Abstract
Background:
The clotting system abnormalities are the common complication in cancer patients. The aim of this retrospective study was to evaluate the coagulation state, clinical features, and treatment in cancer patients by routine tests.
Methods:
A total of 2328 patients with different types of cancer were classified as the positive group (n = 1419, including 53 patients with thrombosis) and the negative group (n = 909) based on D-dimer (DD) value. Of the 2328 cases, 354 were admitted for chemotherapy. Hemostasis test and complete blood count (CBC) were performed during treatment or following-up.
Results:
This study showed that the hypercoagulable state was affected not only by clinical staging (P < 0.0001) but also by metastasis site (P < 0.0001 for bone vs. lung). Compared to negative DD group, the higher fibrinogen level, the extended activated partial thromboplastin time, and prothrombin time interacted markedly with disease clinical stage (P < 0.05) in the positive group. Between positive DD groups with and without thrombus, the significantly statistic difference in white blood cell (WBC) and DD (P < 0.05) rather than in red blood cell (RBC) and platelet count was observed. However, the higher DD level was not correlated with WBC, RBC, and platelet count in the positive DD group. Furthermore, the hypercoagulable plasma profile in cancer patients was moderated 2–3 weeks after chemotherapy (P < 0.05 for first six cycles).
Conclusions:
The routine hemostatic parameters and CBC are valuable to assessment for thrombosis and chemotherapy even for disease prognosis.
Keywords: Cancer, Chemotherapy, Coagulation Disorder, Thrombosis
INTRODUCTION
The coagulation disorder is most common complication in patients with cancer, which presents as thromboembolic manifestations such as deep vein thrombosis (DVT) and pulmonary embolism (PE), intravascular disseminated coagulation (DIC), but mostly as abnormalities in the clotting system in the absence of clinical manifestations.[1,2] Postmortem studies have shown a markedly increased incidence of developing venous thromboembolism (VTE) in patients with cancer than in patients without malignancy.[2,3] It was also found that the diagnosis of cancer was much higher in patients presenting with idiopathic than in patients with secondary VTE. The risk for developing cancer significantly increases, particularly in the 1st year after the diagnosis of VTE.[4,5] The risk of thrombosis varies with different type of tumors, in which the tumors of the ovary, pancreas, and central nervous system were thought to be ranked on the top.[6,7,8] Many factors also contribute to the risk of VTE, including the primary tumor site, age, immobility, and type of therapeutic intervention.[3,9,10,11] Khorana et al. tried to establish a model to predict the chemotherapy-associated thrombosis. Unfortunately, this model can only identify patients with a 7% short-term risk of symptomatic VTE.[12]
In this study, we retrospectively investigated 2328 patients with cancer and systematically analyzed their clinical features, laboratory tests variable for hemostasis and peripheral hemogram, exploring the coagulable plasma profiles and evaluating the factors related to the occurrence of hypercoagulable and thrombotic events.
METHODS
Patients
This retrospective study was performed at the Changzhou Second People's Hospital. The study was approved by the Local Ethics Committee, which waived the need for informed written consent. The authors had access to identifying information during and after data collection. The patients (aged 16–90 years) with different types of cancer admitted for the adjuvant or palliative chemotherapy and the supportive treatment from 2011 to 2015 were recruited. The cancer was defined according to pathological findings. Other inclusion criteria were measurable disease by image examinations. Disease clinical staging (I-IV) depended on the systems recommended by the National Comprehensive Cancer Network for different types of cancer. Exclusion criteria were DIC, which was diagnosed on the parameters issued by the Chinese hematology society in 1999. The patients who were being treated with the medications (such as heprin or hemocoagulase) were also excluded. The patients with severe liver diseases, such as hepatocirrhosis, were not included except that with liver cancer.
