Abstract
All episodes of Clostridium difficile associated diarrhea (CDAD) diagnosed in a defined population of 274,000 including one tertiary and two primary hospitals and their catchment areas were studied during 12 months. The annual CDAD incidence in the county was 97 primary episodes per 100,000, and 78% of all episodes were classified as hospital associated with a mean incidence of 5.3 (range, 1.4 to 6.5) primary episodes per 1,000 admissions. The incidence among hospitalized individuals was 1,300-fold higher than that in the community (33,700 versus 25 primary episodes per 100,000 persons per year), reflecting a 37-fold difference in antibiotic consumption (477 versus 13 defined daily doses [DDD]/1,000 persons/day) and other risk factors. Three tertiary hospital wards with the highest incidence (13 to 36 per 1,000) had CDAD patients of high age (median age of 80 years versus 70 years for other wards, P < 0.001), long hospital stay (up to 25 days versus 4 days), or a high antibiotic consumption rate (up to 2,427 versus 421 DDD/1,000 bed days). PCR ribotyping of C. difficile isolates available from 330 of 372 CDAD episodes indicated nosocomial acquisition of the strain in 17 to 27% of hospital-associated cases, depending on the time interval between index and secondary cases allowed (2 months or up to 12 months), and only 10% of recurrences were due to a new strain of C. difficile (apparent reinfection). In other words, most primary and recurring episodes were apparently caused by the patient's endogenous strain rather than by one of hospital origin. Typing also indicated that a majority of C. difficile strains belonged to international serotypes, and the distribution of types was similar within and outside hospitals and in primary and relapsing CDAD. However, type SE17 was an exception, comprising 22% of hospital isolates compared to 6% of community isolates (P = 0.008) and causing many minor clusters and a silent nosocomial outbreak including 36 to 44% of the CDAD episodes in the three high-incidence wards.
Toxin-producing Clostridium difficile contributes to many cases of antibiotic-associated diarrhea (AAD). Its fecal colonization rate in healthy individuals ranges up to 3% (1, 5, 48) and is markedly increased in the hospital environment due to transmission of the organism and frequent disruption of the patient colonic flora by antibiotics or by therapeutic interventions affecting the gut flora (3, 46). Since the 1980s the incidence of C. difficile-associated diarrhea (CDAD) has increased dramatically, presumably due to the introduction of new antibiotics prone to select this organism. A study in the United States based on national hospital discharge data from 1980 to 1994 showed an almost 10-fold increase of age-adjusted death rates due to “non-classical diarrhea pathogens” apparently dominated by C. difficile (19). In Sweden, about 50 cases of CDAD per 100,000 inhabitants were diagnosed in 1995, outnumbering all other etiologically diagnosed domestic cases of diarrhea taken together (27). A variety of typing methods have been used to study C. difficile epidemiology in hospitals, and mainly epidemic (10, 11) but occasional endemic (17) situations have been reported. In recent years PCR ribotyping has become established as a highly reproducible reference method for the typing of C. difficile isolates. In a unique nationwide study from the United Kingdom including over 1,000 isolates, one PCR ribotype (UK1) predominated and comprised 55% of hospital isolates compared to only 7.5% of community isolates, indicating an extensive nosocomial clonal spread of UK1 (49). The molecular epidemiology of C. difficile in Sweden is sparsely known, and comprehensive studies of CDAD epidemiology including typing of isolates in a large defined geographical area have not been reported. We therefore studied all CDAD patients and applied PCR ribotyping to a majority of C. difficile isolates of hospital or community origin in Örebro County in Sweden during 1 year of apparently normal endemic conditions.
MATERIALS AND METHODS
Study population and material.
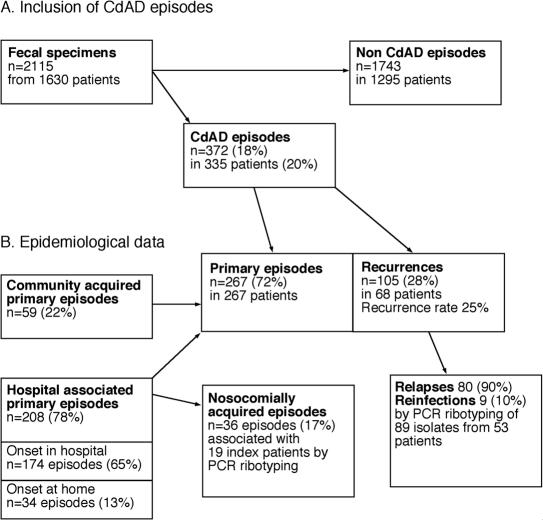
We studied all diagnosed CDAD cases in Örebro County, located in central Sweden, from February 1999 through January 2000. The county had 274,000 inhabitants served by Örebro University Hospital (ÖUH), Karlskoga City Hospital (KCH), and Lindesberg City Hospital (LCH); ÖUH is the largest with 668 beds and a catchment area population of 171,000 (Table 1). A total of 335 patients, contributing a total of 372 CDAD, episodes were investigated (Fig. 1).
TABLE 1.
Population studied and occurrence of CDAD in Örebro County during 12 months
| Characteristica | Value for groupb
|
|||
|---|---|---|---|---|
| ÖUH | LCH | KCH | County | |
| Study population data | ||||
| Inhabitants | 171,000 | 47,000 | 55,000 | 274,000 |
| Hospital beds | 668 | 117 | 126 | 911 |
| Hospital admissions | 26,560 | 5,920 | 6,906 | 39,386 |
| Occupied bed days | 186,013 | 38,315 | 40,451 | 264,779 |
| Hospital-associated episodes | ||||
| Primary episodes (incidencec) | 172 (6.5) | 26 (4.4) | 10 (1.4) | 208 (5.3) |
| Recurrences (% of episodes) | 64 (27) | 5 (16) | 4 (29) | 73 (26) |
| Patients with recurrences (%) | 41 (24) | 3 (11) | 2 (20) | 46 (22) |
| Total episodes (incidencec) | 236 (8.9) | 31 (5.2) | 14 (2.0) | 281 (7.1) |
| Primary CDAD episodes | ||||
| Community acquired (%, incidenced) | 37 (14, 8) | 11 (4, 27) | 11 (4, 23) | 59 (22, 25) |
| Hospital associated | ||||
| Onset in hospital (%) | 145 (54) | 24 (9) | 5 (2) | 174 (65) |
| Onset outside hospital (%) | 27 (10) | 2 (1) | 5 (2) | 34 (13) |
| All (%, incidenced) | 172 (64; 33,748) | 26 (10; 26,995) | 10 (4; 9,023) | 208 (78; 28,670) |
| Total episodes (%) | 209 (78) | 37 (14) | 21 (8) | 267 (100) |
| Nosocomially acquired episodese | ||||
| 2 months (%) | 35 (20, 17) | 1 (4, 3) | 0 | 36 (17, 13) |
| 12 months (%) | 52 (30, 25) | 4 (15, 11) | 0 | 56 (27, 21) |
| All CDAD episodes | ||||
| Primary episodes (incidenced) | 209 (122) | 37 (78) | 21 (40) | 267 (97) |
| Recurrences (% of episodes) | 88 (30) | 11 (23) | 6 (22) | 105 (28) |
| Patients with recurrence (%) | 57 (27) | 7 (19) | 4 (19) | 68 (25) |
| Total episodes (incidenced) | 297 (170) | 48 (100) | 27 (50) | 372 (135) |
For definitions see Materials and Methods.
