Abstract
We developed a PCR-based assay to differentiate medically important species of Aspergillus from one another and from other opportunistic molds and yeasts by employing universal, fungus-specific primers and DNA probes in an enzyme immunoassay format (PCR-EIA). Oligonucleotide probes, directed to the internal transcribed spacer 2 region of ribosomal DNA from Aspergillus flavus, Aspergillus fumigatus, Aspergillus nidulans, Aspergillus niger, Aspergillus terreus, Aspergillus ustus, and Aspergillus versicolor, differentiated 41 isolates (3 to 9 each of the respective species; P < 0.001) in a PCR-EIA detection matrix and gave no false-positive reactions with 33 species of Acremonium, Exophiala, Candida, Fusarium, Mucor, Paecilomyces, Penicillium, Rhizopus, Scedosporium, Sporothrix, or other aspergilli tested. A single DNA probe to detect all seven of the most medically important Aspergillus species (A. flavus, A. fumigatus, A. nidulans, A. niger, A. terreus, A. ustus, and A. versicolor) was also designed. Identification of Aspergillus species was accomplished within a single day by the PCR-EIA, and as little as 0.5 pg of fungal DNA could be detected by this system. In addition, fungal DNA extracted from tissues of experimentally infected rabbits was successfully amplified and identified using the PCR-EIA system. This method is simple, rapid, and sensitive for the identification of medically important Aspergillus species and for their differentiation from other opportunistic fungi.
Invasive aspergillosis (IA) is a leading cause of infection among patients undergoing hematopoietic stem cell transplantation, solid organ transplantation, and treatment for hematological malignancies (11, 45). Although the incidence of IA differs from one host group to another, the mortality rate from this disease is high and ranges from 58 to 99% in immunocompromised patients (14, 38). Aspergillus fumigatus remains the most frequent cause of IA; however, at least 20 other species, including A. flavus, A. terreus, A. niger, A. nidulans, A. versicolor, and A. ustus have been reported to cause human infection (13). In some centers, A. terreus is becoming an increasingly common cause of IA (2, 27). This species is of concern because it is less susceptible to amphotericin B in vitro than A. fumigatus (37, 62) and has the potential to cause fulminant invasive infections in immunocompromised patients (27).
The high mortality rate of IA is caused in part by the rapid progression of infection and by difficulties in its diagnosis, particularly in the early stages of disease. Although there has been some progress in attempts to expedite the radiological diagnosis of IA (4, 7), early diagnosis remains difficult. Definitive diagnosis requires histopathologic evidence of deep-tissue invasion or a positive culture from a sterile site (1, 47). However, invasive diagnostic procedures can be dangerous for thrombocytopenic patients, and blood cultures are seldom positive (47, 66). In addition, the morphological similarities of many filamentous fungi in tissue make their specific identification difficult (25, 32, 47). The detection of galactomannan antigen in serum by enzyme immunoassay (EIA) has been an important adjunct in the diagnosis of IA (44, 64). However, test sensitivity and specificity have been reported to vary from 56 to 100% and from 71 to 94%, respectively, depending upon variations in the patient population studied, the test conditions used, and/or the immunosuppressive agents employed (18, 44, 47, 57, 64). Although this test is promising as a screening tool, the effect on specificity of a newly introduced lower positive cutoff value is still under investigation. In addition, this test has not been shown to detect circulating antigen from all medically important Aspergillus species or to discriminate among Aspergillus species (61).
PCR-based methods, which show potential as rapid, sensitive means to diagnose infections, have been used in the detection of DNA from some Aspergillus species (6, 15, 23, 33, 69, 70). Such tests may provide increased sensitivity compared to antigen detection assays (21, 30, 33, 70). However, most tests to date have been directed to the detection of only one Aspergillus species (12, 52, 72) or to the detection of only single-copy genes (8, 23, 29). Others have used primers or probes which are pan-fungal and directed to highly conserved, multicopy gene targets in an attempt to increase test sensitivity but, by doing so, have forfeited species specificity (15, 31, 36, 60, 65, 69, 70). In addition, most amplicon detection methods have used technologies that are not easily adapted for use in a clinical microbiology laboratory, such as gel electrophoresis with or without restriction fragment length polymorphism analysis (33, 60, 65, 72), Southern blotting (6, 15, 65, 70), single-strand conformational polymorphism analysis (34, 56, 67), or DNA sequence analysis (12, 24, 55, 63).