Blood sampling and handling
Blood was sampled into sterile sodium citrate- or ethylenediaminetetraacetic acid- contained tubes (Greiner Bio-One), respectively, for analysis of hemostasis-related parameters and thrombin generation measurement and complete blood count (CBC). Blood was immediately centrifuged at room temperature for 3 min at 3500 g, and plasma was aliquoted. Routine hemostasis parameters were determined directly with immunoturbidimetry. Sysmex CA-8000 was used for blood coagulation determination. CBC was performed with Sysmex XE-2100 or XS-800i (Symex, Japan).
Routine clinical parameter
All routine hemostasis parameters such as prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen (FBG), thromobin time (TT), and D-dimer (DD) were determined using the commercial kits (Sysmex, Japan) including the corresponding normal and standard plasma. These parameters were tested before or 2–3 weeks after chemotherapy. The level of DD ≥0.55 mg/L was set as positive baseline.
Computed tomography (CT) and magnetic resonance imaging were used for disease clinical staging. The status of tumor metastasis was detected by emission CT and positron emission tomography-CT. The DVT and PE were diagnosed with color Doppler, spiral CT and venography, respectively.
Statistical analysis
All of patients were divided into different groups according to diseases clinical staging, metastasis sites, thrombosis, and routine coagulation parameters. The data related to interaction or correlation were dealt with two-way ANOVA or Monte Carlo. The unpaired samples were analyzed by t-test. A value of P < 0.05 was considered statistically significant. Graphpad Prism version 6 (GraphPad Software, Inc. USA) was used for the above data analysis.
RESULTS
A total of 2328 patients (1262 males and 1066 females) with different types of cancer were involved in this study, 1419 with positive DD value and other 909 with negative DD value. Of the 2328 patients, 354 accepted 2–8 cycles of chemotherapy. In the positive DD group, 53 patients were diagnosed as thrombosis, which constituted of 40 with VTE and 13 with PE. As shown in Table 1, all of 2328 distributed among 21 different types of patients with cancer. Overview, the identified evidence of thrombosis was about 2.28% in spite of a significantly higher proportion of positive DD cases (60.95%) in the included patients.
Table 1.
Patients’ distribution in 21 types of tumor (n)
| Types | Positive DD without thrombus | Positive DD with thrombus | Negative DD |
|---|---|---|---|
| Gastric tumor | 313 | 6 | 174 |
| No small cell lung cancer | 279 | 13 | 123 |
| Colon cancer | 184 | 2 | 167 |
| Rectal cancer | 134 | 5 | 101 |
| Breast cancer | 85 | 6 | 91 |
| Cardia cancer | 64 | 1 | 37 |
| Lymphoma | 45 | 42 | |
| Ovarian cancer | 41 | 3 | 17 |
| Pancreatic cancer | 36 | 2 | 14 |
| Esophageal cancer | 29 | 1 | 47 |
| Hepatocellular cancer | 27 | 6 | 5 |
| Nasopharyngeal carcinoma | 26 | 23 | |
| Small cell lung cancer | 17 | 8 | |
| Cervical cancer | 15 | 1 | 14 |
| Bladder cancer | 14 | 9 | |
| Gallbladder cancer | 14 | 1 | 7 |
| Prostate cancer | 13 | 1 | 12 |
| Kidney cancer | 10 | 2 | 5 |
| Melanoma | 9 | 7 | |
| Biliary tract carcinoma | 7 | ||
| Fallopian tube cancer | 4 | 6 | |
| Other | 4* |
*Three endometrial cancer and one carcinoma vulvae. DD: D‑dimer.
The incidence of positive DD events in different cancer types is illustrated in Figure 1. There was a higher incidence of 86.84% and 73.08% in 38 hepatocellular carcinoma cases and 52 pancreatic cancer cases, respectively. This is not completely consistent with the incidence of thrombosis as shown in Table 1. Interestingly, the types of tumors with high risk of thrombosis[3,4,5,6,7,8] such as kidney cancer, hematocellular carcinoma, pancreatic cancer, and ovarian cancer were included in our data.
Figure 1.
