Abstract
Background:
Anemia of chronic disease is defined as anemia occurring in chronic infections, inflammatory conditions, or neoplastic disorders which are not due to marrow deficiencies or other diseases, and occurring despite the presence of adequate iron stores and vitamins.
Aims:
To evaluate the relation between anemia and periodontitis by estimation of blood parameters and to assess whether periodontitis like other inflammatory conditions can lead to anemia. It is a randomized controlled clinical trial.
Materials and Methods:
A total of 50 healthy controls, 50 chronic generalized gingivitis, and 50 chronic generalized periodontitis patients were selected. Hemoglobin levels (Hb), erythrocyte count red blood cell, erythrocyte sedimentation rate (ESR), mean corpuscular volu e (MCV), mean corpuscular Hb (MCH) and MCH concentration (MCHC), gingival index, plaque index, probing pocket depth, and clinical attachment level were recorded. Intergroup comparison of blood parameters is by one-way ANOVA. Intergroup pair wise comparison of the three groups is by Newman–Keuls multiple post-hoc procedures. Karl Pearsons's correlation coefficient method is used for correlation between different parameters for three groups.
Results:
The results revealed a decrease in Hb and erythrocyte counts and increase in white blood corpuscles counts in chronic generalized periodontitis when compared to healthy controls and chronic generalized gingivitis group. There was no statistically significant difference in MCV, MCH, MCHC, and ESR among the groups.
Conclusions:
The treatment of periodontitis can lead to an improvement in hematocrit and other related blood parameters in chronic generalized periodontitis patients with anemia. This provides evidence that periodontitis like other chronic diseases may also cause anemia.
Key words: Anemia, anemia of chronic disease, hemoglobin, periodontitis
INTRODUCTION
Periodontitis is an inflammatory disease of the supporting tissues of the tooth caused by specific microorganisms in a susceptible host. Gram-negative anaerobic bacteria are most commonly associated in the initiation of periodontitis.[1] Localized infections which are characteristic of periodontitis can have a significant effect on the systemic health of humans and animals. Just as the periodontal tissues mount an immune inflammatory response to bacteria and their products, systemic challenges with these agents also induce a major vascular response.[2]
The sulcular epithelium acts as a protective barrier and prevents entry of microorganisms and other irritants into the systemic circulation. The host-microbial interaction in periodontitis leads to ulceration of sulcular epithelium. The ulcerated pocket epithelium acts as a portal of entry for the bacteria to enter the connective tissue and thus into the systemic circulation and causes bacteremia. Bacteremia has been observed in patients with periodontitis and has been directly related to the severity of the inflammation.[3]
The host response may offer explanatory mechanisms for the interaction between periodontitis and a variety of systemic disorders.[4] Infections, malignant cells, and autoimmune dysregulation all lead to the activation of the immune system and production of cytokines most notably tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) and IL-6 (IL-6).[5] Such inflammatory cytokines can depress erythropoietin (Epo) production leading to the development of anemia.[6]
The disorders associated with anemia of chronic disease (ACD) are characterized by the production of certain inflammatory cytokines primarily, macrophage-derived which includes IL-1α, IL-β, IL-6, transforming growth factor-β and TNF-α. In chronic inflammatory disorders, cellular or humoral factors including TNF and IL-1 cause suppression of the bone marrow response to Epo and thus possibly contribute to this anemia.
Anemia is defined as an Hb concentration in blood below the lower limit of the normal range for the age and sex of the individual. It is usually classified as either acute or chronic. Acute anemia occurs in a quick time, and chronic anemia occurs over a long period of time.
ACD is the most common form of anemia observed in clinical medicine.[7] ACD is defined as “anemia occurring in chronic infections, inflammatory conditions, or a neoplastic disorder which is not caused by marrow deficiencies or other diseases and occurring despite the presence of adequate iron stores and vitamins.”[8] ACD is considered the most frequent cause of anemia in rheumatoid arthritis (RA), which demonstrates a pattern of hard and soft tissue destruction caused by an inflammatory process which is similar to the inflammatory process seen in chronic periodontitis.[9]
ACD is a multifactorial anemia often coexistent with iron deficiency. ACD is the second most prevalent after anemia caused by iron deficiency. It is a mild to moderate anemia associated with chronic infections, chronic noninfectious inflammatory diseases, and malignancies.[10] The anemia develops after a month or two of active disease.
