Abstract
Context:
Host's immune response elicits cytokines in response to bacterial challenge. We explore role of one such cytokine interleukin-18 (IL-18) in periodontal health and disease.
Aims:
IL-18 is a pro-inflammatory and tumor suppressive cytokine. Dental literatures suggest that IL-18 might have a role to play in the progression from oral health to periodontal disease. Therefore, this study was undertaken to elucidate the level and role of IL-18 in the gingival crevicular fluid (GCF) and serum of individuals with healthy gingiva, chronic gingivitis, chronic periodontitis, and aggressive periodontitis before and after periodontal therapy.
Settings and Design:
Eighty individuals chosen for the study were divided into healthy control group (1A), chronic gingivitis (2A), chronic periodontitis (3A), and aggressive periodontitis (4A) with twenty individuals each. Criteria for the division were the subject's gingival index, probing pocket depth, clinical attachment loss, and radiographic evidence of bone loss.
Materials and Methods:
The individuals underwent treatment (scaling in case of Groups 1A and 2A and scaling and root planing followed by flap surgery in Groups 3A and 4A) to form posttreatment Groups 1B, 2B, 3B, and 4B, respectively. Thus, a total of 160 GCF and 160 serum samples were collected and tested by ELISA.
Statistical Analysis Used:
Intergroup comparison was done by post hoc Tukey's test.
Results:
The mean IL-18 concentration was greatest in Group 3A (GCF 144.61 pg/μl, serum 55.12 pg/ml) followed by Group 4A (GCF 98.55 pg/μl, serum 39.06 pg/ml), Group 2A (GCF 22.27 pg/μl, serum 27.73 pg/ml) and lowest (GCF 17.94 pg/μl, serum 11.49 pg/ml) in Group 1A. Posttreatment groups (1B–4B) showed reduction in the mean IL-18 concentration in both GCF and serum.
Conclusions:
As the inflammation increased, there was a concomitant increase in the level of IL-18 and vice versa following periodontal therapy.
Key words: Gingival crevicular fluid, gingivitis, interleukin, periodontitis, serum
INTRODUCTION
Traditionally, bacterial biofilms have been believed to be the primary etiological factor for the commencement of gingival inflammation and progression to periodontal tissue destruction in a susceptible host. The host's immunoinflammatory responses elicited to overcome these bacterial challenges are mediated by numerous cytokines.[1] This study was designed to explore the role, if at all any of one such cytokine interleukin-18 (IL-18) in periodontal health and disease in Indian Bengali population.
Though initially referred to as interferon gamma (IFN-γ) inducing factor, its name was changed to IL-18 after molecular cloning and later to IL-1F4 due to its resemblance in structure, receptor family, and signal transduction pathways with IL-1.[2,3,4] IL-18, a pro-inflammatory cytokine, is produced mainly by the antigen-presenting cells, monocytes/macrophages, Kupffer cells, and also by nonimmune cells such as intestinal and airway epithelial cells.[5,6] It is expressed at the sites of chronic inflammation, autoimmune diseases, in a variety of cancers and in numerous diseases.[7] IL-18, in the presence of IL-12 induce IFN-γ production from natural killer (NK) and T-cells and stimulate a Th1 cell response, while in its absence, IL-18 induce the production of Th2 cytokines such as IL-4, IL-5, IL-10, and IL-13, stimulate allergic inflammation and induce prostaglandin E2 production.[8,9] Thus, IL-18 has the unique capacity to induce either Th1 or Th2 differentiation, depending on the immunological context.[10]
MATERIALS AND METHODS
A total of 80 subjects were included in the study. They were of the age group 20–50 years, nonsmokers, free from any known systemic disease and had not undergone any periodontal therapy or had received any antibiotics and anti-inflammatory drugs in the previous 6 months. Written informed consent from the individuals participating in the study along with ethical clearance from the Institution's Ethical Committee was obtained. This study was conducted in accordance with the Helsinki Declaration of 1975 as revised in 2000. Orthopantomograph supplemented by intraoral periapical radiographs were done to assess the bony architecture. Based on their gingival index (GI), probing pocket depth (PD), clinical attachment (CA) loss, and radiographic evidence of bone loss, these eighty individuals were then divided equally into four groups of twenty members each as follows:
Group 1A (healthy control) – individuals having clinically healthy periodontium, GI score – 0, PD of ≤3 mm, and CA loss – 0 with no indication of bone loss on radiographs
Group 2A (chronic gingivitis) – individuals with clinical signs of gingival inflammation, GI score >l, PD ≤3 mm with the absence of attachment loss and radiographic bone loss
Group 3A (chronic periodontitis) – individuals with signs of clinical inflammation consistent with local etiological factors, GI score >l, PD ≥5 mm, CA loss of ≥3 mm, with radiographic evidence of bone loss [11,12]
Group 4A (aggressive periodontitis) – individuals with noncontributory medical history, rapid attachment loss and bone destruction, familial aggregation of cases, amount of deposits, which are inconsistent with the severity of periodontal tissue destruction and showing generalized interproximal attachment loss affecting at least three permanent teeth other than first molars and incisors.[11,13]
The individuals of Groups 1A and 2A underwent nonsurgical periodontal therapy, which was completed in two sessions within 24 h to form posttreatment Groups 1B and 2B, respectively.[14] Individuals of Groups 3A and 4A underwent periodontal flap surgery after scaling and root planing (SRP) to form Groups 3B and 4B, respectively. Gingival crevicular fluid (GCF) and serum samples were collected 6–8 weeks after scaling or flap surgery from the same sites from where GCF was collected prior to treatment. Thus, a total of 160 GCF samples and 160 serum samples were collected over the study period.
