Abstract
The performance of a commercially available, rapid membrane enzyme immunoassay for influenza A and B virus detection was compared to that of viral culture in 4,092 respiratory specimens collected from patients presenting with respiratory symptoms during the 2002-2003 influenza season. The test's overall sensitivity was 43.83%, lower than previously reported but similar for detection of both influenza A and B viruses (42.98 versus 44.76%). However, specificity, 99.74%, was excellent for both influenza A and B viruses (99.82 versus 99.92%). These values make this test a very good confirmatory test when clinical suspicion is high, but a less accurate screening test for large populations.
Influenza viruses are important causes of morbidity and mortality in individuals of all age groups, especially the elderly and patients with chronic disabilities (5, 15). Influenza virus testing is often a part of the evaluation of febrile respiratory illness in hospital settings, as well as in the practitioner's office and emergency and urgent care centers (1, 5, 8, 10, 16). The reference method is viral culture. However, since the results of viral culture may be delayed for days to weeks, most centers perform rapid testing for influenza virus by immunofluorescence assay or enzyme immunoassay (2-6). A rapid and correct diagnosis is essential to help clinicians with decisions regarding early antiviral treatment, need for additional testing, and cohorting of the patients. Rapid response teams investigating outbreaks of severe respiratory disease also may use rapid tests to help differentiate between infection with influenza virus, biological warfare agents, such as those that cause anthrax and smallpox (2, 8), and other causes of epidemic respiratory illnesses, such as coronaviruses associated with severe acute respiratory syndrome (5, 12, 13). Currently, there are several commercially available rapid influenza virus test kits that can easily be performed in less than 30 min (2, 4, 6, 9). However, these tests have different methodologies and performance characteristics and may be expensive to perform in large numbers (Table 1). Furthermore, the performance of rapid influenza virus tests and their impact on patient care should be evaluated periodically not only by manufacturers, but also by physicians and laboratories who perform the tests in large numbers of patients, in real-world clinical settings (2, 3, 10, 11, 16).
TABLE 1.
Commercially available rapid test kits for detection of influenza virus
| Test name (manufacturer) | Test method | Influenza virus type detected | Distinguishes A from B | Time (min) | CLIA waiveda | Cost per test ($)b |
|---|---|---|---|---|---|---|
| Directigen Flu A (Becton Dickinson) | Membrane immunoassay | A only | 15 | No | 20.50 | |
| Directigen Flu A + B (Becton Dickinson) | Membrane immunoassay | A, B | Yes | 15 | No | 20.50 |
| Flu OIA (Thermo BioStar) | Optical immunoassay | A, B | No | 15 | No | 16.50 |
| QuickVue (Quidel) | Lateral-flow immunoassay | A, B | No | 10 | Yes | 13.80 |
| NOW FluA and NOW FluB (Binax) | Lateral-flow immunoassay | A, B | Yes | 15 | No | 18.00 |
| Xpect FluA&B | Lateral-flow immunoassay | A, B | Yes | 15 | No | 24.75 |
| ZstatFlu (Zyme Tx) | Enzyme-based color-metric assay | A, B | No | 30 | Yes | 14.50 |
CLIA waived: diagnostic tests may be granted a Clinical Laboratory Improvement Amendments (CLIA) waiver from regulatory oversight if they meet certain requirements established by the statute. The section of the statute specifying the criteria for categorizing a test as waived was excerpted without elaboration in the regulations at 42 CFR 493.15(b) and 493.15(c).
Cost is the estimated cost of materials only, to perform testing on one sample, and was obtained by calling manufacturers. Material costs may vary with region. Costs for quality control assurance and improvement, and also cost for technical time to perform testing, were not included.