Our investigators previously described a PCR-based method that employed universal, fungus-specific primers and species-specific oligonucleotide probes, directed to the internal transcribed spacer 2 (ITS2) region of ribosomal DNA (rDNA), to identify Candida species (16, 19, 59) and the dimorphic fungi (39). Amplicons were detected in a colorimetric EIA format (PCR-EIA) that could be easily adapted to clinical laboratory use. We describe here the design and application of specific DNA probes to differentiate medically important species of Aspergillus from one another and from other molds and Candida species using this simple and rapid PCR-EIA format.
MATERIALS AND METHODS
Microorganisms.
Microorganisms and their sources are listed in Tables 1 and 2. Isolates were obtained from the culture collections of the Mycotic Diseases Branch, Centers for Disease Control and Prevention (CDC), Atlanta, Ga., from the American Type Culture Collection (ATCC), Manassas, Va., and from the National Regional Reference Laboratory, Peoria, Ill. A laboratory variant of Candida albicans strain CBS 2730 was obtained as a gift from R. Rüchel (48), and a human clinical isolate of A. ustus from Korea was provided by one of the authors (J. H. Shin). Phenotypic characteristics as well as DNA sequencing were used to identify all isolates for probe development, type cultures and bona fide culture collection isolates were used whenever possible for both positive and negative control isolates, and multiple strains of most organisms were included to validate probe assays (Tables 1 and 2).
TABLE 1.
Source and characteristics of Aspergillus spp. isolates used for probe development and testing
| Organism | Identification no.a | Source and/or characteristic(s) |
|---|---|---|
| A. flavus | CDC 001-96 | Unspecified |
| A. flavus | CDC B-5333b | Clinical isolate; California |
| A. flavus | ATCC 10124 | Aflatoxin-negative strain |
| A. flavus | ATCC 11497 | Proteolytic enzyme production |
| A. flavus | ATCC 34896 | Prosthetic mitral valve, endocarditis |
| A. flavus | ATCC 64025 | Human sputum; exoantigen testing |
| A. flavus var. columnaris | ATCC 44310 | Soy sauce koji, Thailand; aflatoxin negative |
| A. fumigatus | CDC B-1172b | Human clinical isolate; Georgia |
| A. fumigatus | CDC B-2570 | Unspecified |
| A. fumigatus | ATCC 1022 | Chicken lung |
| A. fumigatus | ATCC 14110 | Human sputum |
| A. fumigatus | ATCC 36607 | Preceptrol culture, clinical isolates |
| A. fumigatus | ATCC 42202 | Human sputum |
| A. fumigatus | ATCC 64026 | Human lung |
| A. fumigatus | ATCC 16907 | Spontaneous light-colored mutant |
| A. fumigatus var. ellipticus | ATCC 16903 | Human chest cavity lining; Illinois |
| A. nidulans | CDC B-5446b | Human bronchial wash |
| A. nidulans | ATCC 10074 | Preceptrol culture |
| A. nidulans | ATCC 16855 | Preceptrol culture; type strain |
| A. nidulans | ATCC 64027 | Human lung fluid; exoantigen testing |
| A. niger | CDC 92-243 | Human spinal fluid |
| A. niger | CDC B-5291 | Instrument room filter; Pennsylvania |
| A. niger | CDC B-5331b | Clinical isolate; California |
| A. niger | ATCC 1015 | Citric acid producer |
| A. niger | ATCC 10535 | Preceptrol culture |
| A. niger | ATCC 16404 | Blueberries |
| A. niger | ATCC 16888 | Preceptrol culture |
| A. niger | ATCC 64028 | Human; exoantigen testing |
| A. terreus | CDC B-5309 | Human clinical isolate, South Carolina |
| A. terreus | CDC B-5502b | Human sputum, Colorado |
| A. terreus | ATCC 1012 | Soil; Connecticut |
| A. terreus | ATCC 7860 | Unspecified |
| A. terreus | ATCC 10029 | Soil; Texas |
| A. terreus | ATCC 28301 | Human spinal fluid |
| A. terreus | ATCC 64029 | Human sputum; exoantigen testing |
| A. ustus | ATCC 16801b | Soil; Panama |
| A. ustus | CUH4 | Human clinical isolate; Korea |
| A. ustus | ATCC 14417 | Production of l-lysine |
| A. versicolor | ATCC 10072b | Human clinical isolate |
| A. versicolor | NRRL 238 | Type strain (ATCC 9577) |
| A. versicolor | NRRL 239 | Date fruits; California (ATCC 16856) |
NRRL, National Regional Reference Laboratory; CUH = Chonnam University Hospital.