Values are for the hospitals and their respective catchment areas, which together comprise Örebro county. All incidences were higher for ÖUH and its catchment area than for LCH and KCH (P = 0.001) (see text).
Per 1,000 admissions.
Per 100,000/year. For community-acquired primary CDAD episodes and all episodes, the incidence was given per number of inhabitants minus those who were in hospital. For hospital-associated primary episodes, the incidence was given per 100,000 bed days (beds × 365 × 100,000).
Rate for 2- or up to 12-month interval allowed between index and secondary cases. The nosocomial acquisition rate is given as proportion of the primary hospital-associated episodes (first number) and of all episodes (second number) in the catchment area.
FIG. 1.
Flowchart of the study (A) and epidemiological definitions (B) applied. In the 372 CDAD episodes diagnosed, C. difficile toxin and/or a toxin-producing strain were found in feces (see Materials and Methods). A patient with a primary episode had no prior CDAD episode within 12 months, and recurrence was defined as an episode starting 2 weeks or more after the end of the primary one. Hospital-associated primary episodes had onset in hospital or within 2 months after discharge from hospital. Other primary episodes were defined as community acquired. A CDAD episode was defined as nosocomially acquired when due to the same PCR ribotype and occurring in a patient cared for in the same ward as a previous (index) CDAD patient within 2 months (or 12 months; see text). A recurrence was defined as apparent relapse when the isolate had the same PCR ribotype as the primary isolate. Other recurrences were classified as apparent reinfections.
Definitions.
CDAD was defined as diarrhea plus presence of toxin-positive C. difficile or its toxin in feces. A patient with a primary episode had no history of CDAD within 12 months prior to this episode. A new episode of CDAD with onset 2 weeks or more after the end of the primary episode was classified as recurrence. Relapse was defined as recurrence due to the same genotype as we found in the initial episode, and reinfection was defined as recurrence due to a new type. Hospital-associated CDAD was epidemiologically defined as an episode with onset during hospitalization or within 60 days after hospital stay, whereas patients with community-acquired CDAD had no history of recent hospitalization (56). This definition turned out to be corroborated by data for patients infected by PCR ribotype SE17, a C. difficile strain typical of ÖUH (see Results). CDAD in patients hospitalized in the same ward either within 2 months or at any time during the 12-month study period and excreting C. difficile of an identical PCR ribotype as a previous CDAD patient in the ward was regarded to reflect nosocomial acquisition. The first patient in each cluster was defined as the index case, and the subsequent ones were defined as secondary cases. The nosocomial acquisition rate was given as the proportion (percentage) of secondary cases among all primary hospital-associated episodes of CDAD. PCR ribotypes that were found in at least seven episodes (≥3% of all episodes) were classified as major types. Hospital wards with a CDAD incidence of more than 10 primary episodes per 1,000 admissions were defined as high-incidence wards. Mortality was noted through the study period and followed for 6 months past the last included patient. Antibiotic consumption data were obtained from the Swedish nationwide drug distribution system (Apoteket AB). Each defined daily dose (DDD) represents the average maintenance dose per day for a drug used for its main medical indication as defined by the World Health Organization (www.whocc.no/atcddd/).
Fecal toxin test and culture.
All stools taken for C. difficile diagnostics were processed at the county laboratory at ÖUH. Fecal samples were suspended in sterile water and membrane filtered (0.45-μm-pore-size filter; Millipore). Filtrate was added over a layer of cultured McCoy cells, followed by incubation at 37°C. After 24 and 48 h the cells were examined microscopically, and fecal filtrates causing abnormal morphology of at least 50% of the cells compared with 0% in an antitoxin antibody neutralization control were considered C. difficile toxin positive. All fecal samples were also cultured for C. difficile on cycloserine-cefoxitin-fructose agar at 37°C for 48 h in an anaerobic chamber (ASSAB Whitley type MK II; Shipley, United Kingdom) having an atmosphere of 10% H2, 5% CO2, and 85% N2, and 99% of the toxin-positive ones yielded C. difficile. Only three positive specimens thus failed to yield a viable C. difficile strain but were still regarded as positives in the epidemiological evaluation. Of the toxin-negative specimens, 23 yielded C. difficile cells that after a 48-h incubation in anaerobic chopped meat broth (Huddinge Hospital, Huddinge, Sweden) were positive for toxin in the cell test. From the 372 toxin-positive isolates obtained (see Results), 42 were lost, mainly due to displacement of a rack during a change of freezers but also through an error during transportation between laboratories, yielding 330 C. difficile isolates for molecular study. The species of each isolate was checked by a slide test (MicroScreen; Microgen Bioproducts, Camberley, Surrey, United Kingdom) before storage at −70°C in Trypticase soy broth (BBL Pharmaceuticals) containing yeast extract and horse serum. The presence of C. difficile was further verified by the fact that 100% of the isolates were typeable and yielded expected PCR ribotype patterns.
PCR ribotyping of C. difficile. (i) Sample preparation.
Isolates were grown in 2 ml of peptone yeast broth for 24 h and harvested by centrifugation. DNA templates were prepared by suspending the pellet in 0.2 ml of 10% Chelex-100 in water and boiling for 10 min prior to the PCR.
(ii) PCR.
PCR amplification of target DNA interspaced between the multiple pairs of genes encoding the 16S and 23S ribosomal RNAs was performed as described by Stubbs et al. (49) but by using premade Ready-To-Go PCR tubes (Amersham Pharmacia Biotech). The primers used were 5′-CTGGGGTGAAGTCGTAACAAGG-3′ and 5′-GCGCCCTTTGTAGCTTGACC-3′. PCR was performed by using a PTC-200 machine (MJ Research Inc., Watertown, Mass.) and a thermal cycling procedure with initial denaturation at 94°C for 60 s, 55°C for 60 s, and 72°C for 60 s, repeated for 30 cycles, and ending with a final cycle of 72°C for 7 min, followed by cooling to 4°C.
(iii) Analysis of PCR products.
PCR products were separated by electrophoresis by using precast polyacrylamide gels (GeneGel Excel 12.5/24; Amersham Biosciences, Piscataway, N.J.) and were stained with silver (Amersham). A set of DNA markers of up to a size of 100 bp (Amersham Biosciences) was included in every fifth lane, and two reference strains of serotype A and serotype C from the Culture Collection of the University of Gothenburg (CCUG 37779 and CCUG 37766, respectively) were included as controls in each gel.
(iv) Analysis of banding patterns.
The same technician blinded for the epidemiological data performed image and pattern analysis. We used Molecular Analyst Fingerprinting Plus 1.6 software (Bio-Rad; the software is identical to Gel Compar of Applied Maths) and the unweighted pair group method using arithmetic averages for cluster correlation. The use of silver staining revealed sharper and additional DNA bands and thus yielded a higher discriminatory power (additional types) compared to the standard ethidium bromide staining according to Stubbs et al. (49) For this reason we used our own numbering (prefix SE, indicating Sweden) to label the PCR ribotypes. The clustering of banding patterns obtained was rechecked visually, and each unique pattern was given a group number (SE type), plus occasionally a suffix indicating a closely related but yet distinct PCR ribotype.
(v) Serotype reference strains.
For comparison with the clinical isolates, a set of 22 available C. difficile reference strains (CCUG 37766 through CCUG 37787 representing serogroups C, A2, A3, A4, A5, A6, A7, A8, A9, A10, S1, S3, S4, A, B, D, F, G, H, I, K, and X) were PCR ribotyped by the same technique.
Statistics.