MATERIALS AND METHODS
This study evaluated the performance of a relatively new rapid test for detection of influenza virus (Directigen Flu A+B; Becton Dickinson Diagnostic Systems, Sparks, Md.) in a real clinical setting, in a virology laboratory that serves a large children's hospital (Diagnostic Virology Laboratory of Texas Children's Hospital, Houston, Tex.). This test kit was used routinely during the 2002-2003 influenza season to detect influenza virus infection. The rapid test results were compared to the reference standard of viral culture in all fresh respiratory specimens collected from patients with respiratory viral symptoms who presented to Texas Children's Hospital for admission or evaluation in the Emergency Department between 1 October 2002 and 30 May 2003. Rapid tests were routinely performed by virology laboratory technicians according to the manufacturer's instructions, 24 h daily, 7 days a week, and results were reported within 2 h of specimen receipt. Briefly, the BD Directigen Flu A+B test is a rapid membrane enzyme immunoassay test that involves extraction of influenza A or B viral antigens from the patient specimens. The extracted specimen is expelled through a filter assembly into each of two wells of a triangular plastic test device containing a membrane surface. Viral antigens, if present in the extracted specimens, are bound to the membrane surface and detected by enzyme-conjugated monoclonal antibodies specific for influenza A or B virus nucleoprotein, followed by a stop reagent. A positive test is indicated by the presence of a purple triangle in the A or B well in the plastic device. Absence of a purple triangle in the presence of a positive procedure control dot indicates a negative test. The test was performed using internal kit positive, negative, and procedural controls as well as external laboratory controls for each test kit run.
It was also the laboratory routine to inoculate all specimens within 1 h of receipt, 24 h daily, 7 days a week, into human foreskin fibroblast (HFF), rhesus monkey kidney (RhMK), and human lung carcinoma (A549) cell culture monolayers. One tube each of inoculated HFF, RhMK, and A549 cell cultures was placed on a rotator drum and incubated at 37°C. In addition, one tube of inoculated HFF cell culture was placed on a rotator drum and incubated at 30°C to enhance rhinovirus isolation. Viral cultures were inspected daily for cytopathic effect using light microscopy, and preliminary identification of viruses was made by cytopathic effect. Hemadsorption with a 0.4% suspension of guinea pig red blood cells was performed on days 2, 5, and 14 of incubation of RhMK cell cultures. Virus identification was confirmed by immunofluorescence assays or, in the case of cell cultures exhibiting cytopathic effect characteristic for picornaviruses, acid lability testing was used to differentiate rhinoviruses from enteroviruses (2, 4). Viral cultures positive for influenza virus, type A or B, were considered true positives. Sensitivity, specificity, and positive and negative predictive values were calculated using two-by-two contingency tables. Differences between tests were analyzed using chi-square tests. Differences between ages of multiple groups were analyzed with analysis of variance, assuming equal variances, and Student's t test was used to compare differences between only two groups.
RESULTS AND DISCUSSION
There were 4,092 respiratory specimens processed during the period of investigation. The majority, 4,001 (97.77%), were nasal washes, 9 (0.22%) were nasal-pharyngeal swabs, 69 (1.7%) were tracheal aspirates, 7 (0.17%) were broncho-alveolar lavage specimens, 4 (0.1%) were sinus washes, and 2 (0.06%) were sputum samples.
The mean age of the patients tested was 3.2 years (median, 0.9 years; range, 3 days to 55.8 years). There were 83 (2.02%) patients older than 18 years of age, who were followed by our services for chronic illnesses. The mean distribution of the age of the patients with a negative viral culture for influenza virus was 5.1 years (median, 0.82 years), compared to 4.97 years (median, 1.8 years) for patients with a viral culture positive for influenza A virus and 7.01 years (median, 6.7 years) for patients with a viral culture positive for influenza B virus (P < 0.0045).