Isolate used to obtain DNA sequence for probe development.
TABLE 2.
Source and characteristics of negative control isolates
| Organism | Identification no.a | Source and/or characteristic(s) |
|---|---|---|
| Acremonium recifei | ATCC 64745 | Human mycetoma; India |
| Acremonium strictum | ATCC 10141 | Preceptrol culture |
| Acrinonium strictum | ATCC 46646 | Human mycetoma |
| Aspergillus candidus | NRRL 303 | Type strain (ATCC 1002) |
| Aspergillus candidus | NRRL 312 | Instituto Biologico, Brazil (ATCC 16871) |
| Aspergillus chevalieri | ATCC16443 | Coffee beans |
| Aspergillus chevalieri | ATCC 24546 | Poultry feed; Ohio |
| Aspergillus clavatus | ATCC 18214 | Tarpaulin, fungus resistance testing |
| Aspergillus flavipes | ATCC 11013 | Microbiological oxidation to lactones |
| Aspergillus flavipes | ATCC 16805 | Soil; Haiti |
| Aspergillus flavipes | ATCC 24487 | Possible type strain |
| Aspergillus ochraceus | NRRL 398 | Type strain, produces aniline (ATCC 1008) |
| Aspergillus ochraceus | NRRL 4752 | Human scalp lesions (ATCC 12066) |
| Aspergillus parasiticus | NRRL 502 | Mealy bug on sugar cane |
| Aspergillus parasiticus | ATCC 56775 | Highly aflatoxigenic |
| Aspergillus parasiticus | ATCC 15517 | Preceptrol culture |
| Aspergillus parasiticus | CDC B-4571 | Ear; Hawaii |
| Aspergillus restrictus | NRRL 151 | New Orleans, La. |
| Aspergillus restrictus | NRRL 148 | Cotton fabric; United Kingdom |
| Aspergillus tamariib | ATCC 64841 | Gosling lung; Nigeria |
| Candida albicans | CDC B-311 | Human clinical isolate (ATCC 32354) |
| Candida albicans | CBS 2730 | Gift of R. Rüchel (laboratory variant) |
| Candida glabrata | NRRL Y-65 | Type strain, feces (ATCC 2001) |
| Candida krusei | CDC 259-75 | Unspecified |
| Candida parapsilosis | ATCC 22019 | Type strain; case of sprue; Puerto Rico |
| Candida tropicalis | CDC 38 | Unspecified |
| Exophiala dermatitidis | ATCC 28869 | Preceptrol culture |
| Exophiala jeanselmei | ATCC 18148 | Human foot; Maryland |
| Exophiala moniliae | ATCC 56486 | Human; Japan |
| Fusarium moniliforme | CDC 118A | Plant |
| Fusarium moniliforme | ATCC 38159 | Human; California |
| Fusarium oxysporum | ATCC 48112 | Preceptrol culture |
| Fusarium solani | CDC R2 | Melon |
| Fusarium solani | ATCC 58877 | Air sample; Maryland |
| Fusarium solani | ATCC 62877 | Human skin; Florida |
| Mucor circinelloides | ATCC 1209b | Minus strain |
| Mucor racemosus | ATCC 46130 | Soybean; Taiwan |
| Mucor racemosus | ATCC 7924 | Forest soil under basswood |
| Paecilomyces carneus | ATCC 46579 | Soil; Japan |
| Paecilomyces farinosus | ATCC 18236 | Soil; England |
| Paecilomyces lilacinus | ATCC 10114 | Soil; New York |
| Paecilomyces variotii | ATCC 22319 | Preceptrol culture |
| Penicillium marneffei | ATCC 58950 | Human skin abscess |
| Penicillium marneffei | ATCC 64101 | Human clinical isolate |
| Penicillium marneffei | ATCC 56573 | Unspecified |
| Penicillium notatum | ATCC 9478 | Contaminant; first penicillin producer |
| Penicillium notatum | ATCC 10108 | Rotting wood; Norway |
| Rhizopus oryzae (arrhizus) | ATCC 1230 | Wheat |
| Rhizomucor pusillus | ATCC 36606 | Brain of cat |
| Scedosporium apiospermum | ATCC 36282 | Human lung |
| Scedosporium apiospermum | ATCC 44328 | Human lung; Japan |
| Scedosporium apiospermum | ATCC 64215 | Human lung; Japan |
| Scedosporium prolificans | ATCC 64913 | Human bone; Maine |
| Sporothrix schenckii | ATCC 58251 | Human clinical isolate; Puerto Rico |
| Sporothrix schenckii | ATCC 28184 | Human arm lesion |
Aspergillus species and other filamentous fungi were grown at 35°C for 4 to 5 days on Sabouraud dextrose agar slants (Emmon's modification; BBL, Becton Dickinson Microbiology Systems, Cockeysville, Md.). Two slants were then washed by vigorously pipetting 5 ml of 0.01 M phosphate-buffered saline (PBS; 8.1 mM Na2HPO4, 1.9 mM KH2PO4, 0.85% NaCl; pH 7.2) onto the surface of each slant, and the washes were transferred to 500-ml Erlenmeyer flasks containing 200 ml of Sabouraud dextrose broth (BBL). Flasks were then incubated for 4 to 5 days on a rotary shaker (140 rpm) at ambient temperature. Growth was harvested in a biosafety level 2 cabinet by vacuum filtration through sterile filter paper (11-cm diameter, no. 1 thickness; Whatman International, Ltd., Maidstone, England) which had been placed into a sterile Büchner funnel attached to a 2-liter side-arm Erlenmeyer flask. The resultant cellular mat was washed three times with sterile distilled H2O (dH2O) by filtration, removed from the filter paper by gentle scraping with a rubber policeman, and frozen at −20°C until used.
