Abstract
Background:
The diagnostic value of current prostate-specific antigen (PSA) tests is challenged by the poor detection rate of prostate cancer (PCa) in repeat prostate biopsy. In this study, we proposed a novel PSA-related parameter named PSA density variation rate (PSADVR) and designed a clinical trial to evaluate its potential diagnostic value for detecting PCa on a second prostate biopsy.
Methods:
Data from 184 males who underwent second ultrasound-guided prostate biopsy 6 months after the first biopsy were included in the study. The subjects were divided into PCa and non-PCa groups according to the second biopsy pathological results. Prostate volume, PSA density (PSAD), free-total PSA ratio, and PSADVR were calculated according to corresponding formulas at the second biopsy. These parameters were compared using t-test or Mann-Whitney U-test between PCa and non-PCa groups, and receiver operating characteristic analysis were used to evaluate their predictability on PCa detection.
Results:
PCa was detected in 24 patients on the second biopsy. Mean values of PSA, PSAD, and PSADVR were greater in the PCa group than in the non-PCa group (8.39 μg/L vs. 7.16 μg/L, 0.20 vs. 0.16, 14.15% vs. −1.36%, respectively). PSADVR had the largest area under the curve, with 0.667 sensitivity and 0.824 specificity when the cutoff was 10%. The PCa detection rate was significantly greater in subjects with PSADVR >10% than PSADVR ≤10% (28.6% vs. 6.5%, P < 0.001). In addition, PSADVR was the only parameter in this study that showed a significant correlation with mid-to-high-risk PCa (r = 0.63, P = 0.03).
Conclusions:
Our results demonstrated that PSADVR improved the PCa detection rate on second biopsies, especially for mid-to-high-risk cancers requiring prompt treatment.
Keywords: Prostate-specific Antigen, Prostatic Hyperplasia, Prostatic Neoplasm, Transrectal, Ultrasound
INTRODUCTION
Transrectal ultrasound (TRUS) prostate biopsy is extensively used for prostate cancer (PCa) detection and is usually guided by prostate-specific antigen (PSA) screening. Although a confirmatory cutoff for PSA level is currently unavailable for TRUS biopsy,[1] Thompson et al.[2] reported in a PCa Prevention Trial that the detection rate was 47.4% for patients with PSA levels >4.0 μg/L, and 27.9% for patients with PSA levels at 2.1–4.0 μg/L, indicating that patients with higher PSA level are at higher risk of PCa.
For patients showing negative results for PCa during the first biopsy, second, or even third biopsies are recommended for continued abnormal PSA values or if high-grade prostatic intraepithelial neoplasia is found on the first biopsy. It has been reported that PCa detection rates vary between 10% and 39% on second biopsies.[3,4,5] A prospective study reported that the PCa detection rates of males with a PSA level 4–10 μg/L were 22%, 10%, and 5%, respectively, for the initial, second, and third biopsies.[6] That study suggested that second biopsies produced unnecessary morbidity in a population with PSA 4–10 μg/L.
To improve PCa detection rates, several PSA-related parameters have been applied for repeated biopsies, among which free-total PSA ratio (f/t PSA), PSA density (PSAD), and PSA-prostate transitional zone volume ratio are regarded as having better sensitivity and specificity for PSA-guided second biopsy.[7]
The incidence and mortality of PCa in Chinese males are lower than in a Western population.[8] PCa detection rate is only 15.9% for Chinese with a PSA level 4–10 μg/L, according to one large-scale study.[9] Therefore, it may be inappropriate to use guidelines for biopsy in Chinese patients based on cutoffs for PSA and other parameters using data from a Western population. New cutoff values or new parameters are necessary to reduce the number of noncancer biopsies in the Chinese population. In this study, a new parameter named as PSAD variation rate (PSADVR), which indicated the rate of change of PSAD over 6 months, was presented and evaluated on predictability for guiding second biopsies in Chinese patients.