Collection of gingival crevicular fluid
After isolation by cotton rolls, GCF samples were collected a day after the assessment of clinical parameters to prevent the contamination of the GCF with blood expressed by probing of the inflamed sites. In each case, GCF sample was collected from the site with the deepest probing depth. If two sites showed similar probing depth, then the site showing the greatest CA loss and signs of inflammation, along with radiographic confirmation of bone loss, was selected for the sampling. The same sites were selected for sampling in the posttreatment groups. Supragingival plaque was removed and then 1 µl GCF was collected by placing 1–5 µl calibrated volumetric microcapillary pipette(Sigma-Aldrich Chemical Company, USA) extracrevicularly. The micropipettes contaminated with blood or the sites that did not express any GCF were excluded from the study. The collected GCF was immediately transferred to Eppendorf microcentrifuge tubes (Sigma-Aldrich Chemical Company, USA) containing 199 µl of phosphate buffered saline to make 200 µl of sample volume.
Collection of serum
The skin over the antecubital fossa was disinfected and 2 ml of blood was collected by venipuncture using 20-gauge needle and 2 ml syringe. The blood samples were allowed to clot at room temperature and 1 h later; they were centrifuged at 3000 rpm for 6 min to separate the serum component.
The samples were stored at − 70°C till the time of the assay. The collected GCF and serum samples were analyzed using ELISA technique (RayBio Human IL-18 ELISA Kit, Assaypro, USA). The technician, who performed the ELISA, was oblivious of the study groups.
Statistical analysis
The data were analyzed using statistical software SPSS version 11.0 (Chicago, IL, USA). The data were checked for normal distribution using the Kolmogorov-Smirnov test. Mean and standard deviation of the continuous variables were calculated. Correlational analysis was conducted within the groups by using measures from both the pretreatment groups and the posttreatment groups. Intergroup comparison of the mean levels of IL-18 in GCF and serum samples was performed using a post hoc Tukey's test. The difference in mean was considered statistically significant if P < 0.05. Pearson's correlation test was used to evaluate correlation among GCF and serum IL-18 concentration and the clinical parameters. P < 0.05 was considered statistically significant.
RESULTS
All the individuals maintained their appointments regularly. None of the individuals or the sampling sites was dropped in the course of the study. The descriptive data of the pretreatment and posttreatment groups is presented in [Tables 1 and 2], respectively. Intergroup comparison of the mean levels of IL-18 in GCF and serum samples using a post hoc Tukey's test is presented in Tables 3 and 4.
Table 1.
Descriptive data of the pretreatment groups

Table 2.
Descriptive data of the posttreatment groups

Table 3.
Comparison of interleukin-18 levels in the pretreatment groups
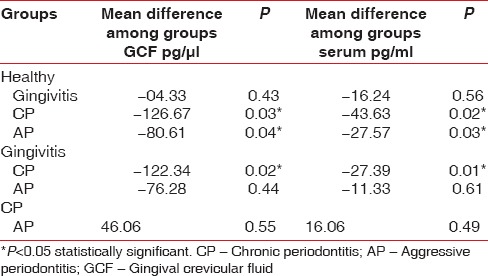
Table 4.