Yeast were grown at 35°C on a rotary shaker (140 rpm) for 18 to 24 h in 10 ml of yeast potato dextrose broth (Difco Laboratories, Detroit, Mich.) in 50-ml Erlenmeyer flasks. Growth was harvested by centrifugation and washed twice with dH2O before DNA extraction as described below.
Extraction of fungal DNA.
Yeast DNA was extracted from overnight cultures using the PureGene gram-positive bacteria and yeast DNA extraction kit (Gentra Systems, Inc., Minneapolis, Minn.) as previously described (16). A mechanical disruption method, using grinding of cellular mats with a mortar and pestle in the presence of liquid nitrogen, was employed to obtain DNA from molds. Just before use, a portion of the frozen cellular mat (approximately equal in size to a quarter) was placed into an ice-cold, sterile mortar (6-in. diameter) in a biosafety level 2 cabinet. Liquid nitrogen was added to cover the mat, which was then ground into a fine powder with a sterile pestle. Additional liquid nitrogen was added as needed to keep the mat frozen during grinding. Filamentous fungal DNA was then extracted and purified using serial proteinase K and RNase treatments followed by phenol-chloroform extraction and ethanol precipitation by conventional methods (51) or by column chromatography using genomic tips as recommended by the manufacturer (QIAGEN Corp., Chatsworth, Calif.).
DNA sequencing.
DNA sequencing used two sets of primer pairs: (i) the fungus-specific, universal primer pair ITS3 and ITS4 (Table 3) was used to amplify a portion of the 5.8S rDNA region, the entire ITS2 region, and a portion of the 28S rDNA region for each fungal species, as previously described (16, 68); and (ii) the fungus-specific, universal primer pair ITS1 and ITS4 (Table 3) was also used to amplify a portion of the 18S rDNA region, the entire 5.8S region, the entire ITS1 and ITS2 regions, and a portion of the 28S rDNA region (68). A DNA reagent kit (TaKaRa Biomedicals, Shiga, Japan) was used for PCR amplification of genomic DNA before sequencing. The PCR was performed using 2 μl (5 ng) of test sample in a total PCR volume of 100 μl consisting of 10 μl of 10× Ex Taq buffer, 2.5 mM (each) dATP, dGTP, dCTP, and dTTP in 8 μl, 0.2 μl of each primer (20 μM), and 0.5 U of TaKaRa Ex Taq DNA polymerase. Thirty cycles of amplification were performed in a Perkin-Elmer 9600 thermal cycler (Emeryville, Calif.) after initial denaturation of DNA at 95°C for 5 min in the thermal cycler. Each cycle consisted of denaturation at 95°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 1 min. A final extension at 72°C for 5 min followed the last cycle. After amplification, samples were stored at −20°C until used. Appropriate measures to prevent PCR contamination were taken (19, 35). The aqueous phase of the primary PCR amplification mixture was purified using QIAquick spin columns (QIAGEN Corp.). DNA was eluted from each column with 50 μl of heat-sterilized Tris-EDTA buffer (10 mM Tris, 1 mM EDTA; pH 8.0).
TABLE 3.