MATERIALS AND METHODS
Patients
A total of 251 Chinese male patients with PSA <10 μg/L and an initial PCa-negative biopsy were included from February 2010 to August 2015 in our hospital. Of these patients, 227 were asked to undergo a second biopsy 6 months later, because their repeat PSA levels were more than 4 μg/L. Twenty-nine patients declined. Patients would be examined and excluded if they took a 5-α-reductase inhibitor for more than 3 months, underwent indwelling catheterization, or had acute prostatitis, which would affect PSA values. Finally, 184 subjects who underwent two biopsies were enrolled in this research to explore the clinical value of PSADVR. Written informed consents following doctor-patient discussion were obtained from all participants, and all procedures were approved by the Medical Ethics Committee of the First Affiliated Hospital of Soochow University. The data for these subjects were collected immediately after their second biopsies and retrieved from the database of our hospital in October 2015.
Prostate-specific antigen measurement and biopsy strategy
Venous blood for PSA measurement was extracted before biopsies or digital rectal examination (DRE). PSA values were measured using an electrochemiluminescence immunoassay kit (Yuande Bio-Medical Engineering Co., Ltd., Beijing, China) according to the manufacturer's instructions, with a normal range of 0–4 μg/L.
The indication for initial biopsy was a PSA level of 4–10 μg/L with f/t PSA ≤0.16, or PSA <4 μg/L with a palpable prostate nodule on DRE. The indication for a second biopsy was a repeat PSA >10 μg/L or PSA 4–10 μg/L with f/t PSA ≤0.16, or new prostate nodule(s) palpated by DRE regardless of PSA. 12-core rectal ultrasound-guided biopsies were performed on the initial set, and an extra 1–2 cores were taken if a prostate nodule was found on ultrasonography. On second biopsies, besides the transrectal punctures as the way on initial biopsy, an extra 1–2 cores were taken in the anterior apical region by a transperineal puncture. All biopsies were performed by a single urologist and ultrasound physician in our medical center.
Calculation of parameters
PSA values and three diameter measurements by rectal ultrasound were recorded during the initial and second biopsies. The formulas for each parameter are as follows:
Prostate volume (PV, ml) = anteroposterior diameter × superior-inferior diameter × left-right diameter × 0.52;
f/t PSA = free PSA/total PSA;
PSAD = PSA/PV;
PSADVR (%) = (second PSAD – first PSAD)/first PSAD × 100%.
A negative or zero value for PSADVR indicates no valid change occurred between the two biopsies. The clinical stage of PCa was evaluated by magnetic resonance imaging and DRE.
Statistical analysis
All data were analyzed using SPSS 17.0 software (IBM, Armonk, NY, USA). Frequency analysis showed that age, PV, PSA, f/t PSA, PSAD, and PSADVR at the second biopsy had an approximately normal distribution. Comparisons of age, PV, PSA, f/t PSA, and PSADVR between the PCa and non-PCa groups were performed using a t-test, but comparisons for PSAD values used the Mann–Whitney U–test due to the heterogeneity of variance between the groups. Comparisons of PCa detection rate among the PSADVR groups were performed using the Chi-square test. We assessed the discriminatory value of PSA, PSAD, and PSADVR by computing the area under the receiver operating characteristic curve (AUC). Logistic regression analysis was used to estimate the value of parameters for PCa prediction. A value of P < 0.05 (bilateral) was considered statistically significant.
RESULTS
On the first biopsy, the average age of 184 participants was 65.1 ± 6.0 years, and all of them were more than 50 years old. The average PSA, PV, f/t PSA and PSAD were 6.76 ± 1.50 μg/L, 43.50 ± 12.60 ml, 0.13 ± 0.08, and 0.17 ± 0.05, respectively. On the second biopsy, 22 subjects had a PSA level >10 μg/L, and the rest had a PSA level 4–10 μg/L with f/t PSA ≤0.16. No new nodules were detected by DRE. PCa was diagnosed in 24 patients (detection rate 13%).