Classification of anemia
Anemia can be classified from three points of view: Pathogenesis [Table 1], red cell morphology [Table 2], and clinical presentation. All are important to guide the diagnosis. Pathogenic mechanisms involved in the production of anemia are very simple: Inadequate production and loss of erythrocytes as a result of bleeding or hemolysis. Based on these pathogenic mechanisms, anemia can be divided into two types. (1) Hypo-regenerative: When bone marrow production is decreased as a result of impaired function, decreased a number of precursor cells, reduced bone marrow infiltration, or lack of nutrients; (2) regenerative: When bone marrow responds appropriately to a low erythrocyte mass by increasing production of erythrocytes.
Table 1.
Etiopathogenic classification of anemia

Table 2.
Morphological classification

Anemia also can be classified according to the form of clinical presentation as acute (usually bleeding or hemolysis) or chronic.
Anemia can be classified as microcytic, normocytic, or macrocytic depending on mean corpuscular volume (MCV).
Morphological classification
The present study was carried out to evaluate the relationship between anemia and periodontitis by estimation of peripheral blood in relation to the number of erythrocytes, hemoglobin (Hb) concentration, erythrocyte sedimentation rate (ESR), MCV, mean corpuscular Hb (MCH) and MCH concentration (MCHC) in Healthy controls, chronic generalized gingivitis and chronic generalized periodontitis patients, and to establish periodontitis as one of the etiological factor leading to ACD.
MATERIALS AND METHODS
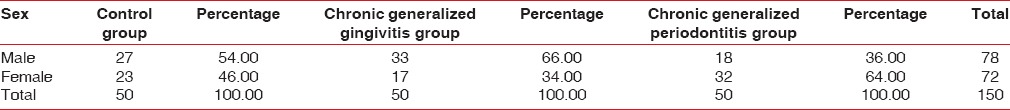
One hundred and fifty patients (men and women) aged between 20 and 55 years who visited the Department of Periodontics and Implantology, Dr. Sudha and Nageswara Rao Siddhartha Institute of Dental Sciences, Chinnaoutpalli, Gannavaram Mandal, were screened and recruited for the study. A total of 50 healthy controls (with gingival index criteria 0–1), 50 chronic generalized gingivitis patients (with gingival index criteria 2–3), and 50 chronic generalized periodontitis patients (30% or more of the teeth examined with ≥5 mm probing depth and ≥2 mm clinical attachment loss [CAL]) were selected for the study.
Inclusion criteria
Patients within age group 20–55 years, systemically healthy patients, healthy controls (with gingival index criteria 0–1), patients diagnosed with chronic generalized gingivitis (with gingival index criteria 2–3), patients diagnosed with generalized chronic periodontitis (30% or more of the teeth examined having ≥5 mm probing depth and ≥2 mm CAL) were included in the study [Table 3].
Table 3.
Distribution of male and females in three groups
Exclusion criteria
Patients with systemic diseases, present and past smokers, patients who have undergone periodontal treatment 6 months prior to the study, patients with <l teeth in the oral cavity were excluded from the study.
Method of collection of data
Healthy controls, chronic generalized gingivitis patients, chronic generalized periodontitis patients were initially screened for inclusion in the study. Periodontal status of the patients included in the study was recorded using gingival index (Loe and Silness 1963), plaque index (Silness and Loe 1964), Probing Pocket Depth, CAL. Under aseptic conditions, about 2 ml of venous blood was drawn from antecubital fossa using a disposable syringe. The collected blood samples were estimated by automated analyzer for a number of erythrocytes red blood cell (RBC), Hb% concentration, white blood corpuscles (WBC), MCV, MCH, MCHC, ESR, and packed cell volume (PCV).
Statistical analysis
Intergroup comparison of blood parameters is by one-way ANOVA. Intergroup pairwise comparison of the three groups is by Newman–Keuls multiple post-hoc procedures. Karl Pearsons's correlation coefficient method is used for correlation between different parameters for three groups.
