Abstract
Background:
Collagen VI-related myopathies are autosomal dominant and recessive hereditary myopathies, mainly including Ullrich congenital muscular dystrophy (UCMD) and Bethlem myopathy (BM). Muscle magnetic resonance imaging (MRI) has been widely used to diagnosis muscular disorders. The purpose of this study was to evaluate the diagnostic value of thigh muscles MRI for collagen VI-related myopathies.
Methods:
Eleven patients with collagen VI gene mutation-related myopathies were enrolled in this study. MRI of the thigh muscles was performed in all patients with collagen VI gene mutation-related myopathies and in 361 patients with other neuromuscular disorders (disease controls). T1-weighted images were used to assess fatty infiltration of the muscles using a modified Mercuri's scale. We assessed the sensitivity and specificity of the MRI features of collagen VI-related myopathies. The relationship between fatty infiltration of muscles and specific collagen VI gene mutations was also investigated.
Results:
Eleven patients with collagen VI gene mutation-related myopathies included six UCMD patients and five BM patients. There was no significant difference between UCMD and BM patients in the fatty infiltration of each thigh muscle except sartorius (P = 0.033); therefore, we combined the UCMD and BM data. Mean fatty infiltration scores were 3.1 and 3.0 in adductor magnus and gluteus maximus, while the scores were 1.3, 1.3, and 1.5 in gracilis, adductor longus, and sartorius, respectively. A “target” sign in rectus femoris (RF) was present in seven cases, and a “sandwich” sign in vastus lateralis (VL) was present in ten cases. The “target” and “sandwich” signs had sensitivities of 63.6% and 90.9% and specificities of 97.3% and 96.9% for the diagnosis of collagen VI-related myopathies, respectively. Fatty infiltration scores were 2.0–3.0 in seven patients with mutations in the triple-helical domain, and 1.0–1.5 in three of four patients with mutations in the N- or C-domain of the collagen VI genes.
Conclusions:
The “target” sign in RF and “sandwich” sign in VL are common MRI features and are useful for the diagnosis of collagen VI-related myopathies. The severity of fatty infiltration of muscles may have a relationship with the mutation location of collagen VI gene.
Keywords: Collagen VI-related Myopathies, Gene Mutation, Muscle Magnetic Resonance Imaging, Sensitivity, Specificity
INTRODUCTION
Collagen VI-related myopathies include autosomal dominant and recessive hereditary myopathies caused by mutations in any of the three collagen VI genes, COL6A1, COL6A2, and COL6A3.[1,2] These myopathies encompass a spectrum of disorders, including Ullrich congenital muscular dystrophy (UCMD), Bethlem myopathy (BM), autosomal dominant limb-girdle muscular dystrophy (LGMD), and autosomal recessive myosclerosis myopathy.[3,4] The most common collagen VI-related myopathies, early-onset UCMD, and late-onset BM, are characterized by muscle wasting and weakness, joint contractures, respiratory dysfunction, and cutaneous abnormalities.[5,6,7] The histopathological changes associated with these myopathies include marked variation in muscle fiber diameter, mild necrosis with muscle fiber regeneration, and increased endomysial connective tissue.[5] Mutations can result in collagen VI deficiency (in UCMD) or dysfunction (in BM) in the extracellular matrices of muscle fibers and collagenous connective tissues.[1] Pathological diagnosis of BM is often difficult because there is usually no loss of collagen VI in the extracellular matrix of muscle fibers.
In recent years, magnetic resonance imaging (MRI) of muscle has been widely used to diagnose muscular disorders and assess their progression.[8,9,10,11,12,13] Collagen VI-related myopathies have been shown to have a distinct pattern of fatty infiltration, which is most severe fatty infiltration in the vasti muscles with relative sparing of gracilis, adductor longus (AL), and sartorius. MRI of patients with collagen VI-related myopathies has shown a peripheral rim of abnormal signal in the vastus lateralis (VL) and a central area of abnormal signal within rectus femoris (RF).[11,14,15,16] Peripheral rims of the abnormal signal have also been shown in soleus and gastrocnemius.[16] Whether these MRI changes are specific to collagen VI-related myopathies and can be used for their diagnosis is still unknown. The purpose of this study was to evaluate the diagnostic value of MRI features in the thigh muscles of patients with collagen VI-related myopathies and to compare these MRI findings to those seen in patients with other neuromuscular disorders.