Table 1 compares PSA and other parameters between the PCa and non-PCa groups. Of these parameters, PSA, PSAD, and PSADVR were greater in the PCa group than in the non-PCa group, with statistical significance. However, age, PV, and f/t PSA showed no significant difference between the groups. The AUC of the receiver operating characteristic curve (ROC) for PSA, PSAD, and PSADVR was 0.702, 0.743, and 0.790, respectively [Figure 1]. PSADVR had the largest AUC. The cutoffs of these parameters are presented in Table 2. The greatest specificity (0.824) was obtained at PSADVR of 10%.
Table 1.
Basic characteristics of PCa and non-PCa groups at second biopsy (mean ± SD)
| Parameters | PCa group (n = 24) | Non-PCa group (n = 160) | t | P |
|---|---|---|---|---|
| Age (years) | 66.9 ± 5.6 | 64.5 ± 6.0 | 1.212 | 0.231 |
| PSA (ng/ml) | 8.39 ± 1.40 | 7.16 ± 1.77 | 3.048 | 0.003 |
| PV (ml) | 44.4 ± 9.5 | 48.5 ± 14.9 | −1.122 | 0.265 |
| f/t PSA | 0.13 ± 0.04 | 0.11 ± 0.05 | −1.476 | 0.147 |
| PSAD | 0.20 ± 0.04 | 0.16 ± 0.06 | −3.526 | <0.001 |
| PSADVR (%) | 14.15 ± 14.05 | −1.36 ± 12.28 | 5.120 | <0.001 |
PSA: Prostate-specific antigen; PV: Prostate volume; f/t PSA: Free-total PSA ratio; PSAD: PSA density; PSADVR: PSAD variation rate; SD: Standard deviation; PCa: Prostate cancer.
Figure 1.
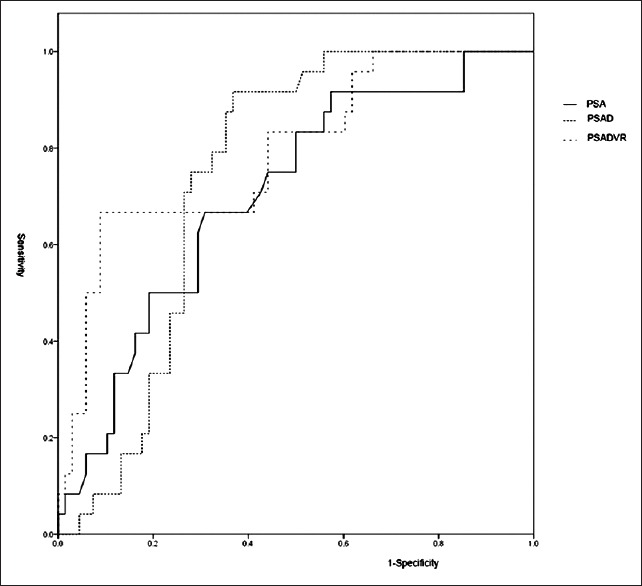
ROCs of PSA, PSAD, and PSADVR. The AUCs of ROCs of PSA, PSAD, and PSADVR were 0.702, 0.743, and 0.790, respectively. ROC: Receiver operating characteristic curve; PSA: Prostate-specific antigen; PSAD: PSA density; PSADVR: PSA density variation rate; AUC: Areas under the curve.
Table 2.
Sensitivity and specificity of cutoffs for PSA, PSAD, and PSADVR
| Parameters | Cutoff | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| PSA (ng/ml) | 8.0 | 0.583 | 0.691 | 0.400 | 0.824 |
| PSAD | 0.18 | 0.750 | 0.676 | 0.439 | 0.882 |
| PSADVR (%) | 10 | 0.667 | 0.824 | 0.571 | 0.875 |
PSA: Prostate-specific antigen; PSAD: PSA density; PSADVR: PSAD variation rate. PPV: Positive predictive value; NPV: Negative predictive value.