Primers and probes used for identification of Aspergillus spp. by PCR-EIA
| Primer or probe | Nucleotide sequence (5′ to 3′)a | Chemistry and location |
|---|---|---|
| Primers | ||
| ITS1 | TCC GTA GGT GAA CCT GCG G | 18S rDNA universal fungal 5′ primer |
| ITS3 | GCA TCG ATG AAG AAC GCA GC | 5.8S rDNA universal fungal 5′ primer |
| ITS4 | TCC TCC GCT TAT TGA TAT GC | 28S rDNA universal fungal 3′ primer |
| Probes | ||
| B58 | GAA TCA TCG A(AG)T CTT TGA ACG | 5′-End-labeled biotin probe; 5.8S region of filamentous fungi |
| AFLA | GAA CGC AAA TCA ATC TTT | 5′-End-labeled digoxigenin probe; ITS2 region of A. flavus |
| AFUM | CCG ACA CCC ATC TTT ATT | 5′-End-labeled digoxigenin probe; ITS2 region of A. fumigatus |
| ANID | GGC GTC TCC AAC CTT ATC | 5′-End-labeled digoxigenin probe; ITS2 region of A. nidulans |
| ANIG | GAC GTT ATC CAA CCA TTT | 5′-End-labeled digoxigenin probe; ITS2 region of A. niger |
| ATER | GCA TTT ATT TGC AAC TTG | 5′-End-labeled digoxigenin probe; ITS2 region of A. terreus |
| AUST | CTT TTA TTT TAC CAG GTT | 5′-End-labeled digoxigenin probe; ITS2 region of A. ustus |
| AVER | CTC CAA CCA TTT TCT TCA | 5′-End-labeled digoxigenin probe; ITS2 region of A. versicolor |
| ASPEN-G | CCT CGA GCG TAT GGG GCT | 5′-End-labeled digoxigenin probe; ITS2 region of Aspergillus and Penicillium spp. |
Patents have been issued or are pending for all Aspergillus species probes.
DNA was then labeled using a dye terminator cycle sequencing kit (ABI PRISM; Applied Biosystems, Foster City, Calif.), amplified, purified, and sequenced in both the forward and reverse directions using an automated capillary DNA sequencer (model 373; ABI Systems, Bethesda, Md.) as described previously (17).
Extraction of DNA from tissue specimens.
Rabbit models of IA and candidiasis developed previously (26, 49) were used to detect fungal DNA in tissues from infected animals. Rabbits were euthanized and necropsied on day 3 after infection, and tissue samples (5 by 5 by 1 mm) were placed immediately after removal onto aluminum foil, wrapped tightly, and submerged into liquid nitrogen for 1 min. Tissue samples were then stored frozen at −70°C until used. Samples of frozen tissue were thawed, weighed, and homogenized for 30 s at a speed setting of 4.5 in a FastPrep cell disruption instrument (Qbiogene, Carlsbad, Calif.), using lysing matrix tubes containing a combination of three beads (1/4-in. ceramic sphere plus garnet matrix plus 1/4-in. ceramic cylinder) and 1 ml of CLS-Y lysing solution as instructed by the manufacturer (Qbiogene). DNA was extracted and purified from the tissue homogenate using the FastDNA kit (Qbiogene) according to the manufacturer's directions before use in the PCR-EIA.
PCR amplification for the PCR-EIA.
The same fungus-specific, universal primer pair, ITS3 and ITS4 (Table 3), that was employed for DNA sequencing was also used to amplify the ITS2 region of DNA for the PCR-EIA. Amplicons from this region then served as the target for probe hybridization reactions as described before (16, 19, 59). Primers and probes were synthesized, and probes were end labeled with digoxigenin or biotin as previously described (39). The PCR was performed using 2 μl (2 ng) of test sample in a total PCR volume of 100 μl consisting of the following reagents (Roche Molecular Biochemicals, Inc., Indianapolis, Ind.): 10 μl of 10× PCR buffer containing 15 mM MgCl2, 2.5 mM (each) dATP, dGTP, dCTP, and dTTP in 8 μl, 0.2 μl of each primer (20 μM), and 2.5 U of Taq DNA polymerase. Thirty cycles of amplification were performed in a Perkin-Elmer 9600 thermal cycler after initial denaturation of DNA in the thermal cycler at 95°C for 5 min. Each cycle consisted of denaturation at 95°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 1 min. A final extension at 72°C for 5 min followed the last cycle. It was previously demonstrated that bacterial and human DNA were not amplified by the fungus-specific primers we employed (19), and therefore DNA from these sources was not tested in this study.