METHODS
Patients
Eleven patients with collagen VI-related myopathies diagnosed by genetic and clinical tests in Peking University First Hospital from June 2012 to January 2015 were recruited for this study. The study was approved by the Ethics Committee of Peking University First Hospital, and written informed consent was obtained from all patients or their parents. Participants consisted of eight males and three females with a median age of 6.8 years (range: 4.7–29.8 years). The median age of disease onset was 0.5 years (range: 0–25.0 years), and the median duration of disease was 5.1 years (range: 2.0–24.0 years). Contracture of the distal joints was present in two of the five BM patients [Supplementary Table 1]. All the patients could walk and had no respiratory dysfunction at the time of diagnosis. Serum creatine kinase levels were between 216 and 700 U/L (normal range: 25–170 U/L). Electromyography was conducted in nine patients; of these, a myopathic pattern was found in six patients and the other three patients were normal.
Supplementary Table 1.
Clinical data and MRI signs of 11 patients with collagen VI-related myopathies
| Patient number | Phenotype | Gender/age (years) | Onset age (years) | Age of walking (months) | Hypotonia at birth | Delayed motor milestone | Contracture/distal hyperlaxity | Muscle strength proximal/distal | MRI signs sandwich/target |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ullrich | Male/5.0 | 0 | 18 | + | + | +/+ | 4–/4 | +/− |
| 2 | Ullrich | Male/5.1 | 0 | 18 | + | + | –/+ | 4–/4 | +/+ |
| 3 | Ullrich | Female/4.7 | 0 | 14 | + | + | +/+ | 4/5 | +/+ |
| 4 | Ullrich | Male/4.8 | 0 | 15 | + | + | +/+ | 4–/4 | +/+ |
| 5 | Ullrich | Male/6.8 | 0.5 | 18 | − | + | +/+ | 4/5 | +/− |
| 6 | Ullrich | Male/13.5 | 0 | 19 | + | + | +/+ | 4/5 | +/+ |
| 7 | Bethlem | Male/6.0 | 3.0 | ≤12 | − | − | –/− | 5–/5 | +/− |
| 8 | Bethlem | Male/9.0 | 7.0 | ≤12 | − | − | − | 4–/5 | –/− |
| 9 | Bethlem | Female/12.8 | 2.0 | ≤12 | − | − | +/− | 4/5 | +/+ |
| 10 | Bethlem | Male/29.0 | 5.0 | ≤12 | − | − | +/− | 4/5 | +/+ |
| 11 | Bethlem | Male/29.8 | 25.0 | ≤12 | − | − | − | 3+/4+ | +/+ |
+: With the symptom or sign; −: Without the symptom or sign; MRI: Magnetic resonance imaging.
Sequence analysis of collagen VI genes
Genomic DNA was extracted from peripheral blood samples from all patients following standard procedures. Mutations in the collagen VI genes (COL6A1, COL6A2, and COL6A3) were analyzed by next generation sequencing, which was conducted using the Agilent Sure Design Panel kit (0.4 Mb, for neuromuscular disorders; Agilent, Santa Clara, CA, USA) and the Illumina HiSeq 2500 sequencer (Illumina, San Diego, CA, USA). Sanger sequencing with specific primers was conducted to confirm the mutations in the patients and their parents, as well as 1000 healthy control participants. Comparison of collagen VI amino acid sequences among species was performed using the National Center for Biotechnology Information HomoloGene Web server (http://www.ncbi.nlm.nih.gov/homologene). Prediction of the impact of new mutations was also performed using tools polymorphism phenotyping 2 (http://www.genetics.bwh.harvard.edu/pph 2/) and sorting intolerant from tolerant (http://www.sift.jcvi.org/).
Muscle magnetic resonance imaging
Muscle MRI examinations at 1.5 and 3.0 T were performed at the thigh level in 11 patients with collagen VI-related myopathies and in 361 patients with other neuromuscular diseases (disease controls). T1-weighted sequences were performed with the following parameters: repetition time = 450 ms, echo time = 12 ms, slice thickness = 5–8 mm, slice gap = 1–2 mm, and field of view = 28–32 cm. The MRI was assessed by two independent and experienced examiners, a radiologist and a neurologist. Using T1-weighted MRI, the degree of fatty infiltration of the muscles was scored using a modified Mercuri's scale.[11,17]
The 361 non-collagen VI-related myopathy cases included 166 cases of dystrophinopathies, 56 cases of inflammatory myopathy, 35 cases of neurogenic muscular atrophy, 33 cases of LGMD2B, 15 cases of congenital myopathy, 15 cases of LGMD2A, 13 cases of metabolic myopathy, 8 cases of LMNA-related myopathy, 7 cases of GNE myopathy, 5 cases of myotonic dystrophy, 3 cases of LGMD2D, 3 cases of LGMD2I, 1 case of LGMD2C, and 1 case of LGMD2Q.