Comparison of interleukin-18 levels in the posttreatment groups

The results suggest that GCF and serum IL-18 levels were lowest in health and highest in periodontitis. Posttreatment, there was a reduction in the level of GCF and serum IL-18 in each group. A statistically significant difference in the mean concentration of IL-18 levels was observed between Groups 1A and 3A, between 1A and 4A and also between 2A and 3A in both GCF and serum. Posttreatment, a statistically significant difference in the mean concentration of IL-18 levels was observed between Groups 1B and 3B in both GCF and serum. IL-18 level in the GCF of Group 3A is positively correlated with PD and CA loss while the serum level in this group is positively correlated with GI, PD, and CA loss [Table 5]. There is no significant correlation between the levels of serum and GCF IL-18 and clinical parameters such as PI, GI, PD, and CA loss in healthy, chronic gingivitis, and aggressive periodontitis subjects.
Table 5.
Pearson correlation coefficient test

DISCUSSION
Enhanced GCF concentration of IL-1 has been reported to be associated with periodontal disease sites as compared to the healthy sites.[15] However, studies relating to the role of IL-18, a member of the IL-1 family in periodontal health, and disease were found to be scanty. Our study was unique and stood apart from other studies because we estimated IL-18 levels both prior and posttreatment in four separate groups-healthy control, chronic gingivitis, chronic periodontitis, and aggressive periodontitis.
The results of the present study indicate that the concentration of IL-18 in the GCF and serum was lowest in periodontal health (Group 1A), higher in gingivitis (Group 2A), even higher in aggressive periodontitis (Group 4A), and highest in the case of chronic periodontitis (Group 3A) subjects. This increase in the IL-18 concentration was in accordance with the reports of previous studies.[16,17,18,19,20,21] Moreover, the concentration of IL-18 in the GCF and serum was observed to be decreased 6–8 weeks after periodontal therapy. This reduction in the concentration in each group could be attributed to the posttreatment reduction in the inflammation of the gingiva which in turn would lead to a diminished release of cytokines into the GCF and serum.
Another finding noted was that despite the reduction in the mean concentration of IL-18 posttreatment, the level did not touch the baseline level of the healthy control group. This observation could be attributed to the nonresolving inflammation of the gingiva influenced by some other cytokine such as IL-6 and IL-12.[17,22] In 2005, Johnson and Serio opined that periodontal inflammation might not successfully resolve because of a decreased concentration of anti-inflammatory cytokines such as IL-12 within the inflamed gingival tissue and accumulation of other cytokines such as IL-6 and IL-18. IL-18 was also reported to play an important role in maintaining the chronicity of inflammation in rheumatoid arthritis, inflammatory bowel disease, and some allergies.[17] Offenbacher et al. analyzed 33 GCF biomarkers in experimental gingivitis in humans and observed that the pattern of biomarker expression during generation and resolution of inflammation differed considerably among subjects with similar clinical responses. Thus, the influence of one cytokine depends on the other cytokines and mediators in the local environment. This may explain the wide variability seen between the individuals in the same group.[22]
Ishida et al. reported that IL-18 increased the chemotaxis of NK cells and induced the production of activated matrix metalloproteinases (MMP) – 2, pro-MMP-2, and MT1-MMP from these cells.[23] Juvenile idiopathic arthritis individuals with incipient connective tissue attachment loss were reported to have higher serum IL-18 levels suggesting a role of IL-18 in periodontitis.[24] In severe inflammatory and septic conditions, high plasma IL-18 concentrations have shown poor clinical outcome. Hence, IL-18 was proposed as a marker in monitoring severe inflammatory conditions particularly in Gram-positive sepsis suspected cases.[25] Kim et al. investigated the effect of IL-18 on the expression of Type I and collagen genes in dermal fibroblasts. Their results suggested that IL-18 down-regulated collagen production in human dermal fibroblasts via extracellular signal-regulated kinase pathway.[26] IL-18 is associated with obesity, atherosclerosis, insulin resistance/glucose intolerance, cardiovascular disease, and multiorgan dysfunction.[27,28,29,30,31,32,33] IL-18 estimation might add prognostic information to lipid and inflammatory markers in patients with or without clinically established atherosclerotic disease.[34]
Literature reports have indicated that IL-18 could induce the release of MMP-9 and IL-1 β, both of which were reported to have pro-inflammatory and tissue degradation effects.[35] IL-18 was found to activate macrophages and other immune cells to secrete pro-inflammatory cytokines and chemokines. IL-18 might promote a priming effect on neutrophils which could upregulate the production of IL-1β, a pro-inflammatory cytokine.[36] Due to its chemotactic, pro-inflammatory, and angiogenic properties, IL-18 might be a factor in the progression of inflammation.[17] Thus, the accumulation of IL-18 in the periodontal tissues might be associated with persistence and maintenance of gingival inflammation. A concomitant rise in the level of IL-18 was observed in this study as the PD increased. This observation was consistent with the findings of Johnson and Serio. They had reported higher concentrations of IL-18 adjacent to the sites with PD > 6 mm than that of the healthy sites.[17] Thus, IL-18 could play an important role in gingival inflammation as relatively higher levels of IL-18 were found in gingival samples with increasing sulcular depth.