RESULTS
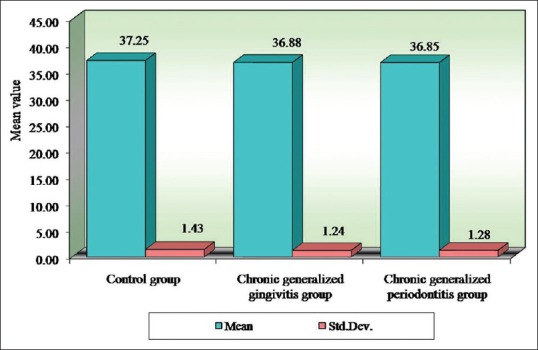
Pairwise comparison of the three groups for RBC, Hb% by Newman–Keuls multiple post-hoc procedures showed no significant difference between control and chronic generalized gingivitis group and control and chronic periodontitis group. There is a statistically significant difference between chronic generalized gingivitis and chronic generalized periodontitis group for RBC [Table 4, Figure 1]. There is a statistically significant difference between control and chronic generalized periodontitis group and chronic generalized gingivitis and chronic generalized periodontitis group for HB% [Table 5, Figure 2]. Pairwise comparison of the three groups for WBC by Newman–Keuls multiple post-hoc procedures showed that there is no significant difference between control and chronic generalized gingivitis group and chronic generalized gingivitis and chronic generalized periodontitis group. There is a statistically significant difference between control and chronic generalized periodontitis group [Table 6, Figure 3]. Pairwise comparison of the three groups for MCH, MCHC, MCV, and ESR by Newman–Keuls multiple post-hoc procedures showed that there is no statistically significant difference between control, chronic generalized gingivitis, and chronic generalized periodontitis groups [Tables 7–10, Figures 4–7]. Pairwise comparison of the three groups for PCV by Newman–Keuls multiple post-hoc procedures showed that there is no significant difference between control and chronic generalized gingivitis group and control and chronic periodontitis group but statistically significant difference was observed between chronic generalized gingivitis and chronic generalized periodontitis groups [Table 11, Figure 8].
Table 4.
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to red blood cell counts by one-way analysis of variance
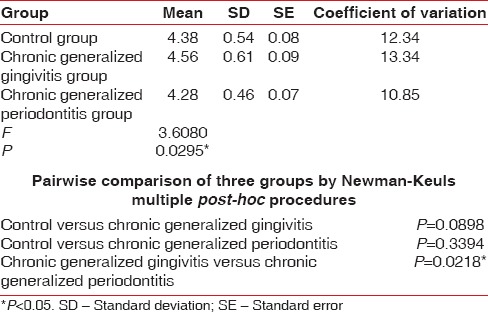
Figure 1.
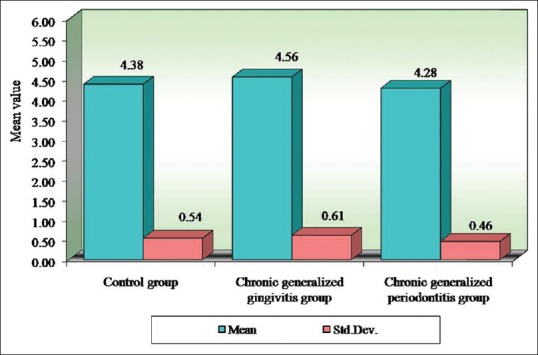
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to red blood cell counts
Table 5.
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to Hemoglobin values by one-way analysis of variance
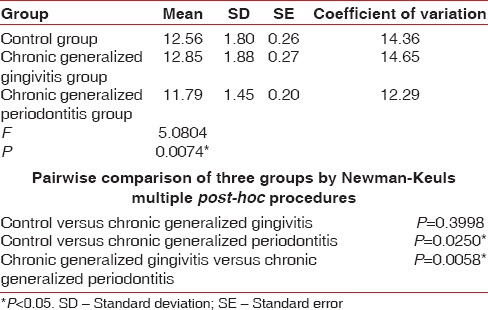
Figure 2.
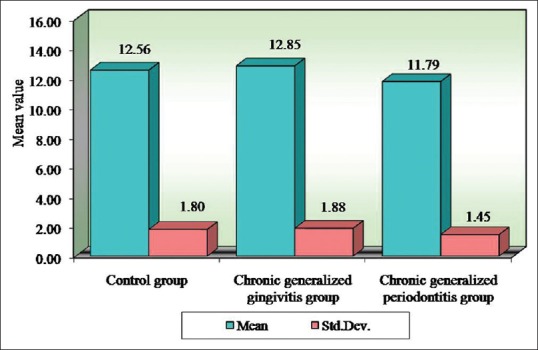
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to hemoglobin values
Table 6.