The PCa detection rates of subjects with PSADVR ≤10% and >10% were 6.5% (8/128) and 28.6% (16/56), respectively. There was a significant increase in PCa detected in subjects with PSADVR >10% (P < 0.001, χ2= 148.42).
In multivariate logistic regression analysis, PSADVR was the only parameter that had a significant relationship with PCa at second biopsy, compared with PSA, PSAD, and f/t PSA [Table 3]. Remarkably, 14 of 24 PCa cases were considered to be mid-to-high-risk cancers according to Gleason scores (>6) or clinical T stage (>T2a). PSADVR had a statistically significant positive correlation with PCa risk level (r = 0.63, P = 0.03, Spearman correlation), but PSA and PSAD did not (r = 0.02, P = 0.945; r = 0.27, P = 0.393, respectively). Of 16 PCa cases with PSADVR >10%, 12 were mid-to-high-risk cancers. However, only two cancers with PSADV ≤10% were mid-to-high-risk (PSADVR = 3%, T1N0M0, Gleason score = 7; PSADVR <0, T2aN0M0, Gleason score = 8).
Table 3.
Multivariate ORs and 95% CIs for PSA, PSAD, and PSADVR for PCa detection at second biopsy by logistic regression analysis
| Parameters | OR | 95% CI of OR | P |
|---|---|---|---|
| PSA | 1.118 | 0.632–1.978 | 0.678 |
| PSAD | 1.052 | 0.902–1.228 | 0.517 |
| f/t PSA | 1.066 | 0.856–1.326 | 0.570 |
| PSADVR | 2.480 | 1.131–5.438 | 0.023 |
PSA: Prostate-specific antigen; f/t PSA: Free-total PSA ratio; PSAD: PSA density; PSADVR: PSAD variation rate; CI: Confidential intervals; ORs: Odds ratios; PCa: Prostate cancer.
DISCUSSION
PSA and related parameters have been widely used for PCa screening and detection. A missed diagnosis of PCa in a biopsy is to some extent unavoidable, because of the multifocal characteristics of PCa and the limitations of prostate biopsies due to sample collection, which can only sample a small part of the entire prostate.[10] However, biopsy remains the most effective clinical method for PCa detection.
Despite tremendous efforts and progress in using PSA cutoffs in guiding prostate biopsy, the benefits remain questionable, and the results of meta-analyses are controversial or inconsistent.[11] Although the current PSA cutoff helps urologists detect and diagnose PCa in millions of patients annually, a much greater number of males who underwent biopsies have shown no cancer, and thus suffered from economic loss and trauma.[12] In addition, there has been no significant reduction in the mortality from PCa since the introduction of PSA screening.[13]
The necessity for a second biopsy should be carefully evaluated in clinical practice because the detection rate of PCa in a second biopsy proved to be much lower than that in the initial biopsy.[5] Repeat biopsy is considered acceptable when serum PSA is higher than 10 μg/L. However, when PSA levels are 4–10 μg/L, repeat biopsy is controversial, because most results are noncancers or low-risk cancers. Moreover, the invasiveness of a repeat TRUS biopsy is not accepted by some subjects. About 15% of recruits in this research refused the second biopsy, complaining of severe pain. Obviously, a new parameter with better sensitivity and specificity for use in guiding second biopsies is greatly needed.