Orozco et al. compared IL-1, IL-12, and IL-18 levels in the GCF of gingivitis (PD ≤ 4 mm) and periodontitis (PD ≥ 6 mm) individuals.[18] They concluded that the concentration of IL-18 was significantly higher than IL-1 at both gingivitis and periodontitis sites and thus inferred a higher significance for this cytokine in periodontal disease. The concentration of IL-18 in the GCF of the individuals of Group 3A was observed to be higher compared to the individuals of Group 2A. This observation of elevated levels of IL-18 in chronic periodontitis individuals could be explained on the basis of the increased probing PD in them. This finding was consistent with the observation by Figueredo et al., who reported higher levels of IL-18 in the individuals with chronic periodontitis as compared to gingivitis.[19] The observations of this study were in accordance with the report of Pradeep et al. who had reported from the southern part of India that the level of GCF IL-18 was highest in chronic periodontitis subjects followed by gingivitis and lowest in periodontal health [20] There was a reduction in the IL-18 levels in the periodontitis group following treatment (SRP).
The change in IL-18 concentration observed in the groups correlated positively with the clinical parameters used in the study. IL-18 level in the GCF of Group 3A is positively correlated with PD and CA loss while the serum level in this group is positively correlated with GI, PD, and CA loss [Table 5]. There is no significant correlation between the levels of serum and GCF IL-18 and clinical parameters such as PI, GI, PD, and CA loss in healthy, chronic gingivitis, and aggressive periodontitis subjects.
GCF was collected using microcapillary pipettes. This avoided loss of GCF sample by evaporation, which was seen with filter paper.[37] The latter could have resulted in a false reduction in the detectable IL-18 level and hence could underestimate the correlation of IL-18 level to the disease severity. However, collection of the GCF by pipette carried the risk of trauma to the marginal gingiva and hence utmost care was taken to avoid it. The microcapillary pipette was gently placed at the entrance of the gingival crevice, and then the sample was collected. Moreover, the loss of GCF due to its sticking to the capillary walls was avoided by flushing the capillary with a fixed amount of diluent, which was contemplated during the final calculations.
The study design had initially four pretreatment groups with four posttreatment groups added 6–8 weeks after the completion of periodontal therapy. Such a designing of the study facilitated better evaluation of the role of IL-18 in the different stages of periodontal health and disease and to evaluate the role of periodontal therapy on the concentration of IL-18 in the GCF and serum. Though most of the healing was supposed to be completed by 6 weeks, the repair process could continue for a longer period. Perhaps, a delay in the posttreatment sample collection by a few weeks might have produced further attenuation in the inflammation of the periodontal tissues and hence further decrease in the IL-18 levels. However, further studies involving larger sample sizes are warranted to properly evaluate the role of IL-18 in periodontal health and disease.
CONCLUSION
Increase in the GCF and serum levels of IL-18 is directly proportional to the increased level of inflammation of the periodontal tissues while a decrease in inflammation following periodontal therapy lead to a reduction in the levels of the IL in GCF and serum. Further longitudinal prospective studies involving larger sample sizes are warranted to affirm the role of IL-18 in periodontal health and disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
I would like to thank Dr. Argha Rudra, Assistant Professor, R.G. Kar Medical College and Hospital, Kolkata, and Mr. Suman, Indian Statistical Institute, Kolkata, for their help and guidance during the study.