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to white blood cell counts by one-way analysis of variance
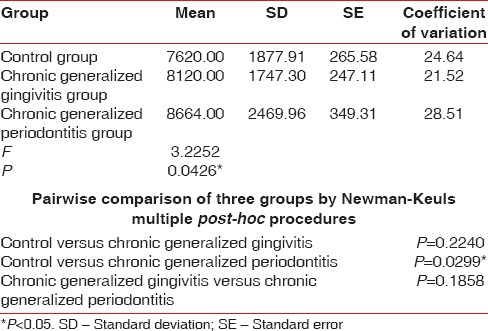
Figure 3.
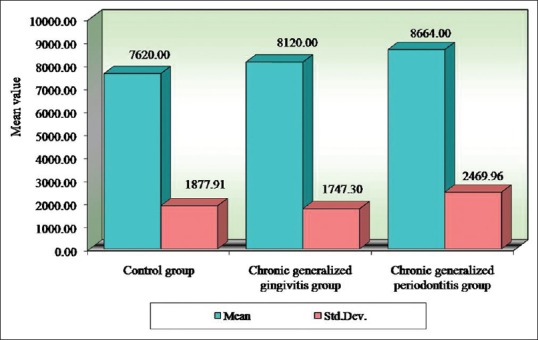
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to white blood corpuscles counts
Table 7.
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to mean corpuscular hemoglobin scores by one-way analysis of variance
Table 10.
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to erythrocyte sedimentation rate scores by one-way analysis of variance
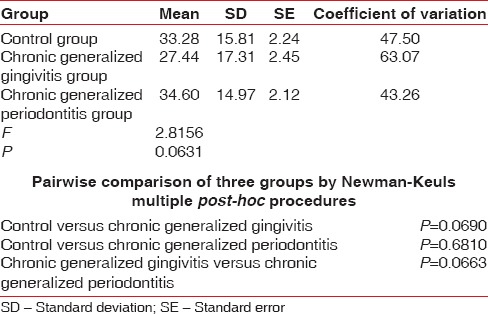
Figure 4.
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to concentration scores
Figure 7.
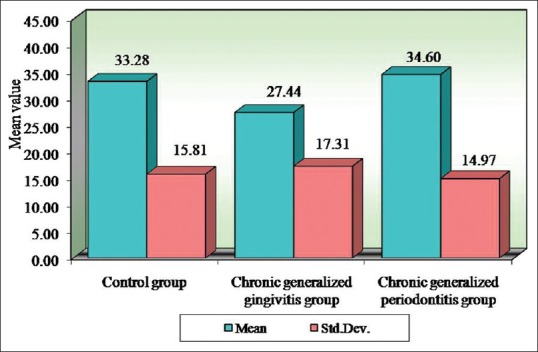
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to erythrocyte sedimentation rate scores
Table 11.
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to packed cell volume scores by one-way analysis of variance
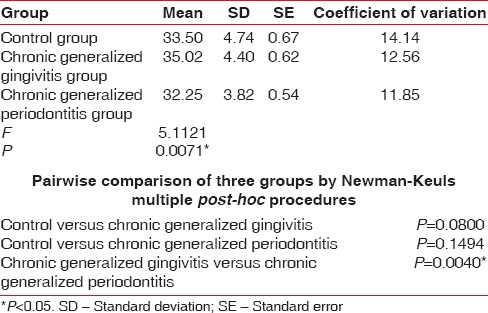
Figure 8.
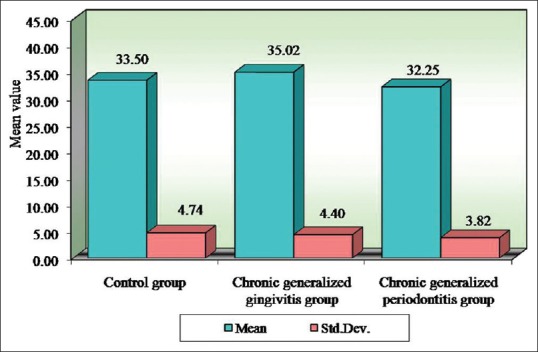
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to packed cell volume scores
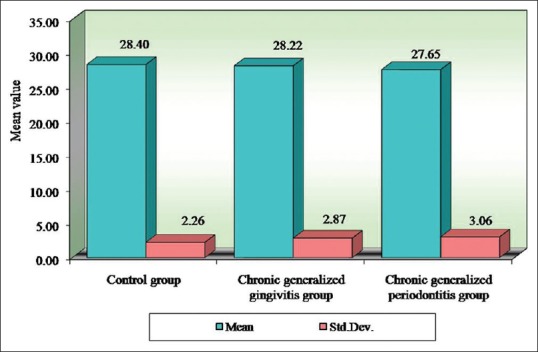
Table 8.