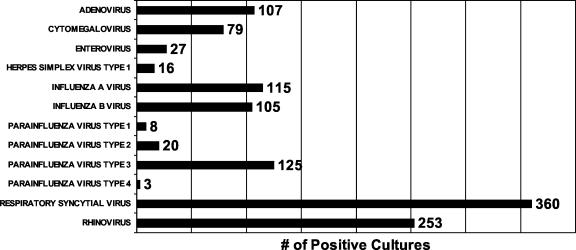
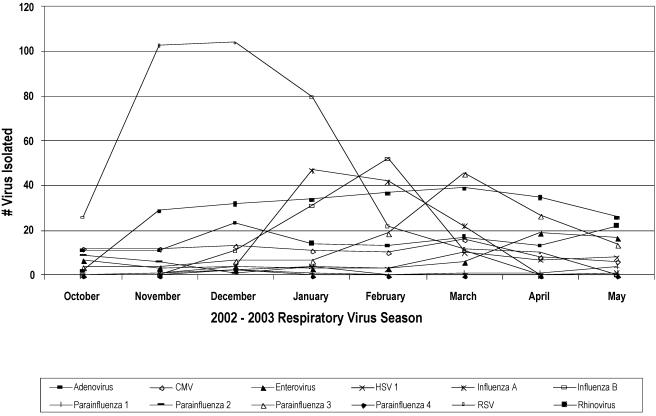
Of the 4,092 specimens submitted for this study, 2,926 (70.6%) had a negative viral culture and 1,166 (29.4%) specimens grew at least one virus. Of 1,218 viruses isolated, 115 (9.44%) were influenza A virus, 105 (8.62%) were influenza B virus, 360 (29.55%) were respiratory syncytial virus (RSV), 253 (20.77%) were rhinovirus, 107 (8.78%) were adenovirus, 79 (6.48%) were cytomegalovirus (CMV), 156 (12.8%) were parainfluenza virus, 27 (2.21%) were enterovirus, and 16 (1.3%) were herpesvirus (Fig. 1). Dual viral infection was present in 50 patients. Five patients had influenza virus in combination with another virus. Influenza A virus was seen in combination with adenovirus once and with CMV once. Influenza B virus was seen in a patient with adenovirus and also in two patients whose viral cultures grew CMV. Other dual infections were adenovirus and rhinovirus (10), RSV and rhinovirus (8), parainfluenza virus type 3 and rhinovirus (8), CMV and RSV (6), CMV and rhinovirus (4), adenovirus and CMV (3), CMV and parainfluenza virus type 3 (2), adenovirus and RSV (1), herpes simplex virus type 1 and parainfluenza virus type 3 (1), and herpes simplex virus type 1 and rhinovirus (1). One patient had three viruses isolated from the respiratory specimen: adenovirus, RSV, and rhinovirus. Influenza season in 2002 in Houston started during the month of December and lasted until March 2003 (Fig. 2).
FIG. 1.
Number and type of viruses isolated from respiratory samples submitted to the Diagnostic Virology Laboratory, Texas Children's Hospital, Houston, Tex., during the period October 2002 to May 2003.
FIG. 2.
Monthly distribution of viruses isolated from respiratory samples submitted to the Diagnostic Virology Laboratory, Texas Children's Hospital, Houston, Tex., during the period October 2002 to May 2003.
The rapid test showed excellent specificity, but only fair sensitivity, when compared to viral culture. Overall, results for both influenza A and B viruses showed similar performances, with no significant difference in the ability of the rapid test to distinguish between the two viruses (Table 2). False-positive results were rare (n = 10; 7 influenza A virus and 3 influenza B virus). False-negative results were more common (n = 123; 58 influenza B virus and 65 influenza A virus).
TABLE 2.
Performance of Directigen Flu A + B membrane immunoassay rapid test compared to that of viral culture for detection of influenza A and B viruses
| Influenza virus type detected | No. of specimens
|
Sensitivity (%) | Specificity (%) | % of specimens with indicated PVa
|
||||
|---|---|---|---|---|---|---|---|---|
| Rapid test +
|
Rapid test −
|
Pos | Neg | |||||
| Culture + | Culture − | Culture + | Culture − | |||||
| A and B | 96 | 10 | 123 | 3,863 | 43.8 | 99.7 | 90.5 | 96.9 |
| A only | 49 | 7 | 65 | 3,971 | 42.9 | 99.8 | 87.5 | 98.4 |
| B only | 47 | 3 | 58 | 3,984 | 44.8 | 99.9 | 94.0 | 98.6 |
PV, predicted value; Pos, positive; Neg, negative.
The performance of the rapid test was influenced by the age of the patient. The test was more sensitive in patients that were <2 years old, both overall (50 versus 35.3%) and in the ability of the test to distinguish the two influenza virus types (45.7 versus 34.4% for influenza A virus; 57.9 versus 35.8% for influenza B virus) (Table 3).
TABLE 3.