PSA-related parameters, such as f/t PSA and PSAD, reportedly improved the sensitivity and specificity of PSA for guiding the second biopsy. Djavan et al.[7] reported a study of 820 participants with PSA 4–10 μg/L, in which patients diagnosed with benign prostatic hyperplasia (BPH) in the initial biopsy underwent a repeat biopsy 6 weeks later. The authors reported that f/t PSA provided a sensitivity of 90% and a specificity of 50% at a cutoff of 0.30, and PSAD had a sensitivity of 74% and a specificity of 44% at a cutoff of 0.13 (when compared to PSA alone, with sensitivity of 80% and specificity of 38% at a cutoff of 5.5 μg/L). However, in our research, the mean values of f/t PSA in the PCa and non-PCa groups showed no significant difference, and f/t PSA showed no significance in logistic regression analysis. The result was contrary to Djavan's study. One possible reason was that the number of PCa patients was much less than those with BPH; another reason was that the levels of some PSAs in the PCa group were higher than 10 μg/L. In the literature, although PSA velocity (PSAV) is statistically associated with outcomes of definite PCa in first or repeat prostate biopsies,[5,14,15] it adds little extra value to PSA itself for PCa detection.[16,17]
On the other hand, optimization of methods may also provide benefits for detection rate in repeat biopsies. One of the potential improvements is to increase the number of cores in a large prostate. Eskew et al.[18] reported that 12–15 core biopsies in prostates larger than 50 ml showed a detection rate 35% greater for PCa compared with a 6-core biopsy. Another extreme example was saturation biopsy. A high incidence of PCa (41%) has reportedly been detected by saturation biopsies in subjects who had at least two previously negative, 8-core biopsy sessions.[19] In addition, the anterior apex of the prostate is an area that can be easily missed in a regular transrectal 12-core biopsy. Although most PCa is located in the lateral peripheral zone of the prostate, it was reported that about 17% of cancers were detected by apical region biopsy.[20] In this study, extra anterior apical cores were performed on all subjects on second biopsies, and three cancers (12.5%) were detected in this region, with Gleason scores of 6, 6, and 7, respectively.
In this study, we designed a new concept of PSADVR to describe the changes in PSAD over 6 months. Compared with static parameters such as PSA, f/t PSA, and PSAD, PSADVR described not only dynamic changes in PSA but also the relationship between PSA and PV. It dramatically demonstrated predictive value in second biopsies performed at a relatively long interval from the initial biopsy.
Theoretically, PSAD should remain constant in males with a benign prostate for a period. In 20 male outpatients under 50 years old without prostatitis or any lower urinary tract symptoms, PSAD varied between individuals but remained constant over 1–3 years. All PSADVR values in these 20 males were <3%. In non-PCa, an increase in PSA was mostly caused by an increase in PV. However, in PCa, PSAD significantly increases along with disease progression, because prostate glandular epithelium invasion causes a large amount of PSA release into the blood. Therefore, we predict that PSADVR will be an excellent parameter for PCa detection, especially for clinically significant cancers. In this study, PSADVR had more specificity than PSA and PSAD, and a significant increase in PCa detection rate was observed in males with PSADVR >10%. Moreover, PSADVR was the only independent factor that aided PCa detection in a second biopsy compared to PSA, PSAD, and f/t PSA.
Chan et al.[21] reported that although biopsies of 9 or more cores could detect earlier stage cancers than those with 8 cores or less, no differences were noted between initial biopsy and repeat biopsy cancer groups in Gleason scores, organ confinement, or extracapsular invasion.[6] Thus, it is still questionable whether second biopsies would help increase the proportion of detection of indolent cancers. In this research, PSADVR was positively correlated with mid-to-high-risk cancers (Gleason score >6 or clinical stage >T2a), and most PCa patients with PSADVR >10% were mid-to-high-risk cancers. Therefore, the new parameter might help identify PCa requiring timely intervention.
We strongly recommended that the time interval between two biopsies should be more than 3 months when calculating PSADVR, based on the calculation method for PSAV,[22,23] since a shorter interval may cause statistical confusion. Thus, PSADVR is mostly appropriate for patients who undergo second biopsies 3 months or more after the first biopsy.
A limitation is the small number of participants in this study, which might affect the accuracy of the conclusions. In addition, the average PVs of PCa and non-PCa groups were both larger than 40 ml, which meant the conclusion was drawn from a prostatic hyperplasia population. Whether PSADVR is practical in small prostate patients’ needs further study. Another limitation of the current study was that not all patients underwent radical prostatectomy; it was reported that there was a remarkable difference between clinical stage and postoperatic pathologic stage.[24] Therefore, the relation of PSADVR and pathological stages were not confirmed.
