Abstract
Aims:
This study was carried out to compare serum erythropoietin (Epo) levels in smokers and nonsmokers with periodontitis.
Materials and Methods:
Fifty-one subjects of both sexes (age range: 30–65 years) with chronic periodontitis (CP) participated in this study. Seventeen patients with generalized CP, nonsmokers without anemia were included in Group I (control group), 17 patients with generalized CP, nonsmokers with anemia were included in Group II, and 17 patients who were smokers, having generalized CP were included in Group III. Peripheral blood samples were obtained and assessed for the number of erythrocytes (total red blood cell [TRBC]), hemoglobin (Hb), and Epo levels.
Statistical Analysis Used:
One-way analysis of variance and Tukey–Kramer multiple comparisons test to assess the statistical difference between groups.
Results:
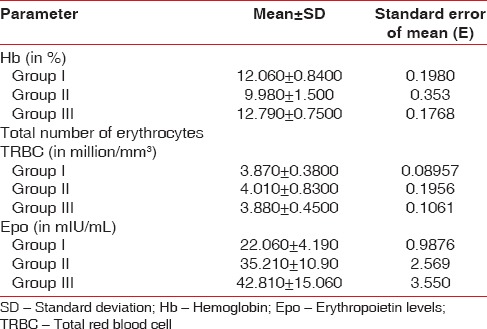
Epo levels varied considerably between the 3 groups. Highest values of Epo were seen in Group III with mean Epo value = 42.81 ± 15, followed by Group II Epo value = 35.21 ± 10.9, then Group I Epo value = 22.06 ± 4.19. Smokers in Group III with CP showed more prevalence toward higher values of Hb% (mean Hb = 12.06 ± 0.84) while there was no statistical difference in the values of TRBC values among the 3 groups (Group I TRBC value = 3.87 ± 0.38, Group II TRBC value = 4.01 ± 0.83, and Group III TRBC value = 3.88 ± 0.45).
Conclusion:
Periodontitis patients were seen to have lower Epo values further strengthening the hypothesis that CP may lead to anemia of chronic disease. In smokers, higher Hb values were seen with higher Epo levels. It indicates that periodontitis individually and along with smoking may affect anemic status of smokers. Thus, Epo levels may be better means to assess anemic status of smokers than relying only on Hb values.
Key words: Anemia, erythropoietin, periodontitis, smoking
INTRODUCTION
For thousands of years, blood has been regarded as the ultimate body fluid that could indicate a disease process. In the past decade, there has been a renewed interest to study the association of periodontitis and changes in the cellular and molecular components of peripheral blood. For example, the relationship of periodontitis with leukocytes,[1,2] thrombocytes,[3] C-reactive protein (CRP),[4] interleukin (IL-6),[5] fibrinogen;[6] erythrocyte sedimentation rate,[7] von Willebrand factor,[8] and red blood cells has been investigated.[9] Some studies found that the total numbers of leukocytes and plasma levels of CRP are consistently higher in periodontitis patients as compared to healthy ones. The aforementioned factors have also been found to have a strong association with cardiovascular diseases. The hypothesis that oral conditions such as periodontal infections may be a risk factor or indicators for important medical outcomes represents a paradigm shift in thinking about causality and directionality of oral and systemic associations.