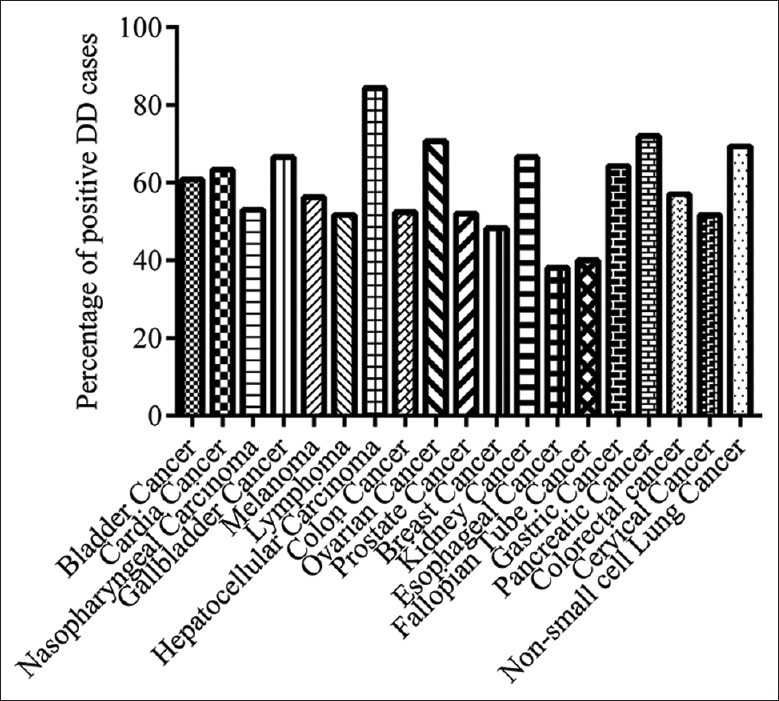
The incidence of positive D-dimer in different types of cancer.
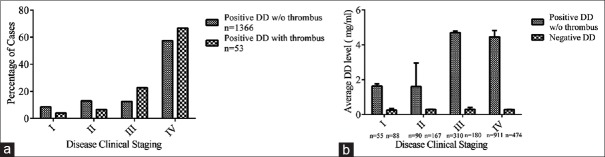
The relationship between positive DD incidence and tumor clinical stage is shown in Figure 2a. It was noted that 57.45% of positive DD cases and 66.69% of thrombosis events occurred at disease clinical Stage IV. There were overall significant differences between positive DD incidence and clinical stages analyzed with two-way ANOVA (P < 0.0001 for interaction, column and row factors) as shown in Figure 2b. However, no statistical significance was given for t-test in negative DD group (P > 0.05), by individually comparing DD level in different stages. Together, these suggest that the disease stage is a key factor for patients’ coagulation status.
Figure 2.
The impact of clinical stage on DD abnormality. Positive DD cases classified by disease clinical staging (I–IV). (a) The distribution. (b) The average DD level, P < 0.0001 for interaction by two-way ANOVA. DD: D-dimer; w/o: without.
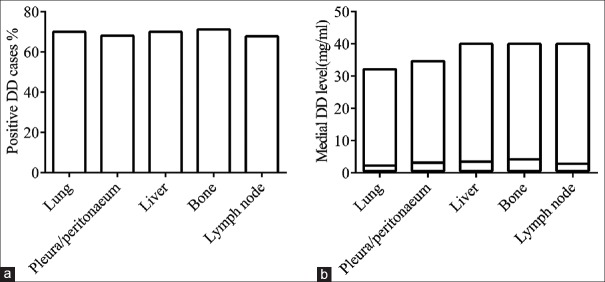
Next, we analyzed the relationship between positive DD development and metastasis site in these 2328 cases. The percentage of positive DD incidence was comparable in bone (71.233%), liver (70.064%), and lung (70.064%) metastasis groups [Figure 3a]. However, the medial DD level in the bone and liver groups was significantly higher (P < 0.0001 and P = 0.0014) as compared to the level in lung group [Figure 3b]. It indicates that the metastasis site is also an important factor affecting positive DD value.