Statistical analysis
Data were analyzed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). Independent t-test or Mann-Whitney U-test were conducted to assess the significance of differences in age of onset, disease duration, and MRI fatty infiltration scores between UCMD and BM patients. Differences were considered statistically significant if P < 0.05. Sensitivity and specificity were calculated to evaluate the diagnostic value of the MRI signs.
RESULTS
Eleven patients with collagen VI gene mutation-related myopathies included six UCMD patients and five BM patients. Six UCMD patients had a median onset age of 1.0 month (range: 0.0–6.0 months), and presented with neonatal hypotonia, delayed motor milestones, distal hyperlaxity, and proximal muscle weakness for a median duration of 5.1 years (range: 4.7–13.5 years); and five BM patients manifested proximal limb weakness, with a median onset age of 5.0 years (range: 2.0–25.0 years) and a median duration of 5.8 years (range: 2.0–24.0 years). Disease onset in UCMD patients was at a significantly earlier age than in BM patients (t = −2.87, P = 0.004), but no significant difference was found in the duration of these diseases (P > 0.05).
Genetic analysis
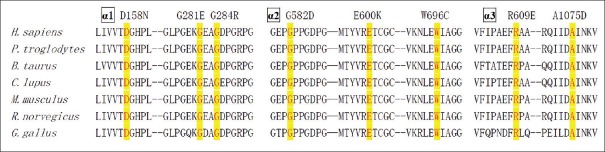
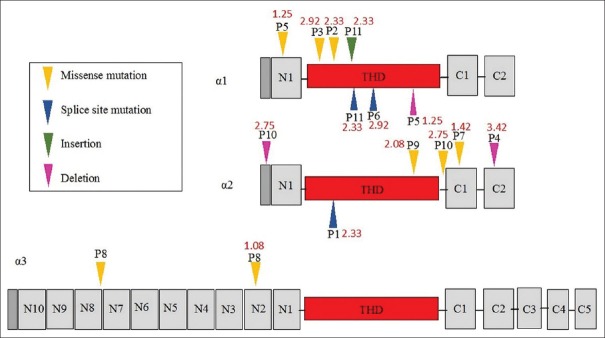
Fifteen different mutations in the collagen VI genes were identified in the patients with collagen VI-related myopathies, and no other pathogenic mutations of other genes were found [Table 1]. Three of these 15 mutations have been previously reported.[18,19] None of the mutations were observed in unaffected family members or in 1000 unrelated healthy controls. The amino acids where missense mutations occurred were highly conserved among species [Figure 1]. The splice site mutations occurred at conserved GT-AG splice junctions. In silico analysis predicted that these missense mutations were likely to be pathogenic [Table 1]. There were seven patients with mutations located in or near the triple helical domain (THD), three with mutations located in the N- or C-terminal globular domain, and one with mutations in both the THD and N-terminal domain [Figure 2].
Table 1.
Collagen VI gene mutations and MRI findings in 11 patients with collagen VI gene mutation-related myopathies
| Patient number | Genes | Substitution | Domain | Source | SIFT/Polyphen2 | MRI findings | ||
|---|---|---|---|---|---|---|---|---|
| Nucleotides | Amino acid | Fatty infiltration | Sandwich/target | |||||
| 1 | COL6A2 | c.955-2A>G(het)* | Splicing | THD | De novo | 2.33 | +/− | |
| 2 | COL6A1 | c.850G>A(het)* | p.G284R | THD | NA | 0/0.98 | 2.33 | +/+ |
| 3 | COL6A1 | c.842G>A(het) | p.G281E | THD | NA | 0/0.969 | 2.92 | +/+ |
| 4 | COL6A2 | c.2515_2516delGA(hom) | Deletion | C2 | Patental | 3.42 | +/+ | |
| 5 | COL6A1 | c.472G>A | p.D158N | N1 | Maternal | 0/0.997 | 1.25 | +/− |
| COL6A1 | c.1576-2_1576-1delAG | Splicing | THD | Patental | ||||
| 6 | COL6A1 | c.1056+1G>T(het) | Splicing | THD | NA | 2.92 | +/+ | |
| 7 | COL6A2 | c.2088G>C(het) | p.W696C | C1 | Maternal | 0.01/0.999 | 1.42 | +/− |
| 8 | COL6A3 | c.5114C>A | p.A1705D | N2 | Patental | 0.01/1 | 1.08 | −/− |
| COL6A3 | c.1826G>A | p.R609Q | N7/N8 | Maternal | 0.57/0.997 | |||
| 9 | COL6A2 | c.1745G>A(het) | p.G582D | THD | De novo | 0/1 | 2.08 | +/+ |
| 10 | COL6A2 | c.1798G>A* | p.E600K | THD | Maternal | 0.28/0.991 | 2.75 | +/+ |
| COL6A2 | c.11_23del | Deletion | SP | Patental | ||||
| 11 | COL6A1 | c.929_930insGCCGT | Insertion | THD | NA | 2.33 | +/+ | |
| COL6A1 | c.930+2T>G | Splicing | THD | |||||
Patients 1–6 and 7–11 were patients with UCMD and BM, respectively. SIFT score ≤0.05 and PolyPhen2 score approximately = 1 indicate a prediction of pathogenicity. *Mutation has been reported. Het: Heterozygosis; hom: Homozygosis; THD: Triple helical domain; N: N-terminal globular domain; C: C-terminal globular domain; SP: Signal peptide; NA: DNAs of parents were not available; +: With the sign; −: Without the sign; MRI: Magnetic resonance imaging.