Performance of Directigen Flu A + B membrane immunoassay rapid test compared to that of viral culture when analyzed by age group (younger than 2 years old and older or equal to 2 years) for detection of influenza A and B viruses
| Age and virus type detected by rapid test | Sensitivity (%) | Specificity (%) | % of specimens with indicated PVa
|
|
|---|---|---|---|---|
| Pos | Neg | |||
| A and B | ||||
| <2 yrs | 50.0 | 99.9 | 93.1 | 98.7 |
| ≥2 yrs | 35.3*b | 99.4 | 83.3* | 94.8* |
| A only | ||||
| <2 yrs | 45.7 | 99.9 | 88.9 | 99.1 |
| ≥2 yrs | 34.4* | 99.7 | 78.6* | 98.1 |
| B only | ||||
| <2 yrs | 57.9 | 100.0 | 100.0 | 99.6 |
| ≥2 yrs | 35.8* | 99.7 | 86.4* | 96.8* |
PV, predictive value; Pos, positive; Neg, negative.
*, P value of <0.001.
The performance of the rapid test did not differ significantly during months when influenza virus was more prevalent (8.4% positive cultures for influenza virus in December, January, and February) than during months when influenza virus was less prevalent (1.7% positive cultures for influenza virus in October, November, March, April, and May) (Table 4).
TABLE 4.
Performance of Directigen Flu A + B membrane immunoassay rapid test during high-prevalence months (December, January, and February) compared to low-prevalence months (October, November, March, April, and May) for detection of influenza A and B viruses
| Prevalence period and test | Culture % positivea | Sensitivity (%) | Specificity (%) | % of specimens with indicated PVb
|
|
|---|---|---|---|---|---|
| Pos | Neg | ||||
| High-prevalence months | |||||
| Both A and B | 8.4 | 40.6 | 99.9 | 86.7 | 99.0 |
| A only | 4.1 | 40.9 | 99.9 | 81.8 | 99.3 |
| B only | 4.3 | 40.0 | 100.0 | 100.0 | 99.7 |
| Low-prevalence months | |||||
| Both A and B | 1.7 | 44.4 | 99.6 | 91.2 | 95.1 |
| A only | 1.2 | 43.5 | 99.8 | 88.9 | 97.6 |
| B only | 0.5 | 45.3 | 99.8 | 93.5 | 97.6 |
For influenza virus, A or B type.
PV, predictive value; Pos, positive; Neg, negative.
Conclusions.
The enzyme immunoassay rapid influenza virus test was highly specific but less sensitive than expected in detecting both influenza A and B virus in respiratory specimens from children. Earlier studies showed this same assay had a higher overall sensitivity (60 to 85%) (2, 3). The reason why the assay performed so differently during the study period is unclear. A difference in specimen collection is not a likely explanation for the differences observed in test performance, because at Texas Children's Hospital nasal washes processed for viral culture and rapid tests are routinely collected by respiratory therapists, who follow a standard protocol that is used for all ages and that has not changed throughout the years (2). Furthermore, recently another study, with a much smaller sample size than our study, also showed that the Directigen A+B rapid influenza virus test performed with lower sensitivity (6).
Most previously reported studies on the performance of rapid assays for detection of influenza virus have been conducted during influenza seasons where influenza A virus predominated (3, 4, 7, 8, 10, 11, 16). The season evaluated in this study had almost an equal number of influenza A and B virus types isolated, providing the opportunity to evaluate the performance of this rapid test for detection of both influenza virus types. Furthermore, the volume of tests performed in The Diagnostic Virology Laboratory at Texas Children's Hospital as well as the routine use of viral culture as a confirmatory test confer strengths to this study. To our knowledge, this is the largest study (n = 4,092) that has evaluated a rapid influenza virus diagnostic method and used viral culture as a confirmatory test. Previously published studies reported that the membrane immunoassay appeared less reliable for detection of influenza type B virus than for influenza type A virus (2, 3). In this study, detection of influenza A and B viruses was similar, but perhaps at the expense of overall sensitivity. However, the membrane immunoassay did perform better in respiratory samples collected from children younger than 2 years of age, most likely because the viral load in this patient population is usually higher (14).
Influenza B virus was more likely to be isolated and detected in older children, as previous studies have shown (2). The 2002-2003 influenza season in Houston started in December and ended in March. The RSV season was seen earlier than influenza, since it peaked in November to December 2002 and ended in March 2003. Other respiratory viruses, such as rhinoviruses and adenoviruses, were seen all through the year, with less seasonal variations. The presence of these respiratory viruses did not appear to influence performance of this rapid test for influenza virus.