Anemia of chronic disease (ACD) is usually defined as the anemia occurring in chronic infectious, inflammatory disorders, or neoplastic disorders that are not due to marrow replacement by tumor, bleeding, or hemolysis.[10] Causes for diminished marrow responses in ACD are: A reduced production of erythropoietin (Epo) or impaired bone marrow response to Epo. EPO is a hormone produced by the kidney that promotes the formation of red blood cells in the bone marrow. Lower EPO levels have been seen with anemia associated with chronic inflammatory conditions such as rheumatoid arthritis, chronic renal failure, acquired immunodeficiency syndrome, cancer, ulcerative colitis, sickle cell disease, and in premature neonates. Periodontitis such as other chronic inflammatory conditions thus can contribute to the prevalence of anemic status due to suppression of erythropoiesis by inflammatory cytokines.[11]
Tobacco smoking is associated with an increased disease rate in terms of periodontal bone loss, periodontal attachment loss, as well as periodontal pocket formation.[12,13] Cigarette smoking is known to cause an increase in hemoglobin (Hb) concentration that is believed to be mediated by exposure to carbon monoxide, leading to hypoxia. In smokers with periodontitis, it may be difficult to ascertain their anemic levels based only on their Hb% levels, as there may be a combined effect of both periodontitis and smoking on the blood parameters. The relative importance of these smoking-related alterations and their precise mode of action in increasing the risk of periodontal disease remain to be elucidated. As, periodontitis and smoking both have influences on erythropoiesis, this study was carried out to investigate the effect of periodontitis and smoking on serum Epo values. To our knowledge, this is the first study to evaluate the effect of periodontitis and smoking on Epo production from the bone marrow.
MATERIALS AND METHODS
Source of data
A total of 51 subjects of both sexes (age range: 30–65 years) with chronic periodontitis (CP) were selected from the outpatient of Department of Periodontology of Darshan Dental College, Udaipur, Rajasthan, India. Of the 51 selected patients, 17 patients with generalized CP, who were nonsmokers without anemia were selected and included in Group I (control group), 17 patients with generalized CP were nonsmokers with anemia were selected and included in Group II, and 17 patients who were smokers having generalized CP were included in Group III. A detailed systemic and family history was recorded. Only those voluntary subjects, who agree to give a written informed consent, were included in the study.
Inclusion criteria
Using criteria established by centers for disease control and prevention smokers were defined as: “Current smokers” those who had smoked 100 or more cigarettes in their lifetime and smoked at the time of interview; “former smokers” those who had smoked 100 or more cigarettes in their lifetime but were not currently smoking; and “nonsmokers” those who had not smoked 100 or more cigarettes in their lifetime.
Patient's age between 25 and 70 years; clinical diagnosis of generalized CP with evident bone loss on radiograph and probing depth (PD) ≥4 mm or more at 30% of proximal sites; clinical attachment loss (CAL) ≥1 mm; no history of periodontal therapy in last 6 months; current smokers – those who had smoked ≥100 cigarettes in their lifetime and smoked at the time of the interview;[14] anemic patients having Hb levels ≤13 g/dl for male adults and ≤12 g/dl for female adults.[15]
Exclusion criteria
The presence of systemic disease that could influence the course of periodontal disease; intake of antibiotics or anti-inflammatory drugs 4 weeks before the study; pregnant women; former smokers – who had smoked 100 or more cigarettes in their lifetime but are not currently smoking were excluded from the study;[14] iron therapy or iron supplements are taken in last 3 months; history of blood loss in previous 6 months; any history of periodontal therapy in last 6 months.
Clinical assessment
Clinical diagnosis of CP was made with an evident bone loss on the radiograph, PD of ≥4 mm at 30% of sites, clinical attachment levels (CAL) of ≥1 mm at 30% of sites. PD and CAL were recorded using Williams's calibrated probe. Other signs of inflammation were recorded using gingival bleeding index (GBI) and plaque index (PI). Current smokers were those who had smoked ≥100 cigarettes in their lifetime and smoked at the time of the interview were included in the study.[14] Anemic patients included those who had Hb levels of ≤13 g/dl for male adults and ≤12 g/dl for female adults.[15]
HEMATOLOGICAL PARAMETERS
After periodontal recordings, venous blood samples were obtained from all patients between 8:30 am and 11:30 am, as diurnal variations of Epo has been reported in literature. The samples were assessed for a number of erythrocytes (hemocytometer), levels of Hb (Sahli's method), and levels of Epo (enzyme immunoassay). Serum samples were then assessed by enzyme-linked immunosorbent assay kit (DRG® EPO ELISA kit)* for Epo. To assay the specimen in duplicate, 400 μL of human serum was required. Whole blood without anticoagulant was collected and allowed to clot between 2°C and 8°C. Then, the serum was separated, centrifuged, and stored at −15°C or lower. Serum samples were stored up to 24 h at 2–8°C until transportation to the laboratory.