Figure 1.
Amino acid sequences where mutations occurred are highly conserved among different species.
Figure 2.
Schematic of the domain structure of three collagen VI chains and related mutations in this study. The red figure represented the mean score of magnetic resonance imaging fatty infiltration in each patient. THD: Triple helical domain.
Magnetic resonance imaging findings
There were no significant differences between UCMD and BM patients in the fatty infiltration scores of each thigh muscle, except sartorius (t = −2.13, P = 0.033). The total fatty infiltration scores for all thigh muscles also did not significantly differ between UCMD and BM patients; therefore, we evaluated the collective MRI changes in all of the patients together.
Mean fatty infiltration scores varied greatly among the different thigh muscles in all patients [Figure 3]. The mean scores were 3.1 and 3.0 in adductor magnus (AM) and gluteus maximus (GM), while the scores were 1.3, 1.3, and 1.5 in gracilis, AL, and sartorius, respectively. Fatty infiltration was unevenly distributed within semitendinosus at different regions in ten (90.9%) patients [Figure 4]. Proximally, the peripheral regions of the muscle were affected more severely than the internal regions; mean fatty infiltration scores were 3.5 and 1.8 for the peripheral and internal regions, respectively. In contrast, distally the scores were 1.6 for the peripheral and 3.4 for the internal portions of the muscle. Changes similar to those seen in the semitendinosus of collagen VI-related myopathy patients appeared in only 18 of the 361 (5.0%) control patients.
Figure 3.
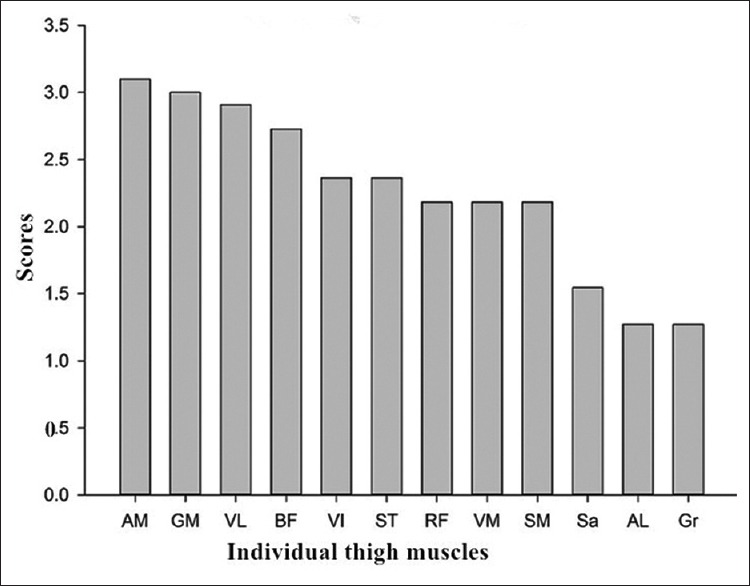
Mean scores of fatty infiltration in individual thigh muscles. AM: Adductor magnus; GM: Gluteus maximus; VL: Vastus lateralis; BF: Biceps femoris long head; VI: Vastus intermedius; ST: Semitendinosus; RF: Rectus femoris; VM: Vastus medialis; SM: Semimembranosus; Sa: Sartorius; AL: Adductor longus; Gr: Gracilis.
Figure 4.