Figure 3.
The influence of metastasis site on DD abnormality. (a) The positive DD incidence based on metastasis site. (b) The medial DD level in positive DD group, P < 0.0001 for bone vs. lung by t-test. DD: D-dimer.
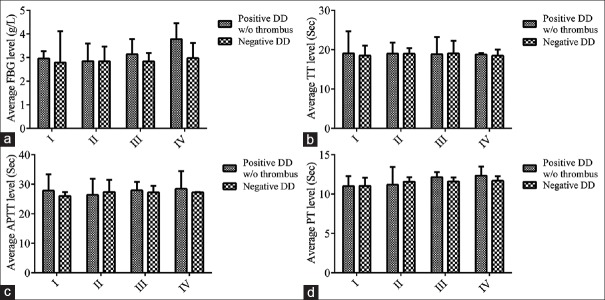
We further analyzed the routine hemostasis parameters such as PT, APTT, TT, and FBG in addition of DD, coarsely exploring the hypercoagulable state and the coagulation cascade in positive DD patients with cancer. In negative DD group, no significantly statistical differences were found in the different stages (P > 0.05) by comparing the individual parameter with t-test described as above. In positive DD group, the interaction as well as the column and row factors between the average level of FBG and the disease stages was significant (P < 0.0001) as shown in Figure 4a. However, there were no significant differences in TT whatever in interaction, column, and row factors [Figure 4b]. Figure 4 also shows the variation of APTT and PT (Biomarkers for intrinsic and extrinsic pathways, respectively) over disease stages in positive DD group. Both of APTT and PT showed a higher level following with disease stages (P = 0.0007 and P < 0.0001 for interaction; P = 0.0053 and P < 0.0001 for column factors; and P = 0.013 and P = 0.007 for row factors). These implicate that both of the intrinsic and extrinsic systems are involved in the development of hypercoagulable plasma profile during the disease progression.
Figure 4.
The interaction of hemostasis parameters with clinical stages in positive DD group without thrombus analyzed by two-way ANOVA. (a and b) Overall comparison: P < 0.0001 and P > 0.05. (c and d) Interaction: P < 0.05 and P < 0.0001. FBG: Fibrinogen; TT: Thromobin time; APTT: Activated partial thromboplastin time; PT: Prothrombin time; DD: D-dimer; w/o: without.
Meanwhile, we compared CBC in addition of DD value between positive DD groups with and without thrombus, evaluating their influence on thrombosis. Between these two groups, the significantly statistical differences were shown in white blood cell (WBC) count (P = 0.026) and DD level (P = 0.043) rather than in red blood cell (RBC) and platelet count (P > 0.05) [Table 2]. The correlation analysis between DD level and CBC in positive DD group revealed that the higher DD level did not correlate with WBC, RBC, or platelet count in these cases.
Table 2.
Comparison of complete blood count between positive DD groups with and without thrombus
| Items | Without thrombus (n = 1366) | With thrombus (n = 53) | t | P |
|---|---|---|---|---|
| DD (mg/ml) | 2.43 ± 18.35 | 14.75 ± 25.87 | 2.53 | 0.043* |
| WBC (×109/L) | 5.73 ± 3.54 | 7.42 ± 3.75 | 2.95 | 0.026* |
| RBC (×1012/L) | 3.74 ± 0.72 | 3.63 ± 0.72 | 0.14 | 0.893* |
| Hb (g/L) | 113.95 ± 21.97 | 110.84 ± 22.52 | 0.54 | 0.609 |
| Platelet (×109/L) | 197.92 ± 107.71 | 218.28 ± 95.29 | 2.26 | 0.672* |
Data were presented as mean ± SD. *No significant correlation between D-dimer level and CBC in positive DD group by Monte Carlo analysis. DD: D-dimer; WBC: White blood cell; RBC: Red blood cell; Hb: Hemoglobin; CBC: Complete blood count.