Expected values
Using the nonparametric method for the analysis of reference values outlined in the National Committee for Clinical Laboratory Standards publication, manufacturers provided the reference ranges (2.5–97.5 percentile) 4.3–32.9 mIU (milli international unit)/ml for Epo in serum.
Statistical analyses
Data were analyzed using one-way analysis of variance (ANOVA) to assess the statistical difference between various groups (Group I, II, and III). Tukey–Kramer multiple comparisons test was done for comparison between the groups. Means and standard deviations for the different parameters were calculated. Mean values for age, GBI, PI, PD, and clinical attachment levels were calculated. P values from all statistical tests were presented but were considered statistically significant at P < 0.05.
RESULTS
The mean age of all the patients included in 3 different groups was comparable and was approximately 42 years. Mean PI values in Group I were 1.57 ± 0.53, for Group II, mean PI = 1.59 ± 0.49, and lowest for Group III, mean PI = 1.32 ± 0.43. Same trend was seen in the mean GBI scores with highest of Group I (96.08%), then Group II (94.66%), and lowest for Group III (83.19%) [Table 1 and Figure 1]. Though the mean PD and CAL value was highest in Group III as compared to the other two groups, but it was not statistically significant (P ≥ 0.05) [Table 2 and Figure 2]. Six point PD was recorded for all the patients before the study using Williams probe. When compared between the groups, highest mean PD%, and CAL% was seen in Group III (mean PD% ≥5 mm = 46.26%, mean CAL% ≥3 mm = 67.15%) [Table 2].
Table 1.
Mean values of plaque index and gingival bleeding index
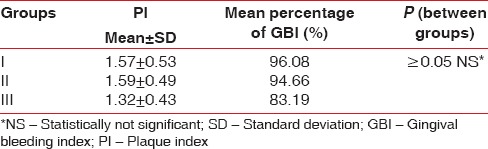
Figure 1.
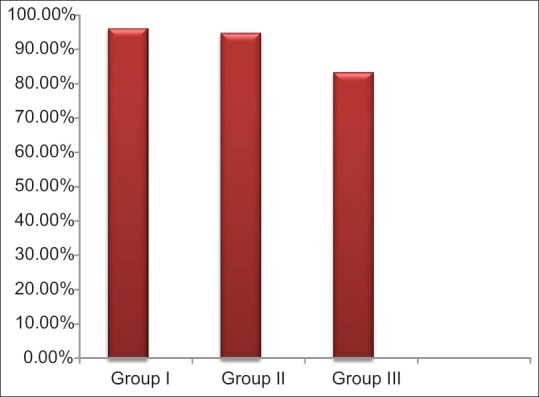
Graph showing mean % of gingival bleeding index. Mean gingival bleeding index values with Group III (smokers) having lowest values followed by Group II and Group I
Table 2.
Mean percentage of probing depth and clinical attachment levels
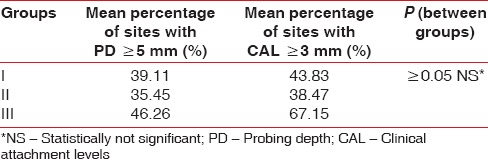
Figure 2.