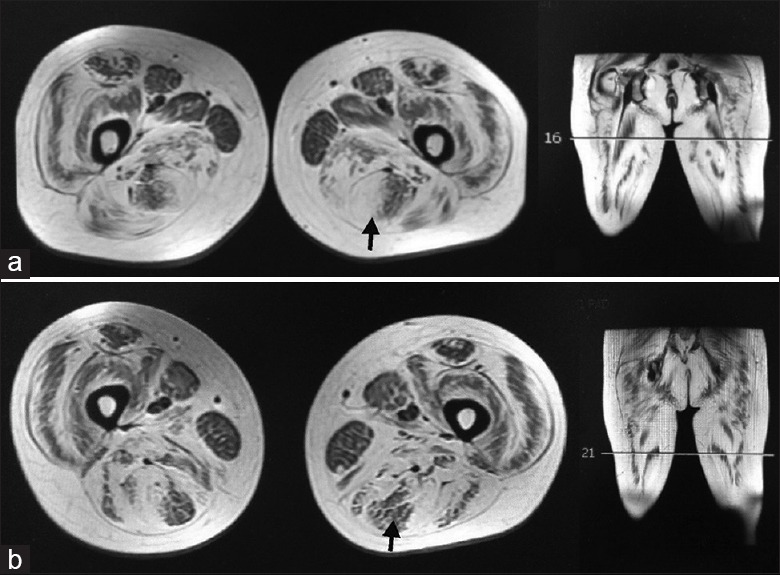
Inhomogeneous fatty infiltration in semitendinosus (shown by arrows). High scores of fatty infiltration appeared in the periphery at upper region (a) and in the central part at lower region (b) of the semitendinosus. The lines on the coronal images represented the different sections of axial images.
Seven patients had a “target” sign due to severe fatty infiltration in the central region of RF, surrounding a mid-point of little infiltration [Figure 5]. The mean fatty infiltration scores were 0.9 in the center, 4.1 in the ring, and 2.0 at the periphery of the muscle. Four patients showed no “target” sign in the less affected RF. Ten cases revealed a “sandwich” sign due to a severe involvement of the periphery of VL, with sparing of the central region [Figure 5]. The fatty infiltration scores were 3.6 in the periphery and 1.4 centrally. There was no obvious clinical difference between patients with and without “target” or “sandwich” signs, which were listed in the Supplementary Table 1. The sensitivities of the “target” sign and “sandwich” sign for the diagnosis of collagen VI-related myopathies were 63.6% and 90.9%, and the specificities were 97.0% and 96.1%, respectively [Table 2]. The control diseases which had “sandwich” sign and “target” sign are shown in Table 2.
Figure 5.
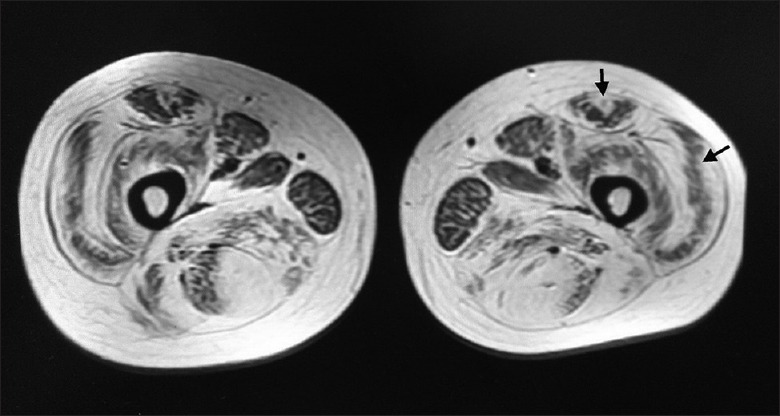
Muscle magnetic resonance imaging of a patient with Bethlem myopathy. Gracilis, adductor longus, and sartorius were less affected. The rectus femoris had a “target” sign and vastus lateralis a “sandwich” sign.
Table 2.
The sensitivity and specificity of “sandwich” and “target” signs for collagen VI-related myopathies and other neuromuscular disorders (disease controls)
| Signs | Collagen VI myopathies (n = 11) | Disease controls (n = 361) | Sensitivity, % | Specificity, % | Disease controls with the signs | |||
|---|---|---|---|---|---|---|---|---|
| Dystrophinopathies (n = 166) | LGMD2B (n = 33) | LGMD2A (n = 15) | LMNA-related myopathy (n = 8) | |||||
| Sandwich | 90.9 | 96.1 | ||||||
| Positive | 10 | 14 | 4 | 4 | 4 | 2 | ||
| Negative | 1 | 347 | 162 | 29 | 11 | 6 | ||
| Target | 63.6 | 97.0 | ||||||
| Positive | 7 | 11 | 7 | 2 | 1 | 1 | ||
| Negative | 4 | 350 | 159 | 31 | 14 | 7 | ||
LGMD2B: Limb-girdle muscular dystrophy 2B; LGMD2A: Limb-girdle muscular dystrophy 2A.