REFERENCES
- 1.Marshall RI. Gingival defensins: Linking the innate and adaptive immune responses to dental plaque. Periodontol 2000. 2004;35:14–20. doi: 10.1111/j.0906-6713.2004.003568.x. [DOI] [PubMed] [Google Scholar]
- 2.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 3.Golab J. Interleukin 18 – Interferon gamma inducing factor – A novel player in tumour immunotherapy? Cytokine. 2000;12:332–8. doi: 10.1006/cyto.1999.0563. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20(5 Suppl 27):S1–13. [PubMed] [Google Scholar]
- 5.Biet F, Locht C, Kremer L. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J Mol Med (Berl) 2002;80:147–62. doi: 10.1007/s00109-001-0307-1. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA. IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103(1 Pt 1):11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 7.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73:213–24. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 8.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–74. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 9.Lotze MT, Tahara H, Okamura H. Interleukin-18 as a novel, distinct, and distant member of the interleukin-1 family promoting development of the adaptive immune response: The interleukin-18 issue of the Journal of Immunotherapy. J Immunother. 2002;25(Suppl 1):S1–3. doi: 10.1097/00002371-200203001-00001. [DOI] [PubMed] [Google Scholar]
- 10.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72. doi: 10.1016/s1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 11.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Flemmig TF. Periodontitis. Ann Periodontol. 1999;4:32–8. doi: 10.1902/annals.1999.4.1.32. [DOI] [PubMed] [Google Scholar]
- 13.Lang NP, Bartold PM, Cullinan M, Jeffcoat M, Mombelli A, Murakami S, et al. Consensus report: Aggressive periodontitis. Ann Periodontol. 1999;4:53. [Google Scholar]
- 14.Quirynen M, De Soete M, Boschmans G, Pauwels M, Coucke W, Teughels W, et al. Benefit of “one-stage full-mouth disinfection” is explained by disinfection and root planing within 24 hours: A randomized controlled trial. J Clin Periodontol. 2006;33:639–47. doi: 10.1111/j.1600-051X.2006.00959.x. [DOI] [PubMed] [Google Scholar]
- 15.Hönig J, Rordorf-Adam C, Siegmund C, Wiedemann W, Erard F. Increased interleukin-1 beta (IL-1 beta) concentration in gingival tissue from periodontitis patients. J Periodontal Res. 1989;24:362–7. doi: 10.1111/j.1600-0765.1989.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 16.de Campos BO, Fischer RG, Gustafsson A, Figueredo CM. Effectiveness of non-surgical treatment to reduce il-18 levels in the gingival crevicular fluid of patients with periodontal disease. Braz Dent J. 2012;23:428–32. doi: 10.1590/s0103-64402012000400020. [DOI] [PubMed] [Google Scholar]
- 17.Johnson RB, Serio FG. Interleukin-18 concentrations and the pathogenesis of periodontal disease. J Periodontol. 2005;76:785–90. doi: 10.1902/jop.2005.76.5.785. [DOI] [PubMed] [Google Scholar]
- 18.Orozco A, Gemmell E, Bickel M, Seymour GJ. Interleukin-1beta, interleukin-12 and interleukin-18 levels in gingival fluid and serum of patients with gingivitis and periodontitis. Oral Microbiol Immunol. 2006;21:256–60. doi: 10.1111/j.1399-302X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 19.Figueredo CM, Rescala B, Teles RP, Teles FP, Fischer RG, Haffajee AD, et al. Increased interleukin-18 in gingival crevicular fluid from periodontitis patients. Oral Microbiol Immunol. 2008;23:173–6. doi: 10.1111/j.1399-302X.2007.00408.x. [DOI] [PubMed] [Google Scholar]
- 20.Pradeep AR, Daisy H, Hadge P, Garg G, Thorat M. Correlation of gingival crevicular fluid interleukin-18 and monocyte chemoattractant protein-1 levels in periodontal health and disease. J Periodontol. 2009;80:1454–61. doi: 10.1902/jop.2009.090117. [DOI] [PubMed] [Google Scholar]
- 21.Hadge P, Daisy H, Pradeep AR, Prasad MV. Interleukin 18: An indicator of inflammatory status in periodontitis. Arch Oral Sci Res. 2011;1:179–84. [Google Scholar]