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to mean corpuscular hemoglobin concentration scores by one-way analysis of variance
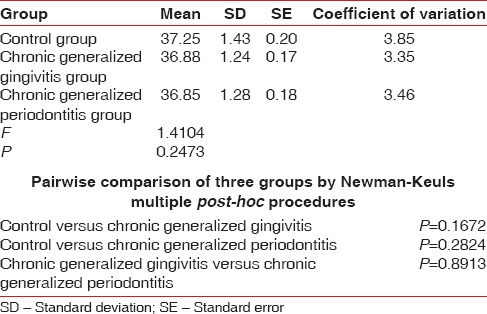
Table 9.
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to mean corpuscular volume scores by one-way analysis of variance
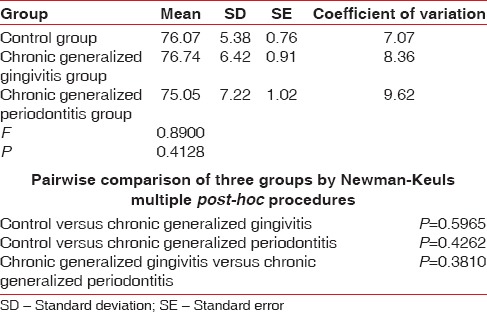
Figure 5.
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to mean corpuscular hemoglobin concentration scores
Figure 6.
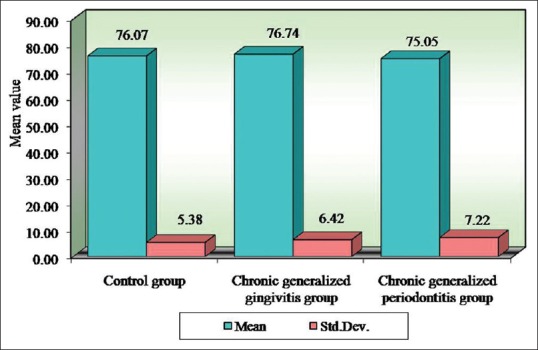
Comparison of three groups (control, chronic generalized gingivitis, and chronic generalized periodontitis group) with respect to mean corpuscular volume scores
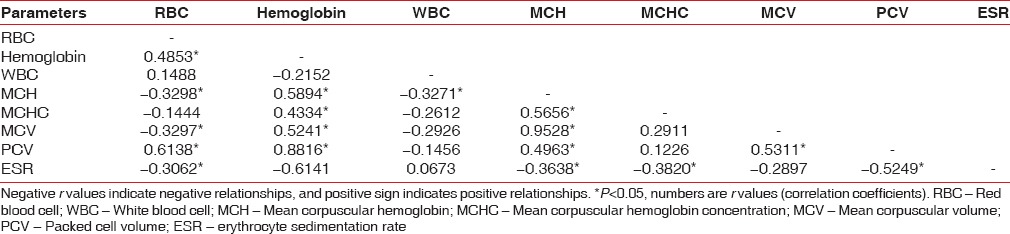
Positive relationship exists among Hb%, RBC, MCH, PCV, and MCV in the control group [Table 12]. A positive relationship exists among Hb%, RBC, WBC in chronic generalized gingivitis group [Table 13]. A positive relationship exists among Hb%, RBC, PCV in chronic generalized periodontitis group [Table 14].
Table 12.
Correlation between different parameters in control group by Karl Pearson's correlation coefficient method
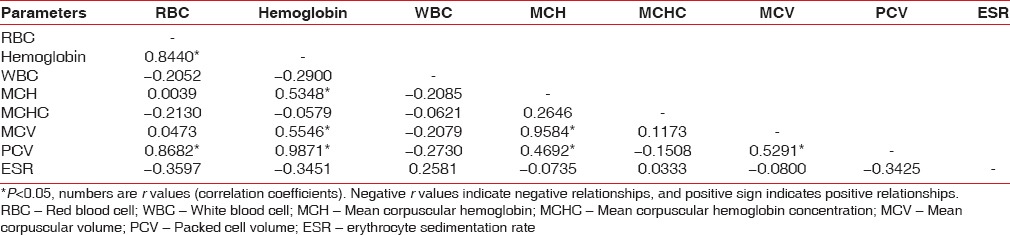
Table 13.