Mean fatty infiltration scores in the thigh muscles ranged from 2.0 to 3.0 in patients with mutations in THD, and from 1.0 to 1.5 in those with mutations in the C- or N-terminal domains. One patient (P4) with a homozygous deletion mutation in the C2 domain had a high score of 3.4.
DISCUSSION
The diagnoses of collagen VI-related myopathies in the patients of this study were confirmed genetically. Most of the mutations in the patients presented in this case series were novel, which were consistent with other reports.[19,20] A previous report indicated that patients with UCMD were more severely affected than those with BM;[16] however, the UCMD and BM patients in our study had no significant difference in disease severity and duration except for age of disease onset at the time of examination. The total MRI fatty infiltration scores for all thigh muscles did not significantly differ between UCMD and BM patients.
We demonstrated a distinct pattern of fatty infiltration in the thigh muscles of patients with collagen VI-related myopathies. AM and GM were the most severely affected muscles, while gracilis, sartorius, and AL were relatively spared. The similar findings were reported by Mercuri et al.[16] in both UCMD and BM patients. Our results also confirmed previous reports of greater peripheral than the central fatty infiltration in vastus lateralis.[14,16,21] We defined the “sandwich” sign, which described the MRI changes in VL and also detected the “central shadow” sign in RF, as reported by others.[14,15,16] A unique finding of our study was the small central region in the RF that was spared fatty infiltration in most of our patients. This region gave the appearance of a “target” on MRI. The “target” sign was not seen as usual as “sandwich” sign. Patients with or without these signs may have no significant difference in demographic and clinical aspects. Moreover, the fatty infiltration in semitendinosus was nonhomogeneous, which has not been previously described.
We found that the “sandwich” sign in VL and “target” sign in RF were common in our patients with collagen VI-related myopathies and were specific to these conditions. These MRI changes had a positive predictive value of 69% for BM,[21] and had high sensitivities and specificities. Supporting our findings, both “sandwich” and “target” signs are very rare in other neuromuscular disorders in the literatures, such as Duchenne muscular dystrophy,[10,22] lipid metabolic myopathy,[23] inflammatory myopathy,[8] LGMD2A,[24] Emery-Dreifuss muscular dystrophy,[25,26,27] and congenital muscular dystrophy with rigid spine syndrome (RSMD1).[17,28]
Our study demonstrated that the fatty infiltration associated with collagen VI-related myopathies was more severe in patients with mutations in the THD than those with mutations in the N- or C-terminal domain. The degree of fatty infiltration seen in the MRI was associated with the severity of clinical symptoms.[16] Homozygous premature termination codon-causing mutations in the triple-helix domains led to the most severe phenotypes.[19] De novo dominant mutations in severe UCMD occurred relatively frequently in all three collagen VI chains. The severity of the phenotype depends on the ability of mutant chains to be incorporated in the multimeric structure of collagen VI.[29]
In conclusion, the muscle MRI changes in collagen VI-related myopathies showed a distinct pattern and can be helpful for the differential diagnosis. The severity of fatty infiltration seen in muscle MRI may have a relationship with the locations of the causative genetic mutations underlying the myopathy.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