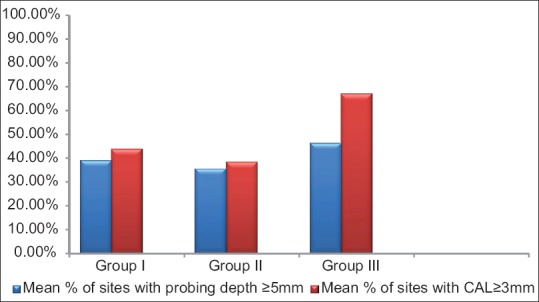
Graph showing difference between the groups with Group III having the highest mean values for probing depths and clinical attachment loss
ANOVA test for all hematological parameters using Tukey–Kramer multiple comparisons test is shown in Tables 3–5. Overall P values showed statistically significant correlations. Epo levels varied considerably between the 3 groups. Highest values of Epo were seen in Group III with mean Epo value = 42.81 ± 15, (n = 17, males = 17 females = 0) followed by Group II Epo value =35.21 ± 10.9, (n = 17, males = 4 females = 13), and then Group I Epo value = 22.06 ± 4.19, (n = 17, males = 5 females = 12) as shown in Table 3. When Group II patients (CP patients with anemia) and Group I patients (CP without anemia) were compared for Epo values, Group II patients showed higher mean values (P ≤ 0.01) Table 3. Group III showed extremely significant values of Epo when compared with Group I or Group II patients (P < 0.001) as shown in Table 3. Smokers in Group III with CP showed more prevalence toward higher values of Hb% (mean Hb = 12.06 ± 0.84) [Table 4] while there was no statistical difference in the values of total red blood cell (TRBC) values among the 3 groups (Group I TRBC value = 3.87 ± 0.38, Group II TRBC value = 4.01 ± 0.83, and Group III TRBC value = 3.88 ± 0.45) as shown in Tables 5 and 6.
Table 3.
One-way analysis of variance for groups I, II, and III (Tukey-Kramer multiple comparison test) for erythropoietin levels
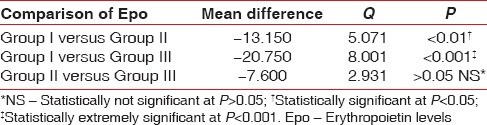
Table 5.
One-way analysis of variance for groups I, II, and III (Tukey-Kramer multiple comparison test) for total erythrocyte count

Table 4.
One-way analysis of variance for groups I, II, and III (Tukey-Kramer multiple comparison test) for hemoglobin levels
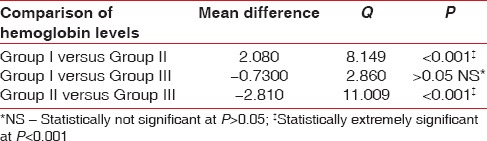
Table 6.
Means and standard deviation values for various blood parameters
DISCUSSION
Differentiation of ACD from iron deficiency is essential, as iron therapy may be harmful in the absence of iron deficiency. Serum iron and total iron binding capacity (TIBC) are both low in the anemia of chronic disorders in contrast to simple iron deficiency anemia where the TIBC is always elevated.[16] Patients with chronic disease can also be genuinely iron deficient, and the combination of the two causes of anemia can produce a more severe anemia. Periodontitis being a chronic inflammatory disease can contribute to anemic status of a patient. An increased production of inflammatory cytokines can directly inhibit erythropoiesis, interfere with Epo production and induce changes in iron homeostasis characterized by reductions of both iron absorption and macrophage iron release.[11] In periodontitis, increased production of inflammatory cytokines such as IL1, IL6, and tumor necrosis factorα are seen which may inhibit erythropoiesis and stimulate Epo production to compensate for the decreased cell mass. This effect was seen in our study as Group II patients which included CP patients with anemia had higher Epo values than Group I patients in which CP patients were included without any anemic status. When Group II patients and Group I patients were compared for mean Epo values, Group II patients showed higher mean values = 35.21 ± 10.9, (n = 17, males = 4, females = 13) than Group I Epo mean values = 22.06 ± 4.19, (n = 17, males = 5, females = 12) with statistically significant P ≤ 0.01.
There have been studies which evaluated the prevalence of anemic status in periodontitis. Gokhale et al.[17] found that the percentage of patients who were anemic in terms of Hb was 16.7% in the test group (periodontitis) compared to 3.3% in control group (without periodontitis). Chawla et al.[18] also suggested that anemia is an important factor in the etiology or pathogenesis of periodontal disease. Hutter et al.[9] evaluated the blood parameters in patients with CP and concluded that these patients show signs of anemia. In their study, the number of erythrocytes and hemoglobin were significantly lower in moderate and severe periodontitis than in controls (P ≤ 0.001, P ≤ 0.001 and P ≤ 0.002, respectively). In our study, no significant difference was found in the number of erythrocytes. Though in our study, the Epo values in Group I (mean = 22.06 ± 4.19) were toward higher value of normal Epo range (5–36 mIU/ml) and Epo levels were still higher in Group II (mean value = 35.21 ± 10.9) than in Group I [Tables 3 and 6]. As evident; Epo levels may be more valuable to assess ACD instead of relying on iron levels.