The Chi-square test, Fisher's exact test, or the Mann-Whitney U test was used when appropriate.
RESULTS
Fecal specimens, patients, and CDAD episodes.
During the 12 months of the study period, a total of 2,115 fecal specimens from 1,630 patients with suspected primary or recurrent CDAD were sent to the laboratory for diagnostics (Fig. 1). These accounted for 42% of all fecal specimens from patients with diarrhea showing the expected dominance of C. difficile as etiological agent. Toxin was found in 349 specimens, and 23 further specimens were culture positive for a toxin-producing strain. An additional 22 specimens were culture positive with a toxin-negative strain but were classified as a non-CDAD episode and excluded from further analysis. Thus, 372 CDAD episodes were diagnosed in 335 patients, or in 20% of those with suspected CDAD (Fig. 1). Of the CDAD patients 55% were female, and the median age was 74 years, with a lower median age among patients with community-acquired disease (median, 64 years; range 1 to 92 years) than among patients with hospital-associated cases (median, 76 years; range 2 to 93 years) (P < 0.001). The 335 patients included 267 with only a primary episode of CDAD and 68 (25%) who experienced a total of 105 recurrences (range, 1 to 5 per patient).
Epidemiological data.
A total of 372 diagnosed episodes of CDAD corresponded to an annual incidence of 135 per 100,000 in the population of Örebro County, including 97 primary episodes per 100,000 (Table 1). The episodes were evenly distributed over the year except for a peak in March (30% over the mean incidence) for both hospital-associated and community-acquired CDAD concomitantly with maximum antibiotic usage both in hospitals and in the community (data not shown). The ÖUH catchment area had a higher incidence of both primary episodes and total episodes (122 and 170 per 100 000, respectively) than the LCH area (78 and 100 per 100,000, respectively; P = 0.01 and 0.001, respectively) and the KCH area (40 and 50 per 100 000, respectively; P = 0.001). Of all primary CDAD episodes in the county, 78% were hospital associated, with onset in hospital (65%) or within 60 days after discharge (13%) (Fig. 1 and Table 1). The rate of hospital-associated primary episodes of CDAD was higher in ÖUH than in LCH and KCH (6.5 versus 0.4.4 and 1.4 per 1,000 admissions, respectively; P = 0.006 and P < 0.002, respectively) (Table 1). Consequently, the proportion of hospital-associated episodes was higher in the ÖUH catchment area (82%) than in the LCH and KCH catchment areas (70 and 47%) (P = 0.001 and P = 0.001, respectively), again emphasizing the role of a large tertiary hospital for the overall CDAD incidence in the county. Among the 33 ÖUH wards, CDAD was diagnosed in only 14 wards (42%). Calculated per patient and year, the incidence of hospital-associated primary episodes at ÖUH was 0.34 or 1,300-fold higher than the incidence outsite the hospital in the community (33,700 versus 25 per 100,000) (Table 1).
Antibiotic consumption.
Although 91% of the DDDs of antibiotics was used in the community, the per capita consumption was 37-fold higher in the hospitals (477 versus 13 DDD per 1,000 bed days). In 98% of the CDAD episodes studied, there was a history of prior antibiotic treatment dominated by cephalosporins (23%), isoxazolyl penicillins (22%), and clindamycin (20%). The usage of these agents was higher in one of the high-incidence wards (infectious diseases) than in other wards (see below and Table 4).
TABLE 4.
CDAD rates and other characteristics of the three high-incidence wards and other selected wards in ÖUH
| ÖUH ward | Primary CDAD ratea | Median stay (days) | Median age (years, range)
|
Antibiotic usage (DDD1000)b
|
||||
|---|---|---|---|---|---|---|---|---|
| CDAD patients | All patients | CLIN | CEP | ISO | Total | |||
| Hematology | 4 | 8 | 73 (66-84) | 78 (20-100) | 12 | 91 | 39 | 607 |
| Oncology | 6 | 6 | 74 (50-81) | 64 (23-98) | 4 | 138 | 10 | 498 |
| Nephrology | 13 | 7 | 82 (57-96) | 70 (19-97) | 8 | 44 | 63 | 316 |
| Infectious diseases | 19c | 6 | 78 (31-92) | 68 (2-95) | 89 | 314 | 387 | 2,427 |
| Geriatrics | 36 | 25 | 78 (64-86) | 80 (60-100) | 8 | 15 | 100 | 336 |
| Other wards (n = 28) | 3.1 | 4 | 70 (1-79) | 72 (1-94) | 7 | 62 | 64 | 421 |
| Total | 6.5 | 5 | 74 (2-96) | 73 (1-100) | 9 | 75 | 72 | 477 |
Number of hospital-associated CDAD cases per 1,000 admissions.
Antibiotic consumption is given as DDD per 1,000 bed days. CLIN, clindamycin; CEP, cephalosporins; ISO, isoxazolyl penicillins.
Excluding six cases referred to the ward of infectious diseases with CDAD already diagnosed.
PCR ribotypes of C. difficile and their epidemiology.
A total of 241 isolates of C. difficile from 241 patients with primary CDAD plus 89 isolates from 54 patients with recurrence(s) were available for PCR ribotyping (Fig. 1). These 330 isolates yielded 53 distinct PCR ribotypes (Table 2). No seasonal variation of PCR ribotypes was noted (data not shown). Except for type SE17 (see also below) the pattern of PCR ribotypes of C. difficile was similar within and outside of the hospitals. Nine major ribotypes, each associated with 7 to 65 episodes, comprised 67% of the isolates from hospital-associated cases compared to 59% of those causing community-acquired disease and were found in 65% of both primary and recurring CDAD episodes (Table 2). The major types were, in ranking order, SE17, SE21, SE21b, SE12, SE20, and SE16 and were identical to those of the reference strains for serotypes C, H, A8, A2, G, and A, respectively, followed by SE25, SE30, and SE7 with an unknown relation to C. difficile serotypes. The major ribotypes SE17, SE21, and the closely related SE21b were the most frequent ones and comprised 38% of isolates from primary episodes and 37% of those from all CDAD episodes (Table 2).
TABLE 2.
PCR ribotypes of C. difficile isolates from 330 episodes of CDAD in Örebro County during 12 months
| PCR ribotypea | Serotype matchb | No. of episodes (% of isolates [n = 330])
|
||||||
|---|---|---|---|---|---|---|---|---|
| Primary
|
Recurrencesc | All | ||||||
| Hospital-associated
|
Community-acquired | All | ||||||
| Hospital onset | Community onset | All | ||||||
| Major types | ||||||||
| SE7 | 4 | 0 | 4 | 3 | 7 | 4 | 11 | |
| SE12 | A2 | 9 | 1 | 10 | 5 | 15 | 9 | 24 |
| SE16 | A | 5 | 3 | 8 | 3 | 11 | 1 | 12 |
| SE17 | C | 33 (21) | 9 (29) | 42 (22)f | 3 (6) | 45 (19) | 20 (22) | 65 (20) |
| SE20 | G | 9 | 3 | 12 | 3 | 15 | 8 | 23 |
| SE21 | H | 16 | 3 | 19 | 6 | 25 | 6 | 31 |
| SE21b | A8 | 18 | 1 | 19 | 3 | 22 | 3 | 25 |
| SE25 | 7 | 1 | 8 | 2 | 10 | 6 | 16 | |
| SE30 | 5 | 0 | 5 | 2 | 7 | 1 | 8 | |
| All (n = 9) | 106 (67) | 21 (68) | 127 (67) | 30 (59) | 157 (65) | 58 (65) | 215 (65) | |
| Minor types (n = 23)d | 44 | 7 | 51 | 14 | 65 | 28 | 93 | |
| Unique types (n = 21)e | 9 (6) | 3 (9) | 12 (6) | 7 (14) | 19 (8) | 3 (3) | 22 (6) | |
| All types (n = 53) | 159 (100) | 31 (100) | 190 (100) | 51 (100) | 241 (100) | 89 (100) | 330 (100)g | |
Current Swedish designations. Major types were each found in 7 to 65 episodes.