In conclusion, PSADVR could serve as a new PSA-related parameter with a valuable clinical impact; PSADVR has better sensitivity and specificity for PCa detection, and avoids many negative biopsies. It also has satisfactory predictive value for mid-to-high-risk cancers requiring prompt treatment. However, prospective and multicenter researches with large sample size should be conducted to confirm the PCa predictability of PSADVR on second biopsy.
Financial support and sponsorship
This work was supported by a grant from the Jiangsu Province's Key Medical Talents Program (No. RC2011108).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Dr. Xu-Feng Chen from UCLA for critical reading.
Footnotes
Edited by: Ning-Ning Wang
REFERENCES
- 1.Semjonow A, Brandt B, Oberpenning F, Roth S, Hertle L. Discordance of assay methods creates pitfalls for the interpretation of prostate-specific antigen values. Prostate Suppl. 1996;7:3–16. doi: 10.1002/(SICI)1097-0045(1996) [PubMed] [Google Scholar]
- 2.Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS, et al. Assessing prostate cancer risk: Results from the prostate cancer prevention trial. J Natl Cancer Inst. 2006;98:529–34. doi: 10.1093/jnci/djj131. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 3.Djavan B, Zlotta AR, Ekane S, Remzi M, Kramer G, Roumeguère T, et al. Is one set of sextant biopsies enough to rule out prostate cancer?Influence of transition and total prostate volumes on prostate cancer yield. Eur Urol. 2000;38:218–24. doi: 10.1159/000020282. doi: 10.1159/000020282. [DOI] [PubMed] [Google Scholar]
- 4.Pepe P, Aragona F. Saturation prostate needle biopsy and prostate cancer detection at initial and repeat evaluation. Urology. 2007;70:1131–5. doi: 10.1016/j.urology.2007.07.068. doi: http://dx.doi.org/10.1016/j.urology.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 5.Auprich M, Augustin H, Budäus L, Kluth L, Mannweiler S, Shariat SF, et al. A comparative performance analysis of total prostate-specific antigen, percentage free prostate-specific antigen, prostate-specific antigen velocity and urinary prostate cancer gene 3 in the first, second and third repeat prostate biopsy. BJU Int. 2012;109:1627–35. doi: 10.1111/j.1464-410X.2011.10584.x. doi: 10.1111/j.1464-410X.2011.10584.x. [DOI] [PubMed] [Google Scholar]
- 6.Djavan B, Ravery V, Zlotta A, Dobronski P, Dobrovits M, Fakhari M, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: When should we stop? J Urol. 2001;166:1679–83. doi: 10.1097/00005392-200111000-00015. [PubMed] [Google Scholar]
- 7.Djavan B, Zlotta A, Remzi M, Ghawidel K, Basharkhah A, Schulman CC, et al. Optimal predictors of prostate cancer on repeat prostate biopsy: A prospective study of 1,051 men. J Urol. 2000;163:1144–8. doi: 10.1097/00005392-200004000-00018. [PubMed] [Google Scholar]
- 8.Zhang HY, Cui J, Zhang Y, Wang ZL, Chong T, Wang ZM. Isoflavones and prostate cancer: A review of some critical issues. Chin Med J. 2016;129:341–347. doi: 10.4103/0366-6999.174488. doi: 10.4103/0366-6999.174488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M, Na YQ. The detection rate of prostate cancer in different prostate-specific antigen (PSA) levels in Chinese men (in Chinese) Natl Med J China. 2008;88:16–8. [PubMed] [Google Scholar]
- 10.Djavan B, Susani M, Bursa B, Basharkhah A, Simak R, Marberger M. Predictability and significance of multifocal prostate cancer in the radical prostatectomy specimen. Tech Urol. 1999;5:139–42. [PubMed] [Google Scholar]
- 11.Roobol MJ, Carlsson S, Hugosson J. Meta-analysis finds screening for prostate cancer with PSA does not reduce prostate cancer-related or all-cause mortality but results likely due to heterogeneity-the two highest quality studies identified do find prostate cancer-related mortality reductions. Evid Based Med. 2011;16:20–1. doi: 10.1136/ebm1165. doi: 10.1136/ebm1165. [DOI] [PubMed] [Google Scholar]