Clinicians, laboratory personnel, and epidemiologists, using rapid tests for influenza virus detection, should be aware of the performance characteristics of each rapid test, so that they may choose the best tool for their specific needs. This test, in the setting of high clinical suspicion, would be best used as a confirmatory test. Our study demonstrated that for screening purposes in large populations this membrane enzyme immunoassay rapid influenza virus test may miss many infected patients and, therefore, may not be the most reliable laboratory test for this indication.
REFERENCES
- 1.Bonner, A. B., K. W. Monroe, L. I. Talley, A. E. Klasner, and D. W. Kimberlin. 2003. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics 112:363-367. [DOI] [PubMed] [Google Scholar]
- 2.Cazacu, A. C., J. Greer, M. Taherivand, and G. J. Demmler. 2003. Comparison of lateral-flow immunoassay with viral culture for rapid detection of influenza virus in nasal wash specimens from children. J. Clin. Microbiol. 41:2132-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan, K. H., N. Maldeis, W. Pope, A. Yup, A. Ozinskas, J. Gill, W. H. Seto, K. F. Shortridge, and J. S. Peris. 2002. Evaluation of the Directigen Flu A+B test for rapid diagnosis of influenza virus type A and B infections. J. Clin. Microbiol. 40:1675-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demmler, G. J.2002. Laboratory diagnosis of influenza: recent advances. Semin. Pediatr. Infect. Dis. 13:85-90. [DOI] [PubMed] [Google Scholar]
- 5.Effler, P., M.-C. Ieong, T. Tom, and M. Nakata. 2002. Enhancing public health surveillance for influenza virus by incorporating newly available rapid diagnostic tests. Emerg. Infect. Dis. 8:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landry, M. L., and D. Ferguson. 2003. Suboptimal detection of influenza virus in adults by the Directigen Flu A+B enzyme immunoassay and correlation of results with the number of antigen-positive cells detected by cytospin immunofluorescence. J. Clin. Microbiol. 41:3407-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noyola, D. E., B. Clark, F. T. O'Donnell, R. L. Atmar, J. Greer, and G. J. Demmler. 2000. Comparison of a new neuraminidase detection assay with an enzyme immunoassay, immunofluorescence, and culture for rapid detection of influenza A and B viruses in nasal wash specimens. J. Clin. Microbiol. 38:1161-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noyola, D. E., and G. J. Demmler. 2000. Effect of rapid diagnosis on management of influenza A infection. Pediatr. Infect. Dis. J. 19:303-307. [DOI] [PubMed] [Google Scholar]
- 9.Noyola, D. E., A. J. Paredes, B. Clark, and G. J. Demmler. 2000. Evaluation of a neuraminidase detection assay for the rapid detection of influenza A and B virus in children. Pediatr. Dev. Pathol. 3:162-167. [DOI] [PubMed] [Google Scholar]
- 10.Poehling, K. A., M. R. Griffin, R. S. Dittus, et al. 2002. Bedside diagnosis of influenza virus infections in hospitalized children. Pediatrics 110:83-88. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez, W. J., R. H. Schwartz, and M. M. Thorne. 2002. Evaluation of diagnostic tests for influenza in a pediatric practice. Pediatr. Infect. Dis. J. 21:193-196. [DOI] [PubMed] [Google Scholar]
- 12.Schlagenhauf, P. 2003. Influenza vaccine enlisted to prevent SARS confusion. Lancet 362:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shortridge, K. F. 2003. SARS exposed, pandemic influenza lurks. Lancet 361:1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steininger, C., M. Kundi, S. W. Aberle, J. H. Aberle, and T. Popow-Kraupp. 2002. Effectiveness of reverse-transcription PCR, virus isolation, and enzyme-linked immunosorbent assay for diagnosis of influenza A virus infection in different age groups. J. Clin. Microbiol. 40:2051-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troendle, J. F., G. J. Demmler, W. P. Glezen, et. al. 1992. Fatal influenza B virus pneumonia in pediatric patients. Pediatr. Infect. Dis. J. 11:117-121. [DOI] [PubMed] [Google Scholar]
- 16.Wilde, J. A. 2002. Rapid diagnostic testing for the identification of respiratory agents in the emergency department. Clin. Pediatr. Emerg. Med. 3:181-190. [Google Scholar]