Cigarette smoking is known to cause an increase in Hb concentration mediated by exposure to carbon monoxide which bonds to Hb to form carboxyhemoglobin, an inactive form of Hb with no oxygen carrying capacity. As compensation, smokers maintain a higher Hb level than nonsmokers.[19] El-Zayadi [20] stated that hypoxia contributes to the development of secondary polycythemia and in turn to increased red cell mass and turnover. He also found that among smokers, those who have slightly higher Hb concentration have a significantly higher serum Epo than smokers with low Hb concentration. Thus, in smokers with periodontitis it may be difficult to ascertain their anemic levels based only on their Hb% levels, as there may be a combined effect of both periodontitis and smoking on the blood parameters. This is evident from our study as Group III subjects (smokers with CP) showed higher Hb levels (mean Hb value =12.79 ± 0.75) than Group I (12.06 ± 0.84) and II (9.98 ± 1.5) which included nonsmokers. In contrast, Erdemir et al.[21] reported lower Hb values in smokers ranging from 13.48 ± 2.19, whereas 14.98 ± 1.58 in nonsmokers. They also reported mean TRBC values in smokers of 4.88 ± 0.48 million/mm3 and 5.17 ± 0.47 million/mm3 in nonsmokers indicating lower TRBC values in smokers. Other hematological parameters such as Hb, hematocrit, and iron were lower in smokers than in nonsmokers with periodontitis. In our study, Epo levels were found to be highest in Group III (mean Epo value = 42.81 ± 15) suggesting hypoxic conditions leading to stimulation of Epo production to compensate for the lower oxygen levels. In our study, though TRBC values were not statistically significant (P > 0.05) with Group I TRBC value = 3.87 ± 0.38, Group II TRBC value = 4.01 ± 0.83, and Group III TRBC value = 3.88 ± 0.45, but the values were lower in smokers as compared to nonsmokers who may have led to the increased production of Epo levels.
CONCLUSION
As evident from this study, periodontitis patients have a lower Epo values which further strengthens the hypothesis that CP may lead to ACD. Increased levels of Epo were found in smokers in spite of higher Hb values. This suggests that the condition of anemia in smokers is masked by high Hb values. It also indicates that periodontitis individually and along with smoking may affect anemic status of smokers. Thus, Epo levels may be better means to assess anemic status of smokers than relying only on Hb values. These patients may benefit from a more comprehensive treatment approach including recombinant Epo therapy along with scaling, root planning, and iron supplementation. Though, further studies are required to elucidate smoking-related alterations, and their precise mode of action in increasing the risk of periodontal disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnote
DRG International Inc., Springfield, New Jersey, USA.