Identity between the PCR ribotype of a C. difficile serotype reference strain and that of a major SE type. The PCR ribotype of other major SE types was distinctly different from that of each of the 22 serotype reference strains tested (see Materials and Methods). None of the isolates belonged to the PCR ribotype known to be toxin A-B+.
Apparent relapse or apparent reinfection (see Table 5).
Each type found in two to six episodes.
Each type found in only one episode.
Value statistically different from the value in the next column at a P value of 0.008.
Isolates for typing were not available in 42 episodes.
SE17 (serotype C) was the most common ribotype and the only hospital-associated one (see below) found in 22% of hospital-associated primary episodes, including 29% of those with onset after discharge, compared to its detection in only 6% of community-acquired episodes (P = 0.008). Of the SE17 isolates 93% were from episodes classified as hospital associated compared to 76% for the other eight major types together (P = 0.01), again reflecting that SE17, in contrast to other types, was predominantly a nosocomial problem. SE17 tended to be more frequent in ÖUH and comprised 23% of the isolates from hospital-associated CDAD compared to 11 and 0% in LCH and KCH, respectively (P = 0.07). C. difficile isolates having a unique PCR ribotype, i.e., those found in only one episode each (n = 21), comprised 14% of the isolates from the community setting compared to 6% of the hospital-associated CDAD isolates (P = 0.19).
Nosocomial acquisition.
According to our narrower definition, 36 of 208 cases of hospital-associated primary CDAD (17%) and 13% of all primary CDAD cases in the county were nosocomially acquired, i.e., due to a C. difficile PCR ribotype identical to that obtained from a fellow patient cared for in the same ward within the preceding 2 months (number of index patients or episodes, 19) (Fig. 1 and Table 1). The corresponding putative CDAD acquisition rates in ÖUH and its catchment area were 20 and 17% compared to only 4 and 3%, respectively, in LCH and 0% in KCH (P = 0.04). Extending the time span allowed for a possible nosocomial relationship from 2 months to up to 12 months increased the number of secondary cases and, thus, the apparent hospital acquisition rates by 1.5- to 3.8-fold (Table 1). With this broader definition 30% of the primary episodes in ÖUH, 27% in all hospitals, and 21% of all primary CDAD episodes diagnosed in the county were nosocomially acquired (Table 1).
The 36 patients with nosocomially acquired CDAD within the 2 months mentioned above carried eight different ribotypes and were mainly confined to ÖUH (94%), with major transmission (75% of cases; see below) in two wards. We found ribotype SE17 in 19 of these 36 patients (53%). All secondary type SE17 cases occurred in ÖUH wards and were related to 7 of the 19 index cases (Table 3), giving a mean of 2.6 (range, 1 to 7) apparent secondary cases per index patient. Seven additional ribotypes were associated with 11 minor clusters of nosocomial spread involving on average only 1.2 (range, 1 to 4) secondary cases per index case (Table 3). The nephrology ward in ÖUH contributed 9 out of the 36 secondary cases (25%), dominated by a cluster of 7 SE17 cases over 6 months apparently related to one index patient. The ward of infectious diseases at UHÖ presented 9 index cases that contributed 18 secondary cases or 2.0 (range, 1 to 3) per index case, evenly distributed over the study period and involving six different PCR ribotypes. The remaining five ÖUH wards with secondary cases had only one to two clusters each. Only two secondary cases of CDAD were identified outside ÖUH, one in LCH and another in a nursing home (Table 3).
TABLE 3.
PCR ribotypes of C. difficile and their epidemiology in primary episodes of CDAD indentified in Örebro County during 12 months and classified as nosocomially acquired
| Hospital warda | No. of patients per PCR ribotype (no. of index cases)b
|
Total no. of cases | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SE7b | SE11 | SE12 | SE17 | SE20 | SE21 | SE21b | SE23a | All | ||
| ÖUH | ||||||||||
| Lung diseases | 1 (1) | 1 (1) | 2 | |||||||
| Hematology | 1 (1) | 1 (1) | 2 | |||||||
| Geriatrics | 2 (2) | 2 (2) | 4 | |||||||
| Nephrology | 2 (1) | 7 (1) | 9 (2) | 11 | ||||||
| Infectious diseases | 2 (2) | 8 (3) | 2 (1) | 2 (1) | 2 (1) | 2 (1) | 18 (9) | 27 | ||
| ICU | 1 (1) | 1 (1) | 2 | |||||||
| Orthopedics | 2 (1) | 2 (1) | 3 | |||||||
| LH | 1 (1) | 1 (1) | 2 | |||||||
| Nursing home | 1 (1) | 1 (1) | 2 | |||||||
| All | 2 (1) | 1 (1) | 2 (2) | 19 (7) | 2 (1) | 3 (2) | 4 (3) | 3 (2) | 36 (19) | 55 |
The wards of geriatrics, nephrology, and infectious diseases were classified as high-incidence wards (see the text).
For designations of ribotypes, see Materials and Methods and Table 1. The first patient in each cluster of CDAD episodes in a ward due to the same PCR ribotype of C. difficile was defined as the index case. CDAD in patients hospitalized on the same ward within 2 months after the index case and excreting C. difficile of an identical PCR ribotype as the index patient was here defined as nosocomially acquired (see the text).
Presumed index case plus secondary cases.
High-incidence wards.
The wards of geriatrics, infectious diseases (excluding six referred patients with CDAD already diagnosed), and nephrology had a CDAD incidence of 36, 19, and 13 hospital-associated primary episodes per 1,000 admissions, respectively, compared to a mean of 3.1 per 1,000 admissions and a median of 0 per 1,000 admissions for the other ÖUH wards (P < 0.001) (Table 4), and were thus defined as high-incidence wards. Each of these wards had two to nine clusters of apparent acquisition compared to one to two or usually no clusters in other wards (Table 3). Furthermore, the recurrence rate was 45% among the patients in the infectious diseases ward versus 19% for all the other studied CDAD patients at ÖUH (see below). The median age of the CDAD population in the high-incidence wards was higher than that of the other CDAD patients in ÖUH (78, 82, and 78 in the geriatrics, infectious diseases, and nephrology wards, respectively, versus 70 years; P < 0.001). Furthermore, the patients admitted to the geriatric ward had a higher median age than ÖUH patients in the other wards (80 versus 72 years, respectively, P < 0.001) and a much longer stay in the ward (median, 25 versus 4 days, respectively) (Table 4). Antibiotic consumption rates in the geriatrics and nephrology wards differed little from those of ÖUH in general, whereas the usage of drugs associated with increased CDAD risk, such as clindamycin, cephalosporins, and isoxazolyl penicillins, and the total consumption of antibiotics was 4- to 10-fold higher than average in the infectious diseases ward (Table 4).