A total of 354 patients with the different types of cancer were admitted for chemotherapy. The cycle of chemotherapy ranged from 2 to 8, but usually about 4–6 cycles. The DD level during chemotherapy is listed in Figure 5. In contrast to the baseline tested before chemotherapy (C0), the average DD level in first six cycles (C1–C6) was markedly decreased (P = 0.0075, P = 0.0302, P = 0.00357, P = 0.0351, P = 0.0006, P = 0.0041, respectively). Furthermore, no one in 53 thrombosis cases was diagnosed during or after chemotherapy. Therefore, these data suggest that the effective chemotherapy can alleviate the hypercoagulable plasma state of cancer patients.
Figure 5.
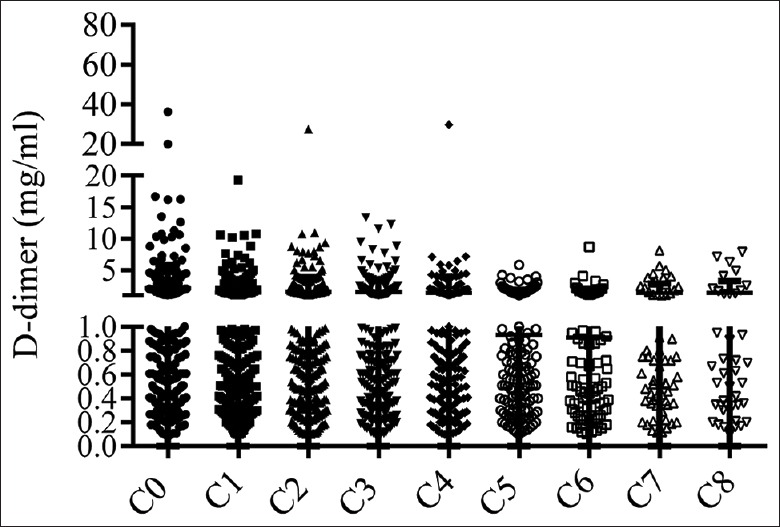
Effect of chemotherapy on D-dimer level P < 0.05 for first 6 cycles (C1–C6) vs. baseline (C0); P > 0.05 for C7–C8 vs. C0.
DISCUSSION
The balance between anti-thrombosis and thrombosis is physiologically normalized by a series of factors in blood. In cancer patients, the decreased levels of coagulation inhibitors, the presence of antiphospholipid antibodies, tumor-derived tissue factor (TF), the impaired fibrinolysis, and the acquired activated protein C resistance contribute to the hypercoagulable state.[3,13,14] Other factors, such as medication and treatment as clotting inducers, are involved in the complex and multifactorial pathogenesis of thrombosis.
This retrospective study showed a great linkage between hypercoagulable abnormality and disease clinical stage based on the positive DD incidence and DD value. Of note, the higher tumor volume is a key risk factor of DVT, intravascular DIC, and abnormalities in the clotting system in the absence of clinical manifestations. Some investigators regarded tumor stage and its primary site as top two predictive factors for the risk of thrombosis.[6,10,15] Otherwise, the site of cancer metastasis is another major risk related to hypercoagulable state according to this observational study (especially, bone vs. lung metastasis). Obviously, the encroachment of sinusoidal circulation in bone marrow or liver has an impact on the coagulation cascade.