Microtitration plate EIA for the detection of PCR products (PCR-EIA).
Seven Aspergillus species probes, directed to the ITS2 region of Aspergillus rDNA and 5′-end labeled with digoxigenin, and an all-filamentous fungal capture probe, directed to the 5.8S rDNA region and 5′-end labeled with biotin (Table 3), were used to detect PCR amplicons in a colorimetric streptavidin-coated microtiter plate format (16, 19, 59). A DNA probe to detect all of the most medically important Aspergillus species (Tables 3 and 4), directed to a more conserved ITS2 region upstream from the binding region of the species probes, was also 5′-end labeled with digoxigenin and used in the PCR-EIA. The absorbance at 650 nm for each well was determined using a microtitration plate reader (UV Max; Molecular Devices, Inc., Menlo Park, Calif.), and the absorbance value for the reagent blank, where DNA was absent and replaced with dH2O, was subtracted from each test sample. To determine the limit of sensitivity of the PCR-EIA compared to detection of amplicons by agarose gel electrophoresis and ethidium bromide staining, DNA was serially diluted, amplified by PCR as described above, and tested in both systems.
TABLE 4.
Specificity of probes to detect Aspergillus species DNA by PCR-EIA
| Target DNA | Mean A650 ± SE using probe fora:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| A. flavus | A. fumigatus | A. nidulans | A. niger | A. terreus | A. ustus | A. versicolor | Aspen-Gb | |
| A. flavus | 1.727 ± 0.164 (7) | 0c | 0 | 0 | 0 | 0 | 0 | 1.247 ± 0.092 (3) |
| A. fumigatus | 0 | 2.368 ± 0.174 (9) | 0 | 0 | 0 | 0 | 0 | 1.269 ± 0.114 (6) |
| A. nidulans | 0 | 0 | 0.868 ± 0.127 (4) | 0 | 0 | 0 | 0 | 1.175 ± 0.140 (4) |
| A. niger | 0 | 0 | 0 | 1.242 ± 0.167 (8) | 0 | 0 | 0 | 1.800 ± 0.169 (5) |
| A. terreus | 0 | 0 | 0 | 0 | 1.512 ± 0.241 (7) | 0 | 0 | 0.440 ± 0.065 (3) |
| A. ustus | 0 | 0 | 0.547 ± 0.034 (3) | 0 | 0 | 1.410 ± 0.203 (3) | 0 | 0.910 ± 0.040 (3) |
| A. versicolor | 0 | 0 | 0 | 0 | 0 | 0 | 1.642 ± 0.080 (3) | 1.407 ± 0.092 (2) |
The mean A650 value ± SE for each of the given probes after subtraction of the corresponding reagent blank (no DNA) for the number of strains tested in parentheses; each strain was tested in multiple experiments.
The Aspen-G probe cross-reacted with DNA from P. marneffei (1.24 ± 0.15; n = 3) and showed some reactivity with P. notatum DNA (0.17 ± 0.03; n = 2) but did not react with DNA from any other yeast or mold tested. DNA from Paecilomyces and Exophila spp. and from Scedosporium prolificans was not tested using the Aspen-G probe.
Mean absorbance value ± SE after subtraction of reagent blank for all negative control samples listed above: A. flavus probe, 0.002 ± 0.0006 (n = 37); A. fumigatus probe, 0.002 ± 0.0005 (n = 35); A. nidulans probe (excluding A. ustus DNA), 0.003 ± 0.0009 (n = 36); A. niger probe, 0.006 ± 0.001 (n = 34); A. terreus probe, 0.009 ± 0.003 (n = 37); A. ustus probe, 0.005 ± 0.002 (n= 38); and A. versicolor probe, 0.004 ± 0.001 (n = 41). P < 0.001 for difference between means of positive and negative control samples (excluding A. nidulans probe versus A. ustus DNA, where P > 0.05).
Statistical analyses.
Student's t test was used to determine significant differences between means for test and control samples. A P value of ≤0.05 was considered significant.
Nucleotide sequence accession number.
DNA sequencing results for isolates used for probe design were deposited with GenBank, and the accession numbers are as follows: A. flavus strain CDC B-5333, AF117920; A. fumigatus strain CDC B-1172, U93683; A. nidulans strain CDC B-5446, U93686; A. niger strain CDC B-5331, U93685; A. terreus strain CDC B-5502, U93684; A. ustus strain ATCC 16801, AF454136; and A. versicolor strain ATCC 10072, AF454142.