SE17 was the major PCR ribotype among the isolates from patients with primary CDAD in the three high-incidence wards and comprised 39% of the C. difficile isolates (mean value; range, 36 to 44%) compared to only 14% of those from the other ÖUH wards with CDAD patients taken together (P < 0.001). Of the 31 hospital-associated primary episodes of C. difficile with onset after discharge (Fig. 1 and Table 2), 14 (45%) were associated with prior stay in the high-incidence wards and included all nine hospital-associated SE17 episodes with onset in the community (data not shown).
Taken together, 40% of the CDAD episodes diagnosed in the three high-incidence wards could be ascribed to PCR ribotype SE17, reflecting part of a hyperendemic CDAD situation apparently associated also with the high age of the patients, excessive length of hospital stay, or high antibiotic consumption.
Relapses and reinfections.
Of the 267 patients with primary CDAD, 68 (25%) experienced recurrence (median, 1; range, 1 to 5 per patient), and the 105 recurrences comprised 28% of all CDAD episodes diagnosed (Fig. 1 and Table 1). The recurrence rate was highest among CDAD patients in the ÖUH ward of infectious diseases (45%) (see above), followed by other patients at ÖUH (19%), patients at LCH and KCH (both 19%) (Table 1), and 17% of community-acquired cases. Isolates from 89 recurrences in 53 patients were available for PCR ribotyping, yielding 28 different PCR ribotypes.
PCR ribotyping showed that in 90% (80 of 89) of the recurrent episodes the isolate was of the same ribotype as the patient's initial one, indicating relapse, with a median time interval to relapse of 28 days (range, 14 to 264 days). Recurrence was thus due to a new strain and defined as an apparent new infection in only 10% of these episodes, with a median time to reinfection of 41 days (range, 14 to 122 days). Only one patient experienced both apparent relapse and apparent reinfection. The nine major types (see above) comprised 65% of the isolates associated with recurrence, compared to 65% of the isolates in primary episodes (Table 2). Similarly, the nosocomial type SE17 comprised 22% of the isolates from both primary episodes and recurrences (Table 2). Thus, the spectrum of ribotypes was the same in primary and recurring CDAD cases, supporting the notion that no particular strain(s) of C. difficile was especially prone to cause relapses or new infections. On the other hand, types SE16 and SE30 were relatively uncommon in apparent relapses (about 2% of relapse episodes compared to about 5% in primary CDAD) (Table 2). Multiple apparent relapses (2 to 5 per patient) occurred in 42% (23 of 54) of the patients with recurrences but, again, without association with any particular type (Table 5), although one patient had five apparent relapses due to type SE17 within 264 days after the primary episode.
TABLE 5.
Recurrent episodes of CDAD related to PCR ribotype and place of onset of disease during 12 months in Örebro county
| Recurrence PCR ribotypea | No. of patients | No. of patients with the following no. of recurrences
|
No. of episodesb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Hospital | Community | Relapse | Reinfection | Total | ||
| SE 1 | 2 | 2 | 2 | 2 | 2 | ||||||
| SE 7 | 1 | 1 | 1 | 1 | 4 | 4 | 4 | ||||
| SE 7b | 2 | 2 | 2 | 4 | 4 | 4 | |||||
| SE 11 | 1 | 1 | 1 | 2 | 2 | 2 | |||||
| SE 12 | 4 | 4 | 3 | 2 | 9 | 8 | 1 | 9 | |||
| SE 14 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | ||||
| SE 16d | 1 | 1 | 1 | 2 | 2 | 2 | |||||
| SE 17 | 8 | 8 | 7 | 3 | 1 | 1 | 20 | 19 | 1 | 20 | |
| SE 19 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | ||||
| SE 20 | 4 | 4 | 2 | 2 | 8 | 6 | 2 | 8 | |||
| SE 21 | 4 | 4 | 1 | 1 | 4 | 2 | 5 | 1 | 6 | ||
| SE 21b | 4 | 4 | 2 | 2 | 3 | 1 | 4 | ||||
| SE 22c | 1 | 1 | 1 | 2 | 2 | 2 | 2 | ||||
| SE 23a | 1 | 1 | 1 | 2 | 2 | 2 | |||||
| SE 25 | 4 | 4 | 1 | 1 | 4 | 2 | 6 | 6 | |||
| SE 26 | 2 | 2 | 2 | 2 | 2 | ||||||
| SE 41 | 1 | 1 | 1 | 2 | 2 | 2 | |||||
| Sporadicc | 10 | 10 | 8 | 2 | 8 | 2 | 10 | ||||
| Total | 53 | 53 | 23 | 10 | 1 | 1 | 75 | 14 | 80d | 9 | 89 |
See Materials and Methods and Fig. 1 for definitions. Hospital, hospital-associated CDAD; Community, community-acquired CDAD; Relapse, recurrence due to a PCR ribotype identical to that of the initial or previous episode; Reinfection, recurrence due to a new PCR ribotype.
PCR ribotypes associated with only one recurrence.
Apparent relapse rate, 90% (80 of 89 episodes).
Mortality.
The overall mortality among the CDAD patients during the 12-month study period plus 6-month follow-up was 13% (34 of 267), and mortality was higher in hospital-associated than in community-acquired cases (15 versus 4%, P = 0.01). The median age and proportion of males among these cases were 76 years and 53%, respectively, compared to 73 years and 44% males among survivors. The median time period from onset of the CDAD episode to death was 14 days (range, 1 to 180 days), and as many as 11 of these 34 patients died within a week of the CDAD diagnosis. Of those who died, 14 (41%) patients had severe concomitant disease (5 patients with cancer in the bladder or lung, 4 with uremia, 2 with chronic pulmonary disease, and 1 with inflammatory bowel disease). The cause of death was obscure in 11 patients, and only 2 patients had the diagnosis fulminant C. difficile-colitis as the cause of death; obstructive ileus was also present in 1 patient (data not shown). No specific PCR ribotype was associated with mortality (data not shown).
DISCUSSION
To our knowledge the present study represents the only published comprehensive epidemiological study of both hospital-associated and community-acquired CDAD in a large, defined population. Furthermore, the analysis was supported by culture of C. difficile and typing of isolates according to the recently developed reference method PCR ribotyping (49). Over the years many typing procedures for C. difficile have been used and evaluated, and PCR ribotyping has emerged as superior with regard to typeability, reproducibility, discriminatory power, stability, and epidemiological concordance (6, 8, 9, 11, 28, 32, 43, 47). Our data, therefore, can also be used for comparative studies. Many attempts have been made to clarify the epidemiology of C. difficile in selected hospital settings during an outbreak usually caused by a predominating type (12, 13, 37, 45), while few studies address the baseline of endemic disease in hospitals or in the community. A recent study claiming an endemic situation and using PCR ribotyping included only two geriatric wards and was focused on recolonization from the hospital environment (17). Other nonoutbreak reports focus on risk factors for CDAD without addressing patient and genotype epidemiology (33, 41, 44).