There are two physiological pathways, intrinsic pathway and extrinsic pathway, to activate coagulation cascade. Our results showed that APTT and PT, as the biomarkers for intrinsic and extrinsic pathways, respectively, presented significant higher level in cancer patient with positive DD, which was related to the disease clinical stage.[15] In the intrinsic pathway, the activation of factor XII, as the initial step, is triggered by the endothelial damages, which is caused directly by tumor invasion or indirectly by cytokines such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor released by macrophages and monocytes as result of their interaction with tumor cells.[3,14] Moreover, TF and cysteine proteases in cancer cells can function as procoagulant factors and efficiently activate factor VIIa (the first step in extrinsic pathway) and factor X, respectively.[3,14,16,17] Meanwhile, the higher level of FBG is another clinical feature in positive DD group rather than in negative DD group based on our study. It is reported that the defect of fibrinolysis in cancer patients is also involved in hypercoagulable development and that FBG increases metastatic potential.[18,19,20] Taken together, the hemostasis abnormalities in cancer are referred to complex mechanism and reflect clinical features, but its significance for treatment optimization and prognosis prediction remains to be further investigated.
A series of other factors has been considered as risk factors referred to clotting, including completed blood count, erythropoietin or granulocyte colony-stimulating factor administration, and chemotherapy.[7,12,14] Kawaguchi et al. and Pulivarthi and Gurram reported that DD was set as cutoff value for prediction of DVT before treatment in ovarian cancer.[21,22] In 1419 positive DD patients, 53 were diagnosed as thrombosis with Doppler or spiral CT and other of cases lived without any clinical findings after our long-term following-up. We found that it was hard to identify thrombotic event by DD value because of its huge deviation although the average DD value in thrombosis patients was much higher than that in positive DD group without thrombosis. On the other hand, the higher platelet and WBC counts seemed to characterize patients in thrombosis group by comparing to positive DD group without thrombosis. However, our study further indicated that whatever blood count (platelet, WBC, and RBC or hemoglobin) did not correlate the higher DD level in positive DD group. It is not consisted with the previous reports in which complete blood count has be taken as one of the multiple factors to evaluate thrombogenesis risk in cancer patients.[7,12] Recently, it was found that the cancer patients had elevated levels of circulating TF-positive microparticles (MPs) derived from tumor, especially in pancreatic and gastric cancer.[23] Moreover, these MPs correlated with venous thrombosis in patients with cancer.[24,25,26] Unfortunately, we did not have chance detecting MPs in our 2238 cases with different types of cancer.
The chemotherapy, particularly platinum regimen, has been considered as an inducer of thrombosis in cancer patients.[3,7,11,12] However, none of the thrombosis events in 53 patients occurred during or after the chemotherapy in our data. The chemotherapy significantly decreased the hypercoagulable state, but this effect was exerted only in first 6 cycles that were commonly recommended. The laboratory research demonstrated that cytotoxic chemotherapy treatment increased cellular phosphatidylserine (PS) exposure and release of TF-positive MPs.[27] However, no increase in MP TF activity was observed in patients during 1 week.[28,29] Therefore, the PS exposure and free DNA release, caused by cytotoxic damage, were taken as activators of coagulation in 1st week of undergoing chemotherapy.[14,27,30] In fact, the DD was tested 2–3 weeks after chemotherapy in our data. Several clinical groups reported that systemic chemotherapy successfully controlled disseminated intravascular coagulation (DIC) complicated with gastric cancer.[31,32] We wonder whether the abnormal DD value caused by chemotherapy also emerges in the 1st week of treatment and whether the phenomenon above in our observational study correlates with the efficiency of chemotherapy.