- 22.Offenbacher S, Barros S, Mendoza L, Mauriello S, Preisser J, Moss K, et al. Changes in gingival crevicular fluid inflammatory mediator levels during the induction and resolution of experimental gingivitis in humans. J Clin Periodontol. 2010;37:324–33. doi: 10.1111/j.1600-051X.2010.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishida Y, Migita K, Izumi Y, Nakao K, Ida H, Kawakami A, et al. The role of IL-18 in the modulation of matrix metalloproteinases and migration of human natural killer (NK) cells. FEBS Lett. 2004;569:156–60. doi: 10.1016/j.febslet.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 24.Miranda LA, Fischer RG, Sztajnbok FR, Johansson A, Figueredo CM, Gustafsson A. Increased interleukin-18 in patients with juvenile idiopathic arthritis and early attachment loss. J Periodontol. 2005;76:75–82. doi: 10.1902/jop.2005.76.1.75. [DOI] [PubMed] [Google Scholar]
- 25.Tschoeke SK, Oberholzer A, Moldawer LL. Interleukin-18: A novel prognostic cytokine in bacteria-induced sepsis. Crit Care Med. 2006;34:1225–33. doi: 10.1097/01.CCM.0000208356.05575.16. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Song SB, Lee JY, Cho BK, Cho DH, Park HJ. Interleukin-18 down regulates collagen production in human dermal fibroblasts via ERK pathway. J Invest Dermatol. 2010;130:706–15. doi: 10.1038/jid.2009.302. [DOI] [PubMed] [Google Scholar]
- 27.Jawien J. New insights into immunological aspects of atherosclerosis. Pol Arch Med Wewn. 2008;118:127–31. [PubMed] [Google Scholar]
- 28.Bruun JM, Stallknecht B, Helge JW, Richelsen B. Interleukin-18 in plasma and adipose tissue: Effects of obesity, insulin resistance, and weight loss. Eur J Endocrinol. 2007;157:465–71. doi: 10.1530/EJE-07-0206. [DOI] [PubMed] [Google Scholar]
- 29.Olusi SO, Al-Awadhi A, Abraham M. Relations of serum interleukin 18 levels to serum lipid and glucose concentrations in an apparently healthy adult population. Horm Res. 2003;60:29–33. doi: 10.1159/000070824. [DOI] [PubMed] [Google Scholar]
- 30.Thorand B, Kolb H, Baumert J, Koenig W, Chambless L, Meisinger C, et al. Elevated levels of interleukin-18 predict the development of type 2 diabetes: Results from the MONICA/KORA Augsburg Study, 1984-2002. Diabetes. 2005;54:2932–8. doi: 10.2337/diabetes.54.10.2932. [DOI] [PubMed] [Google Scholar]
- 31.Rau B, Baumgart K, Paszkowski AS, Mayer JM, Beger HG. Clinical relevance of caspase-1 activated cytokines in acute pancreatitis: High correlation of serum interleukin-18 with pancreatic necrosis and systemic complications. Crit Care Med. 2001;29:1556–62. doi: 10.1097/00003246-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Straczkowski M, Kowalska I, Nikolajuk A, Otziomek E, Adamska A, Karolczuk-Zarachowicz M, et al. Increased serum interleukin-18 concentration is associated with hypoadiponectinemia in obesity, independently of insulin resistance. Int J Obes (Lond) 2007;31:221–5. doi: 10.1038/sj.ijo.0803421. [DOI] [PubMed] [Google Scholar]
- 33.Everett BM, Bansal S, Rifai N, Buring JE, Ridker PM. Interleukin-18 and the risk of future cardiovascular disease among initially healthy women. Atherosclerosis. 2009;202:282–8. doi: 10.1016/j.atherosclerosis.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Packard RR, Libby P. Inflammation in atherosclerosis: From vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 35.Jablonska E, Izycka A, Wawrusiewicz N. Effect of IL-18 on IL-1beta and sIL-1RII production by human neutrophils. Arch Immunol Ther Exp (Warsz) 2002;50:139–41. [PubMed] [Google Scholar]
- 36.Jablonska E, Izycka A, Jablonska J, Wawrusiewicz N, Piecuch J. Role of IL-18 in the secretion of Il-1beta, sIL-1RII, and IL-1Ra by human neutrophils. Immunol Invest. 2001;30:221–9. doi: 10.1081/imm-100105066. [DOI] [PubMed] [Google Scholar]
- 37.Griffiths GS. Formation, collection and significance of gingival crevice fluid. Periodontol 2000. 2003;31:32–42. doi: 10.1034/j.1600-0757.2003.03103.x. [DOI] [PubMed] [Google Scholar]