In Örebro County the incidence of CDAD has been stable during the past 10 years, with an incidence rate ranging from 281 to 352 cases per year (unpublished data). Thus, 335 cases diagnosed during the 12 months studied here did not indicate an outbreak situation. The overall incidence of 97 primary episodes per 100,000 in the population and 135 episodes per 100,000, including recurrences, was twice the average in a Swedish nationwide study from 1995 (27). Published CDAD incidences in resident populations are rare and often restricted to selected cohorts (15, 48), whereas a majority of the studies refer to hospitalized populations only, with incidence rates ranging from 10 to 30 cases per 1,000 admissions or discharges (1, 22, 30, 34, 41, 43-45). The importance of highly specialized hospital care for the size of the CDAD problem was emphasized by the fact that one large tertiary hospital, ÖUH, contributed 56% of all CDAD episodes in the county and had a total incidence of 8.9 episodes per 1,000 admissions, up to 4.7-fold higher than that of the smaller hospitals. In fact, the rate of CDAD per occupied bed day in ÖUH was 2,000-fold higher than that per person and day in the community, reflecting the combined impact of higher total antibiotic consumption, particularly of CDAD-prone agents, high age and lower immunity of patients, and high population density, and, thus, transmission of C. difficile. According to our definition 22% of the episodes were community acquired, similar to the number found in the previous Swedish study (28%) (27), leaving 78% classified as hospital-associated episodes, including 13% with onset after discharge from hospital. Only four published studies have addressed community-associated disease. Two found that CDAD was among the most common identified causes of diarrheal disease in the ambulatory setting (5.5 and 10.7%) (40, 42), whereas two others reported incidences of 7.7 and 12.2 cases per 100,000 in the community (21, 31) compared to 25 cases per 100,000 found here. A very low median age in a university catchment area population (37 [31] versus 64 years in the present study) and voluntary health care insurance system in contrast to the Swedish system covering all residents may have contributed to the differences found in CDAD incidences. Riley and colleagues (40) suggested that CDAD incidence was influenced by the diagnostic awareness of the clinician since an educational program for general practitioners increased the proportion of CDAD in community-acquired diarrhea from 2.6 to 10.7%. In our study, there were no striking differences between the departments and institutions with regard to the proportion of positive specimens among those sent for C. difficile diagnostics (ambulatory care, 25%; infectious diseases, 24%; internal medicine, 18%; and general surgery at ÖUH, 18%; KCH, 18%; and LHC, 23%), suggesting that diagnostic routines did not account for any major differences in CDAD incidence. The lowest positivity rate was found in community-acquired disease (13%). Since 98% of the health care system in Sweden is financed by the county council, there should be little or no cost constraints limiting the testing of patients with AAD for C. difficile toxin. Thus, liberal testing could contribute to the low toxin positivity rate in community-acquired AAD. Other possible explanations are that these patients generally have milder disease and lower toxin levels, thus increasing the risk of false-negative toxin tests, and that the proportion of CDAD cases among patients with AAD is truly lower outside compared to inside hospitals.
Risk factors for CDAD such as high age, long hospital stay, and extensive use of antibiotics and, particularly, certain drugs have been well documented by others (7) and were supported here, both by comparison of the high-incidence wards and other ÖUH wards and hospital-associated and community-acquired disease. For example, the antibiotic consumption rate among ÖUH patients was 477 DDD/1,000 bed days compared to 13 DDD per 1,000 inhabitants per day for the community county population, a 37-fold difference illustrating the role of antibiotic usage for the risk of CDAD. As for clindamycin, found to be a high-risk antibiotic for CDAD in many studies (23, 36, 51) and particularly for clindamycin-resistant C. difficile strains (25, 39), the usage of this drug was 26 DDD per 1,000 bed days in the high-incidence wards compared to 7.4 DDD in the other ÖUH wards and 0.2 DDD per 1,000 inhabitants per day in the community. Clindamycin usage could be one reason for the high prevalence of ribotype SE17 in these wards, since the SE17 isolates were uniformly resistant to clindamycin (unpublished data) due to carriage of the ermB gene (38).
An overall recurrence rate of 25% was similar to that in other published studies (18, 55). In contrast, only 10% of recurrences or 3% of all episodes were apparent reinfections as shown by typing of the isolates and apparently reflecting a low acquisition rate, compared to corresponding rates of about 50 and 10 to 15% of recurrences reported by others (4, 54). The low rate of apparent reinfection found here could explain also why only 42% of our patients had multiple recurrences compared to 62 to 64% in other reports (14, 18, 35). The overall apparent relapse rate in the present study (90% of 25%, or 22.5% of all episodes) was twice that in other studies (about 50% of 20%, or 10%) (2, 4, 54). This could indicate a greater impact of antibiotics disrupting the colonic microflora or host factors increasing the apparent relapse rate, such as diverticulosis (50) or poor immune response (29, 53) in our patients. An alternative explanation for a high apparent relapse rate and a low rate of apparent reinfections in our patients compared to other published studies could be ascertainment bias related to the definitions and typing methods used. For example, the superior reproducibility of PCR ribotyping could account for the high relapse/reinfection ratio among the recurrences. This was supported by the fact that while the ratio of relapse to reinfection was higher than usual, the overall recurrence rate was typical in the present study.
PCR typing indicated that 17 to 27% of all hospital-associated primary episodes of CDAD were due to strains isolated also from potential index cases. Thus, despite that CDAD mainly was a hospital-associated problem, a majority of the inpatients apparently fell ill due to their endogenous C. difficile strain rather than from a hospital-acquired one. This has been suggested also by others (16), although most authors emphasize nosocomial transmission as the factor underlying most cases of hospital-associated CDAD (13, 14, 22, 45). The low rate of nosocomial transmission of C. difficile strains including a low risk of apparent reinfection may have contributed to the relatively low CDAD incidence found in the present study.
Among the 33 wards at ÖUH, potential acquisition of C. difficile strains was observed in only 7 wards, and of these the 3 high-incidence wards (geriatrics, nephrology, and infectious diseases) contributed 80% (29 of 36) of all cases having a strain classified as nosocomially acquired. Unlike results found in other studies (15, 20, 26), the hematology and oncology wards had only an average CDAD rate (4 to 6 primary episodes/1,000 admissions versus 6.5 primary episodes/1,000 admissions for ÖUH) although it was higher than the median rate for ÖUH wards (0/1,000). In contrast, the rate was high in the nephrology and geriatrics wards (13 and 36, respectively, per 1,000 admissions) and apparently associated with the high age of the patients and very long hospital stays. The infectious diseases unit also had a high CDAD rate (19/1,000 admissions) even after exclusion of the referred cases (4/1,000). This rate was apparently due to heavy antibiotic usage, and, surprisingly, as many as nine clusters of nosocomial acquisition were observed in the ward despite its specially designed facilities and a staff trained in isolation precautions. A high acquisition rate of the type SE17 strain contributed to the high CDAD rate among the nephrology and infectious diseases patients, as this strain was found in 40% of the primary CDAD episodes in these wards compared to 18% of the other hospital-associated CDAD episodes and 6% of the community-acquired episodes. The bacterial factors underlying this local clonal spread of SE17, also observed during the past 4 years prior to the study (unpublished data), are unclear apart from its resistance to multiple antibiotics (38), making this strain a target for selection by drugs such as clindamycin.
In summary, this 12-month study pinpointed the total scope of CDAD morbidity and strain epidemiology in a defined population area, thus enabling comparison of the data with those from other geographical areas. It showed that CDAD remains a clinical problem in Örebro County, the risk being the highest in hospitals and particularly in certain wards characterized by the high age and prolonged hospital stay of the patients or by extensive use of antibiotics. Nevertheless, CDAD was usually due to endogenous infection and apparent relapse rather than to primary and recurring episodes of apparent exogenous origin. Apart from the type SE17 strain, no C. difficile strains with superior transmission ability were observed, and despite this silent localized outbreak, the overall CDAD rate observed was in the lower range. A more active attitude towards wards with a high disease incidence including reviews of antibiotic use (52) and hygienic interventions (24) could have lowered this incidence.