REFERENCES
- 1.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(11 Suppl):2106–15. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 2.Kweider M, Lowe GD, Murray GD, Kinane DF, McGowan DA. Dental disease, fibrinogen and white cell count; links with myocardial infarction? Scott Med J. 1993;38:73–4. doi: 10.1177/003693309303800304. [DOI] [PubMed] [Google Scholar]
- 3.Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000;71:1528–34. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- 4.Ebersole JL, Machen RL, Steffen MJ, Willmann DE. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clin Exp Immunol. 1997;107:347–52. doi: 10.1111/j.1365-2249.1997.270-ce1162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raja KB, O Latunde-Dada G, Peters TJ, McKie AT, Simpson RJ. Role of interleukin-6 in hypoxic regulation of intestinal iron absorption. Br J Haematol. 2005;131:656–62. doi: 10.1111/j.1365-2141.2005.05814.x. [DOI] [PubMed] [Google Scholar]
- 6.Sahingur SE, Sharma A, Genco RJ, De Nardin E. Association of increased levels of fibrinogen and the -455G/A fibrinogen gene polymorphism with chronic periodontitis. J Periodontol. 2003;74:329–37. doi: 10.1902/jop.2003.74.3.329. [DOI] [PubMed] [Google Scholar]
- 7.Mercado FB, Marshall RI, Klestov AC, Bartold PM. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001;72:779–87. doi: 10.1902/jop.2001.72.6.779. [DOI] [PubMed] [Google Scholar]
- 8.Salvi GE, Lawrence HP, Offenbacher S, Beck JD. Influence of risk factors on the pathogenesis of periodontitis. Periodontol 2000. 1997;14:173–201. doi: 10.1111/j.1600-0757.1997.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 9.Hutter JW, van der Velden U, Varoufaki A, Huffels RA, Hoek FJ, Loos BG. Lower numbers of erythrocytes and lower levels of hemoglobin in periodontitis patients compared to control subjects. J Clin Periodontol. 2001;28:930–6. doi: 10.1034/j.1600-051x.2001.028010930.x. [DOI] [PubMed] [Google Scholar]
- 10.Means RT, Jr, Krantz SB. Progress in understanding the pathogenesis of the anemia of chronic disease. Blood. 1992;80:1639–47. [PubMed] [Google Scholar]
- 11.Naik V, Acharya A, Deshmukh VL, Shetty S, Shirhatti R. Generalized, severe, chronic periodontitis is associated with anemia of chronic disease: A pilot study in urban, Indian males. J Investig Clin Dent. 2010;1:139–43. doi: 10.1111/j.2041-1626.2010.00028.x. [DOI] [PubMed] [Google Scholar]
- 12.Bergström J, Preber H. Tobacco use as a risk factor. J Periodontol. 1994;65(5 Suppl):545–50. doi: 10.1902/jop.1994.65.5s.545. [DOI] [PubMed] [Google Scholar]
- 13.Haffajee AD, Socransky SS. Relationship of cigarette smoking to attachment level profiles. J Clin Periodontol. 2001;28:283–95. doi: 10.1034/j.1600-051x.2001.028004283.x. [DOI] [PubMed] [Google Scholar]
- 14.Centre for Disease Control Classification of Smokers. [Last accessed on 215 Aug 22]. Available from: http://www.cdc.gov/tobacco/data_statistics/fact_sheets/
- 15.Haemoglobin Values for Anaemic Patients. Available from: Ministry of Health and Family Welfare. [Last accessed on 2015 Aug 22]. http://www.rchiips.org/nfhs/report.shtml .
- 16.Samson D. The anaemia of chronic disorders. Postgrad Med J. 1983;59:543–50. doi: 10.1136/pgmj.59.695.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gokhale SR, Sumanth S, Padhye AM. Evaluation of blood parameters in patients with chronic periodontitis for signs of anemia. J Periodontol. 2010;81:1202–6. doi: 10.1902/jop.2010.100079. [DOI] [PubMed] [Google Scholar]
- 18.Chawla TN, Kapoor KK, Teotia SP, Singh NK. Anaemia and periodontal disease – A correlative study. J Indian Dent Assoc. 1971;43:67–78. [PubMed] [Google Scholar]
- 19.Nordenberg D, Yip R, Binkin NJ. The effect of cigarette smoking on hemoglobin levels and anemia screening. JAMA. 1990;264:1556–9. [PubMed] [Google Scholar]
- 20.El-Zayadi AR. Heavy smoking and liver. World J Gastroenterol. 2006;12:6098–101. doi: 10.3748/wjg.v12.i38.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdemir EO, Nalcaci R, Caglayan O. Evaluation of systemic markers related to anaemia of chronic disease in peripheral blood of smokers and non smokers with chronic periodontitis. Eur J Dent. 2008;2:102–9. [PMC free article] [PubMed] [Google Scholar]