- 12.Pataky R, Gulati R, Etzioni R, Black P, Chi KN, Coldman AJ, et al. Is prostate cancer screening cost-effective? A microsimulation model of prostate-specific antigen-based screening for British Columbia, Canada. Int J Cancer. 2014;135:939–47. doi: 10.1002/ijc.28732. doi: 10.1002/ijc.28732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andriole GL, Crawford ED, Grubb RL, Andriole GL, Crawford ED, Grubb RL, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: Mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–32. doi: 10.1093/jnci/djr500. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Amico AV, Renshaw AA, Sussman B, Chen MH. Pretreatment PSA velocity and risk of death from prostate cancer following external beam radiation therapy. JAMA. 2005;294:440–7. doi: 10.1001/jama.294.4.440. doi: 10.1001/jama.294.4.440. [DOI] [PubMed] [Google Scholar]
- 15.D'Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125–35. doi: 10.1056/NEJMoa032975. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 16.Vickers AJ, Till C, Tangen CM, Lilja H, Thompson IM. An empirical evaluation of guidelines on prostate-specific antigen velocity in prostate cancer detection. J Natl Cancer Inst. 2011;103:462–9. doi: 10.1093/jnci/djr028. doi: 10.1093/jnci/djr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vickers AJ, Wolters T, Savage CJ, Cronin AM, O’Brien MF, Pettersson K, et al. Prostate-specific antigen velocity for early detection of prostate cancer: Result from a large, representative, population-based cohort. Eur Urol. 2009;56:753–60. doi: 10.1016/j.eururo.2009.07.047. doi: 10.1016/j.eururo.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eskew LA, Bare RL, McCullough DL. Systematic 5 region prostate biopsy is superior to sextant method for diagnosing carcinoma of the prostate. J Urol. 1997;157:199–202. doi: http://dx.doi.org/10.1016/S0022-5347(01)65322-9. [PubMed] [Google Scholar]
- 19.Walz J, Graefen M, Chun FK, Erbersdobler A, Haese A, Steuber T, et al. High incidence of prostate cancer detected by saturation biopsy after previous negative biopsy series. Eur Urol. 2006;50:498–505. doi: 10.1016/j.eururo.2006.03.026. doi: 10.1016/j.eururo.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Wright JL, Ellis WJ. Improved prostate cancer detection with anterior apical prostate biopsies. Urol Oncol. 2006;24:492–5. doi: 10.1016/j.urolonc.2006.03.003. doi: 10.1016/j.urolonc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Chan TY, Chan DY, Stutzman KL, Epstein JI. Does increased needle biopsy sampling of the prostate detect a higher number of potentially insignificant tumors? J Urol. 2001;166:2181–4. doi: 10.1097/00005392-200112000-00036. [PubMed] [Google Scholar]
- 22.O'Brien MF, Cronin AM, Fearn PA, Smith B, Stasi J, Guillonneau B, et al. Pretreatment prostate-specific antigen (PSA) velocity and doubling time are associated with outcome but neither improves prediction of outcome beyond pretreatment PSA alone in patients treated with radical prostatectomy. J Clin Oncol. 2009;27:3591–7. doi: 10.1200/JCO.2008.19.9794. doi: 10.1200/JCO.2008.19.9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vickers AJ, Brewster SF. PSA velocity and doubling time in diagnosis and prognosis of prostate cancer. Br J Med Surg Urol. 2012;5:162–8. doi: 10.1016/j.bjmsu.2011.08.006. doi: 10.1016/j.bjmsu.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen XC, Qiu YQ, Zheng YC, Zhang SZ. Are Partin tables suitable for Chinese patients with prostate cancer? Chin Med J. 2012;125:3795–9. doi: 10.3760/cma.j.issn.0366-6999.2012.21.009. [PubMed] [Google Scholar]