REFERENCES
- 1.Alfa, M. J., T. Du, and G. Beda. 1998. Survey of incidence of Clostridium difficile infection in Canadian hospitals and diagnostic approaches. J. Clin. Microbiol. 36:2076-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso, R., S. Gros, T. Pelaez, D. Garcia-de-Viedma, M. Rodriguez-Creixems, and E. Bouza. 2001. Molecular analysis of relapse vs re-infection in HIV-positive patients suffering from recurrent Clostridium difficile associated diarrhoea. J. Hosp. Infect. 48:86-92. [DOI] [PubMed] [Google Scholar]
- 3.Anand, A., and A. E. Glatt. 1993. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin. Infect. Dis. 17:109-113. [DOI] [PubMed] [Google Scholar]
- 4.Barbut, F., A. Richard, K. Hamadi, V. Chomette, B. Burghoffer, and J. C. Petit. 2000. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J. Clin. Microbiol. 38:2386-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett, J. G. 2002. Clinical practice. Antibiotic-associated diarrhea. N. Engl. J. Med. 346:334-339. [DOI] [PubMed] [Google Scholar]
- 6.Bidet, P., V. Lalande, B. Salauze, B. Burghoffer, V. Avesani, M. Delmee, A. Rossier, F. Barbut, and J. C. Petit. 2000. Comparison of PCR-ribotyping, arbitrarily primed PCR, and pulsed-field gel electrophoresis for typing Clostridium difficile. J. Clin. Microbiol. 38:2484-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bignardi, G. E. 1998. Risk factors for Clostridium difficile infection. J. Hosp. Infect. 40:1-15. [DOI] [PubMed] [Google Scholar]
- 8.Brazier, J. S. 1998. The epidemiology and typing of Clostridium difficile. J. Antimicrob. Chemother. 41(Suppl. C):47-57. [DOI] [PubMed] [Google Scholar]
- 9.Brazier, J. S. 2001. Typing of Clostridium difficile. Clin. Microbiol. Infect. 7:428-431. [DOI] [PubMed] [Google Scholar]
- 10.Brazier, J. S., T. C. Fitzgerald, I. Hosein, C. Cefai, N. Looker, M. Walker, A. C. Bushell, and P. Rooney. 1999. Screening for carriage and nosocomial acquisition of Clostridium difficile by culture: a study of 284 admissions of elderly patients to six general hospitals in Wales. J. Hosp. Infect. 43:317-319. [DOI] [PubMed] [Google Scholar]
- 11.Brazier, J. S., M. E. Mulligan, M. Delmee, S. Tabaqchali, et al. 1997. Preliminary findings of the international typing study on Clostridium difficile. Clin. Infect. Dis. 25(Suppl. 2):S199-S201. [DOI] [PubMed] [Google Scholar]
- 12.Brazier, J. S., G. L. O′Neill, and B. I. Duerden. 1997. Polymerase chain reaction ribotypes of Clostridium difficile in hospitals in England and Wales. Rev. Med. Microbiol. 8:S55-S56. [Google Scholar]
- 13.Cartmill, T. D., H. Panigrahi, M. A. Worsley, D. C. McCann, C. N. Nice, and E. Keith. 1994. Management and control of a large outbreak of diarrhoea due to Clostridium difficile. J. Hosp. Infect. 27:1-15. [DOI] [PubMed] [Google Scholar]
- 14.Clabots, C. R., S. Johnson, M. M. Olson, L. R. Peterson, and D. N. Gerding. 1992. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. J. Infect. Dis. 166:561-567. [DOI] [PubMed] [Google Scholar]
- 15.Cohen, S. H., Y. J. Tang, J. Muenzer, P. H. Gumerlock, and J. Silva, Jr. 1997. Isolation of various genotypes of Clostridium difficile from patients and the environment in an oncology ward. Clin. Infect. Dis. 24:889-893. [DOI] [PubMed] [Google Scholar]
- 16.Do, A. N., S. K. Fridkin, A. Yechouron, S. N. Banerjee, G. E. Killgore, A. M. Bourgault, M. Jolivet, and W. R. Jarvis. 1998. Risk factors for early recurrent Clostridium difficile-associated diarrhea. Clin. Infect. Dis. 26:954-959. [DOI] [PubMed] [Google Scholar]
- 17.Fawley, W. N., and M. H. Wilcox. 2001. Molecular epidemiology of endemic Clostridium difficile infection. Epidemiol. Infect. 126:343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fekety, R., L. V. McFarland, C. M. Surawicz, R. N. Greenberg, G. W. Elmer, and M. E. Mulligan. 1997. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin. Infect. Dis. 24:324-333. [DOI] [PubMed] [Google Scholar]
- 19.Frost, F., G. F. Craun, and R. L. Calderon. 1998. Increasing hospitalization and death possibly due to Clostridium difficile diarrheal disease. Emerg. Infect. Dis. 4:619-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heard, S. R., B. Wren, M. J. Barnett, J. M. Thomas, and S. Tabaqchali. 1988. Clostridium difficile infection in patients with haematological malignant disease. Risk factors, faecal toxins and pathogenic strains. Epidemiol. Infect. 100:63-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirschhorn, L. R., Y. Trnka, A. Onderdonk, M. L. Lee, and R. Platt. 1994. Epidemiology of community-acquired Clostridium difficile-associated diarrhea. J. Infect. Dis. 169:127-133. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, S., C. R. Clabots, F. V. Linn, M. M. Olson, L. R. Peterson, and D. N. Gerding. 1990. Nosocomial Clostridium difficile colonisation and disease. Lancet 336:97-100. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, S., and D. N. Gerding. 1998. Clostridium difficile-associated diarrhea. Clin. Infect. Dis. 26:1027-1034. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, S., D. N. Gerding, M. M. Olson, M. D. Weiler, R. A. Hughes, C. R. Clabots, and L. R. Peterson. 1990. Prospective, controlled study of vinyl glove use to interrupt Clostridium difficile nosocomial transmission. Am. J. Med. 88:137-140. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, S., M. H. Samore, K. A. Farrow, G. E. Killgore, F. C. Tenover, D. Lyras, J. I. Rood, P. DeGirolami, A. L. Baltch, M. E. Rafferty, S. M. Pear, and D. N. Gerding. 1999. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N. Engl. J. Med. 341:1645-1651. [DOI] [PubMed] [Google Scholar]
- 26.Kamthan, A. G., H. W. Bruckner, S. Z. Hirschman, and S. G. Agus. 1992. Clostridium difficile diarrhea induced by cancer chemotherapy. Arch. Intern. Med. 152:1715-1717. [PubMed] [Google Scholar]
- 27.Karlström, O., B. Fryklund, K. Tullus, L. G. Burman, et al. 1998. A prospective nationwide study of Clostridium difficile-associated diarrhea in Sweden. Clin. Infect. Dis. 26:141-145. [DOI] [PubMed] [Google Scholar]
- 28.Kato, H., H. Kita, T. Karasawa, T. Maegawa, Y. Koino, H. Takakuwa, T. Saikai, K. Kobayashi, T. Yamagishi, and S. Nakamura. 2001. Colonisation and transmission of Clostridium difficile in healthy individuals examined by PCR ribotyping and pulsed-field gel electrophoresis. J. Med. Microbiol. 50:720-727. [DOI] [PubMed] [Google Scholar]