Correlation between different parameters in chronic generalized gingivitis group by Karl Pearson's correlation coefficient method
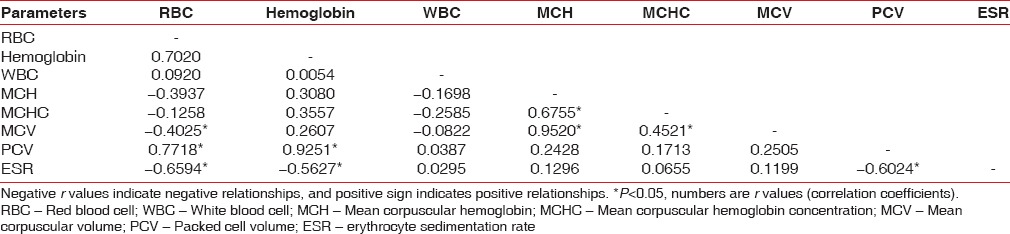
Table 14.
Correlation between different parameters in chronic generalized periodontitis group by Karl Pearson's correlation coefficient method
DISCUSSION
Localized infections of periodontal tissues which result in periodontitis can have a significant effect on the systemic health of an individual. As the periodontal tissues mount an immune inflammatory response to bacteria and their products, systemic challenges with these agents also induce a major vascular response. Infections, malignant cells, and autoimmune dysregulation all lead to the activation of the immune system and production of inflammatory cytokines (TNF-α, IL-1 and IL-6) which can depress Epo production leading to the development of anemia.[11]
The present study was carried out to assess the effect of periodontal inflammation on peripheral blood in chronic generalized periodontitis patients. The relation between anemia and periodontitis has been explored in the latter half of the 20th century. Few studies investigated both the concepts of anemia as an etiological factor of periodontitis and periodontitis as a risk factor of anemia. It was believed that anemia may be a factor in the causation of periodontitis rather than a consequence.[10] Lainson et al. stated that anemia is a systemic cause of periodontitis.[12]
Recently, various studies have tried to evaluate the relation between periodontitis and anemia. Few studies present conflicting results. Hutter et al. showed that periodontitis patients have a lower hematocrit, lower number of erythrocytes, lower Hb levels, and higher ESRs.[13] Whereas, the studies by Wakai et al. failed to show any association between Hb and periodontal status.[14] Balwanth Rai and Kharb (2008) in a 10 weeks intervention study found an increase of blood parameters in Hb and RBC levels in patients with severe periodontitis after scaling and root planning (SRP). A statistically significant increase in the mean Hb of 14.5 mg/dl at baseline to 15 mg/dl at 10 weeks after SRP was observed.
Most of these studies are epidemiological in nature. Our present cross-sectional study was carried out to identify the risk of periodontitis and anemia. It was carried out in 50 healthy controls, 50 chronic generalized gingivitis, and 50 chronic generalized periodontitis (male and female) patients to assess the effect of periodontal inflammation on blood parameters.
Seigel,[15] Wakai et al.,[14] Kowolik et al.,[16] Hutter et al.,[13] Loos,[17] Gokhale et al.,[1] Yamamoto et al.,[18] Pejcic et al.,[19] and Ali [20] showed that periodontal inflammation results in decrease in number of erythrocytes and levels of Hb and increase in WBC count in accordance with our study. In our present study, the mean RBC value for the control group is 4.38 million/cu.mm, chronic generalized gingivitis is 4.56 million/cu.mm and chronic generalized periodontitis is 4.28 million/cu.mm. The mean Hb for the control group is 12.56 g%, chronic generalized gingivitis is 12.85 g%, and chronic generalized periodontitis is 11.79 g%. The mean WBC count in the control group is 7620 cells/cu.mm, chronic generalized gingivitis is 8120 cells/cu.mm, and chronic generalized periodontitis is 8664 cells/cu.mm. This implies that periodontitis like other chronic conditions may lead to anemia. This anemia is mild to moderate, and inflammatory cytokines may play the role in its pathogenesis.
