.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]