- 29.Kyne, L., M. Warny, A. Qamar, and C. P. Kelly. 2001. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 357:189-193. [DOI] [PubMed] [Google Scholar]
- 30.Lai, K. K., Z. S. Melvin, M. J. Menard, H. R. Kotilainen, and S. Baker. 1997. Clostridium difficile-associated diarrhea: epidemiology, risk factors, and infection control. Infect. Control Hosp. Epidemiol. 18:628-632. [DOI] [PubMed] [Google Scholar]
- 31.Levy, D. G., A. Stergachis, L. V. McFarland, K. Van Vorst, D. J. Graham, E. S. Johnson, B. J. Park, D. Shatin, J. C. Clouse, and G. W. Elmer. 2000. Antibiotics and Clostridium difficile diarrhea in the ambulatory care setting. Clin. Ther. 22:91-102. [DOI] [PubMed] [Google Scholar]
- 32.Martirosian, G., S. Kuipers, H. Verbrugh, A. van Belkum, and F. Meisel-Mikolajczyk. 1995. PCR ribotyping and arbitrarily primed PCR for typing strains of Clostridium difficile from a Polish maternity hospital. J. Clin. Microbiol. 33:2016-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McFarland, L. V. 1995. Epidemiology of infectious and iatrogenic nosocomial diarrhea in a cohort of general medicine patients. Am. J. Infect. Control 23:295-305. [DOI] [PubMed] [Google Scholar]
- 34.McFarland, L. V., M. E. Mulligan, R. Y. Kwok, and W. E. Stamm. 1989. Nosocomial acquisition of Clostridium difficile infection. N. Engl. J. Med. 320:204-210. [DOI] [PubMed] [Google Scholar]
- 35.McFarland, L. V., C. M. Surawicz, M. Rubin, R. Fekety, G. W. Elmer, and R. N. Greenberg. 1999. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect. Control Hosp. Epidemiol. 20:43-50. [DOI] [PubMed] [Google Scholar]
- 36.Nath, S. K., S. Salama, D. Persaud, J. H. Thornley, I. Smith, G. Foster, and C. Rotstein. 1994. Drug risk factors associated with a sustained outbreak of Clostridium difficile diarrhea in a teaching hospital. Can. J. Infect. Dis. 5:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nath, S. K., J. H. Thornley, M. Kelly, B. Kucera, S. L. On, B. Holmes, and M. Costas. 1994. A sustained outbreak of Clostridium difficile in a general hospital: persistence of a toxigenic clone in four units. Infect. Control Hosp. Epidemiol. 15:382-389. [DOI] [PubMed] [Google Scholar]
- 38.Norén, T., Y. J. Tang-Feldman, S. H. Cohen, J. Silva, Jr., and P. Olcén. 2002. Clindamycin resistant strains of Clostridium difficile isolated from cases of C. difficile-associated diarrhea (CDAD) in a hospital in Sweden. Diagn. Microbiol. Infect. Dis. 42:149-151. [DOI] [PubMed] [Google Scholar]
- 39.Pear, S. M., T. H. Williamson, K. M. Bettin, D. N. Gerding, and J. N. Galgiani. 1994. Decrease in nosocomial Clostridium difficile-associated diarrhea by restricting clindamycin use. Ann. Intern. Med. 120:272-277. [DOI] [PubMed] [Google Scholar]
- 40.Riley, T. V., M. Cooper, B. Bell, and C. L. Golledge. 1995. Community-acquired Clostridium difficile-associated diarrhea. Clin. Infect. Dis. 20(Suppl. 2):S263-S265. [DOI] [PubMed] [Google Scholar]
- 41.Riley, T. V., G. L. O'Neill, R. A. Bowman, and C. L. Golledge. 1994. Clostridium difficile-associated diarrhoea: epidemiological data from Western Australia. Epidemiol. Infect. 113:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riley, T. V., F. Wetherall, J. Bowman, J. Mogyorosy, and C. L. Golledge. 1991. Diarrheal disease due to Clostridium difficile in general practice. Pathology 23:346-349. [DOI] [PubMed] [Google Scholar]
- 43.Samore, M. H., K. M. Bettin, P. C. DeGirolami, C. R. Clabots, D. N. Gerding, and A. W. Karchmer. 1994. Wide diversity of Clostridium difficile types at a tertiary referral hospital. J. Infect. Dis. 170:615-621. [DOI] [PubMed] [Google Scholar]
- 44.Samore, M. H., P. C. DeGirolami, A. Tlucko, D. A. Lichtenberg, Z. A. Melvin, and A. W. Karchmer. 1994. Clostridium difficile colonization and diarrhea at a tertiary care hospital. Clin. Infect. Dis. 18:181-187. [DOI] [PubMed] [Google Scholar]
- 45.Samore, M. H., L. Venkataraman, P. C. DeGirolami, R. D. Arbeit, and A. W. Karchmer. 1996. Clinical and molecular epidemiology of sporadic and clustered cases of nosocomial Clostridium difficile diarrhea. Am. J. Med. 100:32-40. [DOI] [PubMed] [Google Scholar]
- 46.Sharma, A. K., and F. E. Holder. 1998. Clostridium difficile diarrhea after use of tacrolimus following renal transplantation. Clin. Infect. Dis. 27:1540-1541. [DOI] [PubMed] [Google Scholar]
- 47.Silva, J., Jr., Y. J. Tang, and P. H. Gumerlock. 1994. Genotyping of Clostridium difficile isolates. J. Infect. Dis. 169:661-664. [DOI] [PubMed] [Google Scholar]
- 48.Simor, A. E., S. L. Yake, and K. Tsimidis. 1993. Infection due to Clostridium difficile among elderly residents of a long-term-care facility. Clin. Infect. Dis. 17:672-678. [DOI] [PubMed] [Google Scholar]
- 49.Stubbs, S. L. J., J. S. Brazier, G. L. O'Neill, and B. I. Duerden. 1999. PCR targeted to the 16S-23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J. Clin. Microbiol. 37:461-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tedesco, F. J., D. Gordon, and W. C. Fortson. 1985. Approach to patients with multiple relapses of antibiotic-associated pseudomembranous colitis. Am. J. Gastroenterol. 80:867-868. [PubMed] [Google Scholar]
- 51.Thibault, A., M. A. Miller, and C. Gaese. 1991. Risk factors for the development of Clostridium difficile-associated diarrhea during a hospital outbreak. Infect. Control Hosp. Epidemiol. 12:345-348. [DOI] [PubMed] [Google Scholar]
- 52.Thomas, C., M. Stevenson, D. J. Williamson, and T. V. Riley. 2002. Clostridium difficile-associated diarrhea: epidemiological data from Western Australia associated with a modified antibiotic policy. Clin. Infect. Dis. 35:1457-1462. [DOI] [PubMed] [Google Scholar]
- 53.Wilcox, M., and J. Minton. 2001. Role of antibody response in outcome of antibiotic-associated diarrhoea. Lancet 357:158-159. [DOI] [PubMed] [Google Scholar]
- 54.Wilcox, M. H., W. N. Fawley, C. D. Settle, and A. Davidson. 1998. Recurrence of symptoms in Clostridium difficile infection-relapse or reinfection? J. Hosp. Infect. 38:93-100. [DOI] [PubMed] [Google Scholar]
- 55.Wilcox, M. H., and R. C. Spencer. 1992. Clostridium difficile infection: responses, relapses and re-infections. J. Hosp. Infect. 22:85-92. [DOI] [PubMed] [Google Scholar]
- 56.Wullt, M., and M. H. Laurell. 1999. Low prevalence of nosocomial Clostridium difficile transmission, as determined by comparison of arbitrarily primed PCR and epidemiological data. J. Hosp. Infect. 43:265-273. [DOI] [PubMed] [Google Scholar]