The study was supported by a grant from the Ministry of Science and Technology of China (2011ZX09307-001-07).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Bönnemann CG. The collagen VI-related myopathies:Muscle meets its matrix. Nat Rev Neurol. 2011;7:379–90. doi: 10.1038/nrneurol.2011.81. doi: 10.1038/nrneurol.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lampe AK, Bushby KM. Collagen VI related muscle disorders. J Med Genet. 2005;42:673–85. doi: 10.1136/jmg.2002.002311. doi: 10.1136/jmg.2002.002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bushby K, Collins J, Hicks D. Collagen type VI myopathies. Adv Exp Med Biol. 2014;802:185–99. doi: 10.1007/978-94-007-7893-1_12. doi: 10.1007/978-94-007-7893-1_12. [DOI] [PubMed] [Google Scholar]
- 4.Allamand V, Briñas L, Richard P, Stojkovic T, Quijano-Roy S, Bonne G. ColVI myopathies:Where do we stand, where do we go?Skelet Muscle. 2011;1:30. doi: 10.1186/2044-5040-1-30. doi: 10.1186/2044-5040-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yonekawa T, Nishino I. Ullrich congenital muscular dystrophy:Clinicopathological features, natural history and pathomechanism(s) J Neurol Neurosurg Psychiatry. 2015;86:280–7. doi: 10.1136/jnnp-2013-307052. doi: 10.1136/jnnp-2013-307052. [DOI] [PubMed] [Google Scholar]
- 6.Allamand V, Merlini L, Bushby K. Consortium for Collagen VI-Related Myopathies. 166th ENMC international workshop on collagen type VI-related myopathies, 22-24 May 2009, Naarden, the Netherlands. Neuromuscul Disord. 2010;20:346–54. doi: 10.1016/j.nmd.2010.02.012. doi: 10.1016/j.nmd.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Armaroli A, Trabanelli C, Scotton C, Venturoli A, Selvatici R, Brisca G, et al. Paternal germline mosaicism in collagen VI related myopathies. Eur J Paediatr Neurol. 2015;19:533–6. doi: 10.1016/j.ejpn.2015.04.002. doi: 10.1016/j.ejpn.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Zheng YM, Liu LL, Wang L, Xiao JX, Zhang W, Yuan Y, et al. Magnetic resonance imaging changes of thigh muscles in myopathy with antibodies to signal recognition particle. Rheumatology (Oxford) 2015;54:1017–24. doi: 10.1093/rheumatology/keu422. doi: 10.1093/rheumatology/keu422. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Wang ZX, Hong DJ, Lv H, Zhang W, Yuan Y, et al. Mutational spectrum and clinical features in 35 unrelated mainland Chinese patients with GNE myopathy. J Neurol Sci. 2015;354:21–6. doi: 10.1016/j.jns.2015.04.028. doi: 10.1016/j.jns.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Li WZ, Zheng YM, Zhang W, Wang ZX, Xiao JX, Yuan Y. Progression and variation of fatty infiltration of the thigh muscles in Duchenne muscular dystrophy, a muscle magnetic resonance imaging study. Neuromuscul Disord. 2015;25:375–80. doi: 10.1016/j.nmd.2015.01.003. doi: 10.1016/j. nmd.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Kana V, Kellenberger CJ, Rushing EJ, Klein A. Muscle magnetic resonance imaging of the lower limbs: Valuable diagnostic tool in the investigation of childhood neuromuscular disorders. Neuropediatrics. 2014;45:278–88. doi: 10.1055/s-0034-1381954. doi: 10.1055/s-0034-1381954. [DOI] [PubMed] [Google Scholar]
- 12.Wattjes MP, Kley RA, Fischer D. Neuromuscular imaging in inherited muscle diseases. Eur Radiol. 2010;20:2447–60. doi: 10.1007/s00330-010-1799-2. doi: 10.1007/s00330-010-1799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercuri E, Pichiecchio A, Allsop J, Messina S, Pane M, Muntoni F. Muscle MRI in inherited neuromuscular disorders:Past, present, and future. J Magn Reson Imaging. 2007;25:433–40. doi: 10.1002/jmri.20804. doi: 10.1002/jmri.20804. [DOI] [PubMed] [Google Scholar]
- 14.Morrow JM, Pitceathly RD, Quinlivan RM, Yousry TA. Muscle MRI in Bethlem myopathy. BMJ Case Rep 2013. 2013 doi: 10.1136/bcr-2013-008596. pii: Bcr2013008596. doi: 10.1136/bcr-2013-008596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drakonaki EE, Allen GM. Magnetic resonance imaging, ultrasound and real-time ultrasound elastography of the thigh muscles in congenital muscle dystrophy. Skeletal Radiol. 2010;39:391–6. doi: 10.1007/s00256-009-0861-0. doi: 10.1007/s00256-009-0861-0. [DOI] [PubMed] [Google Scholar]