In summary, this retrospective study demonstrates the positive correlation between hypercoagulable abnormality and tumor types, clinical stage, metastasis site in cancer. The routine hemostatic parameters and CBC may be used as assessment for thrombogenesis and chemotherapy although new biomarkers such as TF, TF-positive MPs have been explored. Finally, the diagnosis of VTE should encompass clinical and laboratory information.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Dr. Lin-Xiao Wang at Neurologic Disease Research Laboratory of Changzhou Second People's Hospital for her statistic assistance.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Gouin-Thibault I, Achkar A, Samama MM. The thrombophilic state in cancer patients. Acta Haematol. 2001;106:33–42. doi: 10.1159/000046587. doi: 10.1159/000046587. [DOI] [PubMed] [Google Scholar]
- 2.Rouzaud D, Alexandra JF, Chauchard M, Delon M, Dossier A, Goulenok T, et al. Frequency of malignancy is high in patients admitted for a first venous thromboembolism episode: An observational study. J Thromb Thrombolysis. 2016 doi: 10.1007/s11239-016-1355-2. doi: 10.1007/s11239-016-1355-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: Pathogenesis and current debate. Neoplasia. 2002;4:465–73. doi: 10.1038/sj.neo.7900263. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron JA, Gridley G, Weiderpass E, Nyrén O, Linet M. Venous thromboembolism and cancer. Lancet. 1998;351(9109):1077–80. doi: 10.1016/S0140-6736(97)10018-6. doi: 10.1016/S0140-6736(97)10018-6. [DOI] [PubMed] [Google Scholar]
- 5.Sørensen HT, Mellemkjaer L, Steffensen FH, Olsen JH, Nielsen GL. The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. N Engl J Med. 1998;338:1169–73. doi: 10.1056/NEJM199804233381701. doi: 10.1056/NEJM199804233381701. [DOI] [PubMed] [Google Scholar]
- 6.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339–46. doi: 10.1002/cncr.23062. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 7.Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27:4839–47. doi: 10.1200/JCO.2009.22.3271. doi: 10.1200/JCO.2009.22.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blom JW, Vanderschoot JP, Oostindiër MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: Results of a record linkage study. J Thromb Haemost. 2006;4:529–35. doi: 10.1111/j.1538-7836.2006.01804.x. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 9.Agnelli G, Bolis G, Capussotti L, Scarpa RM, Tonelli F, Bonizzoni E, et al. Aclinical outcome-based prospective study on venous thromboembolism after cancer surgery: The @RISTOS project. Ann Surg. 2006;243:89–95. doi: 10.1097/01.sla.0000193959.44677.48. doi: 10.1097/01.sla.0000193959.44677.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: Determination of frequency and characteristics. Thromb Haemost. 2002;87:575–9. [PubMed] [Google Scholar]
- 11.Wang Z, Dang C, Zhu K, Zhang Y, Chang D, Xia P, et al. Cyclophosphamide, epirubicin and fluorouracil chemotherapy-induced alteration of haemostasis markers in breast cancer patients. Mol Clin Oncol. 2015;3:1088–92. doi: 10.3892/mco.2015.584. doi: 10.3892/mco.2015.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–7. doi: 10.1182/blood-2007-10-116327. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen VG, Matika RW, Ley ML, Waer AL, Gharagozloo F, Kim S, et al. Tissue-type plasminogen activator-induced fibrinolysis is enhanced in patients with breast, lung, pancreas and colon cancer. Blood Coagul Fibrinolysis. 2014;25:248–53. doi: 10.1097/MBC.0000000000000040. doi: 10.1097/MBC.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 14.Hisada Y, Geddings JE, Ay C, Mackman N. Venous thrombosis and cancer: From mouse models to clinical trials. J Thromb Haemost. 2015;13:1372–82. doi: 10.1111/jth.13009. doi: 10.1111/jth.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilic L, Yildiz I, Sen FK, Erdem MG, Serilmez M, Keskin S, et al. D-dimer and international normalized ratio (INR) are correlated with tumor markers and disease stage in colorectal cancer patients. Cancer Biomark. 2015;15:405–11. doi: 10.3233/CBM-150477. doi: 10.3233/CBM-150477. [DOI] [PubMed] [Google Scholar]