RESULTS
Amplification of DNA from all fungi using the universal, fungus-specific primer pair, ITS3 and ITS4.
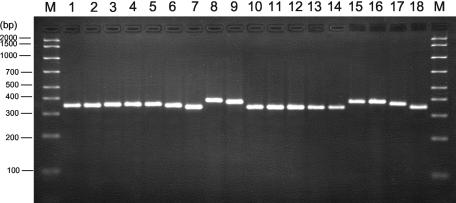
DNA from three to nine strains of each Aspergillus species for which DNA probes were designed (Tables 1 and 3) was PCR amplified using the universal, fungus-specific primer pair, ITS3 and ITS4. Amplicons were detected in agarose gels after electrophoresis and staining with ethidium bromide. DNA from each Aspergillus species for which DNA probes were designed produced amplicons of approximately 350 bp in size, and PCR amplicons from selected Aspergillus species are shown in Fig. 1. No significant differences in amplicon size were noted among the various Aspergillus species tested.
FIG. 1.
PCR amplicons from Aspergillus species compared to those for other molds. DNA was extracted from cultures of the following organisms, PCR amplified, and detected in an agarose gel after ethidium bromide staining as described in Materials and Methods: M, molecular mass markers (AmpliSize ruler; Bio-Rad Laboratories); lane 1, A. fumigatus (ATCC 14110); lane 2, A. tamarii (ATCC 64841); lane 3, A. niger (ATCC 16404); lane 4, A. terreus (ATCC 10029); lane 5, A. strictum (ATCC 10141); lane 6, A. parasiticus (ATCC 56775); lane 7, F. solani (ATCC 58877); lane 8, S. apiospermum (ATCC 64215); lane 9, S. prolificans (ATCC 64913); lane 10, P. marneffei (ATCC 58950); lane 11, P. marneffei (ATCC 64101); lane 12, S. schenckii (ATCC 58251); lane 13, P. farinosis (ATCC 18236); lane 14, P. variotii (ATCC 22319); lane 15, P. lilacinus (ATCC 10114); lane 16, E. jeanselmei (ATCC 18148); lane 17, E. dermatitidis (ATCC 28869); lane 18, E. moniliae (ATCC 56486).
DNA from all non-Aspergillus species molds (Table 2), to be used as negative controls for the Aspergillus species probes, produced amplicons of between 340 and 410 bp (representative examples are shown in Fig. 1). DNA from Fusarium solani, Paecilomyces farinosis, and Paecilomyces variotii, strains of Penicillium marneffei, and Sporothrix schenckii gave the smallest amplicons, whereas DNA from Scedosporium species, Paecilomyces lilacinus, Exophiala jeanselmei, and Exophiala dermatitidis gave the largest amplicons (Fig. 1). DNA from eight Aspergillus species for which no probes were designed, but which were also used as negative controls for the Aspergillus species probes (Table 2), as well as DNA from species of Rhizopus, Rhizomucor, and Mucor, gave amplicons of approximately the same size as those from Aspergillus species for which the probes were designed (approximately 350 to 360 bp). Amplicons from Candida species ranged from approximately 325 bp for C. tropicalis to approximately 410 bp for C. glabrata, confirming previous results from our laboratory (19). Use of amplicon size after gel electrophoresis and ethidium bromide staining afforded some discrimination among the filamentous fungi and yeasts but not among species of Aspergillus. In addition, a specific, unequivocal identification, relying on amplicon size in stained agarose gels alone, was not possible. Therefore, DNA sequence analysis of the ITS2 region of the seven most common Aspergillus species responsible for human infection was conducted. Sequences were aligned, and results were used to design specific DNA probes (Table 3) to differentiate Aspergillus species from one another and from other medically important opportunistic fungal genera.
Specificity of oligonucleotide probes among medically important Aspergillus species.
DNA was amplified as described above and used as the target for Aspergillus species probes in a colorimetric EIA detection format (PCR-EIA). Probe sequences were adjusted in certain cases to introduce minor intentional base pair mismatches, so as to reduce cross-reactivity among the Aspergillus species and mold targets. These mismatches were, however, sufficiently minor so as to maintain significant reactivity to the intended target sequences.