CONCLUSION
The present study showed that periodontal inflammation results in a decrease in a number of erythrocytes and levels of Hb. This implies that periodontitis like other chronic conditions may lead to anemia. This anemia is mild to moderate, and inflammatory cytokines may play the role in its pathogenesis and one of the benefits of its treatment would be an improvement in hematocrit and related hematological values of the patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gokhale SR, Sumanth S, Padhye AM. Evaluation of blood parameters in patients with chronic periodontitis for signs of anemia. J Periodontol. 2010;81:1202–6. doi: 10.1902/jop.2010.100079. [DOI] [PubMed] [Google Scholar]
- 2.DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. BMJ. 1993;306:688–91. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowe GD. The relationship between infection, inflammation, and cardiovascular disease: An overview. Ann Periodontol. 2001;6:1–8. doi: 10.1902/annals.2001.6.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Mealey BL, Klokkevold PR. Periodontal medicine. In: Newman MG, Takei HH, Carranza FA, editors. Carranza's Clinical Periodontology. 9th ed. Philadelphia: Saunders; 2002. pp. 229–44. [Google Scholar]
- 5.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 6.Faquin WC, Schneider TJ, Goldberg MA. Effect of inflammatory cytokines on hypoxia-induced erythropoietin production. Blood. 1992;79:1987–94. [PubMed] [Google Scholar]
- 7.Geoffrey BIHL. Anemia of chronic disease. CME. 2008;26:238–40. [Google Scholar]
- 8.Means RT, Jr, Krantz SB. Progress in understanding the pathogenesis of the anemia of chronic disease. Blood. 1992;80:1639–47. [PubMed] [Google Scholar]
- 9.Swaak A. Anemia of chronic disease in patients with rheumatoid arthritis: Aspects of prevalence, outcome, diagnosis, and the effect of treatment on disease activity. J Rheumatol. 2006;33:1467–8. [PubMed] [Google Scholar]
- 10.Agarwal N, Kumar VS, Gujjari SA. Effect of periodontal therapy on hemoglobin and erythrocyte levels in chronic generalized periodontitis patients: An interventional study. J Indian Soc Periodontol. 2009;13:6–11. doi: 10.4103/0972-124X.51887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pradeep AR, Anuj S. Anemia of chronic disease and chronic periodontitis: Does periodontal therapy have an effect on anemic status? J Periodontol. 2011;82:388–94. doi: 10.1902/jop.2010.100336. [DOI] [PubMed] [Google Scholar]
- 12.Lainson PA, Brady PP, Fraleigh CM. Anemia, a systemic cause of periodontal disease? J Periodontol. 1968;39:35–8. doi: 10.1902/jop.1968.39.1.35. [DOI] [PubMed] [Google Scholar]
- 13.Hutter JW, van der Velden U, Varoufaki A, Huffels RA, Hoek FJ, Loos BG. Lower numbers of erythrocytes and lower levels of hemoglobin in periodontitis patients compared to control subjects. J Clin Periodontol. 2001;28:930–6. doi: 10.1034/j.1600-051x.2001.028010930.x. [DOI] [PubMed] [Google Scholar]
- 14.Seigel EH. Total erythrocyte, leucocyte and differential white cell counts of blood in chronic periodontal disease. J Dent Res. 1945;24:270. [PubMed] [Google Scholar]
- 15.Wakai K, Kawamura T, Umemura O, Hara Y, Machida J, Anno T, et al. Associations of medical status and physical fitness with periodontal disease. J Clin Periodontol. 1999;26:664–72. doi: 10.1034/j.1600-051x.1999.261006.x. [DOI] [PubMed] [Google Scholar]
- 16.Kowolik MJ, Dowsett SA, Rodriguez J, De La Rosa RM, Eckert GJ. Systemic neutrophil response resulting from dental plaque accumulation. J Periodontol. 2001;72:146–51. doi: 10.1902/jop.2001.72.2.146. [DOI] [PubMed] [Google Scholar]
- 17.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(11 Suppl):2106–15. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto T, Tsuneishi M, Furuta M, Ekuni D, Morita M, Hirata Y. Relationship between decrease of erythrocyte count and progression of periodontal disease in a rural Japanese population. J Periodontol. 2011;82:106–13. doi: 10.1902/jop.2010.100211. [DOI] [PubMed] [Google Scholar]
- 19.Pejcic A, Kesic L, Pesic Z, Mirkovic D, Stojanovic M. White blood cell count in different stages of chronic periodontitis. Acta Clin Croat. 2011;50:159–67. [PubMed] [Google Scholar]
- 20.Ali S. The correlation between hemoglobin level and generalized moderate chronic periodontitis. Oral Maxillofac Surg Periodontol. 2012;24:85–8. [Google Scholar]