- 16.Ünlü B, Versteeg HH. Effects of tumor-expressed coagulation factors on cancer progression and venous thrombosis: Is there a key factor. Thromb Res. 2014;133(Suppl 2):S76–84. doi: 10.1016/S0049-3848(14)50013-8. doi: 10.1016/S0049-3848(14)50013-8. [DOI] [PubMed] [Google Scholar]
- 17.Wang JG, Geddings JE, Aleman MM, Cardenas JC, Chantrathammachart P, Williams JC, et al. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012;119:5543–52. doi: 10.1182/blood-2012-01-402156. doi: 10.1182/blood-2012-01-402156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–85. doi: 10.1182/blood-2004-06-2272. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 19.Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104:397–401. doi: 10.1182/blood-2004-02-0434. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- 20.Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. 2002;62:6966–72. [PubMed] [Google Scholar]
- 21.Kawaguchi R, Furukawa N, Kobayashi H. Cut-off value of D-dimer for prediction of deep venous thrombosis before treatment in ovarian cancer. J Gynecol Oncol. 2012;23:98–102. doi: 10.3802/jgo.2012.23.2.98. doi: 10.3802/jgo.2012.23.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pulivarthi S, Gurram MK. Effectiveness of d-dimer as a screening test for venous thromboembolism: An update. N Am J Med Sci. 2014;6:491–9. doi: 10.4103/1947-2714.143278. doi: 10.4103/1947-2714.143278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363–70. doi: 10.1111/j.1538-7836.2012.04754.x. doi: 10.1111/j.1538-7836.2012.04754.x. [DOI] [PubMed] [Google Scholar]
- 24.Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122:1873–80. doi: 10.1182/blood-2013-04-460139. doi: 10.1182/blood-2013-04-460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: A link between cancer and thrombosis. J Thromb Haemost. 2007;5:520–7. doi: 10.1111/j.1538-7836.2007.02369.x. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 26.Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, et al. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15:6830–40. doi: 10.1158/1078-0432.CCR-09-0371. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boles JC, Williams JC, Hollingsworth RM, Wang JG, Glover SL, Owens AP, 3rd, et al. Anthracycline treatment of the human monocytic leukemia cell line THP-1 increases phosphatidylserine exposure and tissue factor activity. Thromb Res. 2012;129:197–203. doi: 10.1016/j.thromres.2011.06.022. doi: 10.1016/j.thromres.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee SD, Swystun LL, Mackman N, Wang JG, Pond G, Levine MN, et al. Impact of chemotherapy on thrombin generation and on the protein C pathway in breast cancer patients. Pathophysiol Haemost Thromb. 2010;37:88–97. doi: 10.1159/000324166. doi: 10.1159/000324166. [DOI] [PubMed] [Google Scholar]
- 29.Tesselaar ME, Romijn FP, van der Linden IK, Bertina RM, Osanto S. Microparticle-associated tissue factor activity in cancer patients with and without thrombosis. J Thromb Haemost. 2009;7:1421–3. doi: 10.1111/j.1538-7836.2009.03504.x. doi: 10.1111/j.1538-7836.2009.03504.x. [DOI] [PubMed] [Google Scholar]
- 30.Swystun LL, Mukherjee S, Liaw PC. Breast cancer chemotherapy induces the release of cell-free DNA, a novel procoagulant stimulus. J Thromb Haemost. 2011;9:2313–21. doi: 10.1111/j.1538-7836.2011.04465.x. doi: 10.1111/j.1538-7836.2011.04465.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee DS, Yoo SJ, Oh HS, Kim EJ, Oh KH, Lee SJ, et al. Advanced gastric cancer associated with disseminated intravascular coagulation successfully treated with 5-fluorouracil and oxaliplatin. J Gastric Cancer. 2013;13:121–5. doi: 10.5230/jgc.2013.13.2.121. doi: 10.5230/jgc.2013.13.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh KH, Cheng AL. Gastric cancer associated with acute disseminated intravascular coagulation: Successful initial treatment with weekly 24-hour infusion of high-dose 5-fluorouracil and leucovorin. Br J Haematol. 1998;100:769–72. doi: 10.1046/j.1365-2141.1998.00613.x. doi: 10.1046/j.1365-2141.1998.00613.x. [DOI] [PubMed] [Google Scholar]