- 16.Mercuri E, Lampe A, Allsop J, Knight R, Pane M, Kinali M, et al. Muscle MRI in Ullrich congenital muscular dystrophy and Bethlem myopathy. Neuromuscul Disord. 2005;15:303–10. doi: 10.1016/j.nmd.2005.01.004. doi: 10.1016/j.nmd.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Mercuri E, Talim B, Moghadaszadeh B, Petit N, Brockington M, Counsell S, et al. Clinical and imaging findings in six cases of congenital muscular dystrophy with rigid spine syndrome linked to chromosome 1p (RSMD1) Neuromuscul Disord. 2002;12:631–8. doi: 10.1016/s0960-8966(02)00023-8. doi: 10.1016/S0960-8966(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 18.Lampe AK, Dunn DM, von Niederhausern AC, Hamil C, Aoyagi A, Laval SH, et al. Automated genomic sequence analysis of the three collagen VI genes:Applications to Ullrich congenital muscular dystrophy and Bethlem myopathy. J Med Genet. 2005;42:108–20. doi: 10.1136/jmg.2004.023754. doi: 10.1136/jmg.2004.023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briñas L, Richard P, Quijano-Roy S, Gartioux C, Ledeuil C, Lacène E, et al. Early onset collagen VI myopathies:Genetic and clinical correlations. Ann Neurol. 2010;68:511–20. doi: 10.1002/ana.22087. doi: 10.1002/ana.22087. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YZ, Zhao DH, Yang HP, Liu AJ, Chang XZ, Hong DJ, et al. Novel collagen VI mutations identified in Chinese patients with Ullrich congenital muscular dystrophy. World J Pediatr. 2014;10:126–32. doi: 10.1007/s12519-014-0481-1. doi: 10.1007/s12519-014-0481-1. [DOI] [PubMed] [Google Scholar]
- 21.ten Dam L, van der Kooi AJ, van Wattingen M, de Haan RJ, de Visser M. Reliability and accuracy of skeletal muscle imaging in limb-girdle muscular dystrophies. Neurology. 2012;79:1716–23. doi: 10.1212/WNL.0b013e31826e9b73. doi: 10.1212/WNL.0b013e31826e9b73. [DOI] [PubMed] [Google Scholar]
- 22.Zheng YM, Li WZ, Lv H, Zhang W, Xiao JX, Yuan Y, et al. The trefoil with single fruit sign in muscle magnetic resonance imaging is highly specific for dystrophinopathies. Eur J Radiol. 2015;84:1992–8. doi: 10.1016/j.ejrad.2015.06.011. doi: 10.1016/j.ejrad.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Xu CX, Zhao YW, Liu J, Zhang W, Wang ZX, Yuan Y. Muscle MRI in neutral lipid storage disease with myopathy carrying mutation c.187+1G>A. Muscle Nerve. 2015;51:922–7. doi: 10.1002/mus.24507. doi: 10.1002/mus.24507. [DOI] [PubMed] [Google Scholar]
- 24.Mercuri E, Bushby K, Ricci E, Birchall D, Pane M, Kinali M, et al. Muscle MRI findings in patients with limb girdle muscular dystrophy with calpain 3 deficiency (LGMD2A) and early contractures. Neuromuscul Disord. 2005;15:164–71. doi: 10.1016/j.nmd.2004.10.008. doi: 10.1016/j.nmd.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Carboni N, Mura M, Marrosu G, Cocco E, Ahmad M, Solla E, et al. Muscle MRI findings in patients with an apparently exclusive cardiac phenotype due to a novel LMNA gene mutation. Neuromuscul Disord. 2008;18:291–8. doi: 10.1016/j.nmd.2008.01.009. doi: 10.1016/j.nmd.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Deconinck N, Dion E, Ben Yaou R, Ferreiro A, Eymard B, Briñas L, et al. Differentiating Emery-Dreifuss muscular dystrophy and collagen VI-related myopathies using a specific CT scanner pattern. Neuromuscul Disord. 2010;20:517–23. doi: 10.1016/j.nmd.2010.04.009. doi: 10.1016/j.nmd.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Mercuri E, Counsell S, Allsop J, Jungbluth H, Kinali M, Bonne G, et al. Selective muscle involvement on magnetic resonance imaging in autosomal dominant Emery-Dreifuss muscular dystrophy. Neuropediatrics. 2002;33:10–4. doi: 10.1055/s-2002-23593. doi: 10.1055/s.2002.23593. [DOI] [PubMed] [Google Scholar]
- 28.Mercuri E, Clements E, Offiah A, Pichiecchio A, Vasco G, Bianco F, et al. Muscle magnetic resonance imaging involvement in muscular dystrophies with rigidity of the spine. Ann Neurol. 2010;67:201–8. doi: 10.1002/ana.21846. doi: 10.1002/ana.21846. [DOI] [PubMed] [Google Scholar]
- 29.Lampe AK, Zou Y, Sudano D, O’Brien KK, Hicks D, Laval SH, et al. Exon skipping mutations in collagen VI are common and are predictive for severity and inheritance. Hum Mutat. 2008;29:809–22. doi: 10.1002/humu.20704. doi: 10.1002/humu.20704. [DOI] [PubMed] [Google Scholar]