Table 4 shows the results of probe binding in the PCR-EIA microtiter plate matrix using Aspergillus DNA from each of the species for which probes were designed. Significant reactivity (P < 0.001) was observed for each of the target DNAs using the homologous species probe (range in mean A650± standard error [SE] for DNA probes versus homologous DNA, 0.868 ± 0.127 to 2.368 ± 0.174; n = 3 to 9). No significant cross-reactivity was observed among these Aspergillus species probes, with the exception of the A. nidulans probe versus A. ustus DNA (Table 4). However, the A. ustus probe did not cross-react with A. nidulans DNA and, by a process of elimination, these two species could be easily differentiated. Background reactivity for all other species probes was insignificant (range in mean A650± SE for DNA probes versus nonhomologous DNA, 0.002 ± 0.0005 to 0.009 ± 0.003; n = 34 to 38).
One isolate obtained from the ATCC as a strain of A. flavus (ATCC 64841) did not react with the A. flavus probe. DNA from this isolate was therefore sequenced in the ITS1 as well as ITS2 rDNA regions (GenBank accession numbers AF453894 and AF454112, respectively). Comparative analysis with reference DNA sequences found in GenBank revealed that DNA from this isolate more closely matched sequences for strains of A. tamarii than those for A. flavus. This isolate is therefore listed in Table 2 as A. tamarii and was not considered to be an isolate of A. flavus, despite its designation as such in the ATCC catalog.
BLAST search analysis, using each Aspergillus species probe sequence to query the GenBank database, revealed no significant homology with ITS2 reference sequences from any unrelated or medically significant fungus for the A. flavus, A. nidulans, A. niger, A. terreus, A. ustus, or A. versicolor probes. Because intentional base changes from the original DNA sequence were designed into the A. fumigatus probe to remove probe cross-reactivity, the A. fumigatus probe sequence did not match any fungal DNA sequences in GenBank.
A probe designed to react with DNA from all of the most medically important Aspergillus species listed in Table 1 (Aspen-G [Table 3]) demonstrated strong reactivity (mean A650± SE, 0.910 ± 0.040 to 1.800 ± 0.169) with DNA from all but one of these species (Table 4). A somewhat lower reactivity of this probe was observed with DNA from isolates of A. terreus compared to that of DNA from the other Aspergillus species (Table 4). Nonetheless, the reactivity of the Aspen-G probe with A. terreus DNA was significantly above background levels (mean A650, 0.440 ± 0.065 versus 0.003 ± 0.002; P < 0.001).
The Aspen-G probe was not completely specific for Aspergillus species, in that it also reacted to varying degrees with DNA from two Penicillium species tested (mean A650± SE versus DNA from Penicillium marneffei, 1.24 ± 0.15 [n = 3] and versus DNA from Penicillium notatum, 0.17 ± 0.03 [n = 2]). However, this probe did not cross-react in the PCR-EIA system with DNA from any of the other yeasts or molds tested (Table 4, footnote) and may therefore provide a means to differentiate Scedosporium apiospermum (Pseudallescheria boydii) and species of Rhizopus and Fusarium from those of the most medically important Aspergillus species.
Specificity of Aspergillus species probes tested against yeasts and other filamentous fungi.
No significant cross-reactivity of the Aspergillus species probes was observed against DNA from any of the organisms listed in Table 2 (mean A650± SE for DNA from all organisms tested using Aspergillus species probes in 8 to 24 sample runs, 0.003 ± 0.005; P < 0.001) with the exception of some minor cross-reactivity observed for the A. versicolor probe versus A. candidus DNA (mean A650± SE, 0.153 ± 0.018). However, examination of rDNA ITS2 regions in the A. candidus DNA sequence upstream of the A. versicolor probe binding region indicated that design of a specific probe for A. candidus DNA should be relatively straightforward. Resolution of these species could therefore be accomplished by using these two probes and a process of elimination in the probe matrix system. The reactivity of the A. versicolor probe with A. candidus DNA gave an A650 value 10-fold lower than that with A. versicolor DNA. Thus, these two species can easily be distinguished in the probe matrix configuration by the 10-fold difference in signal strength for A. candidus DNA compared to that for the A. versicolor positive control included on each test plate.
The agarose gel electrophoresis results, showing successful amplification of DNA from all fungal organisms tested, demonstrated that PCR amplicons were obtained for each of the genera and species of fungi tested, including those used as negative controls (examples are shown in Fig. 1). Therefore, negative probe test results were not caused by insufficient sample DNA or lack of PCR amplification but were the result of sequence nonhomology.
Application of the A. fumigatus probe for the detection of DNA in tissues from infected rabbits.
Rabbit models of disseminated candidiasis and IA, previously described by our laboratory (26, 49), were used as the source of infected tissues. DNA extracted from frozen, homogenized kidney tissue was PCR amplified using primers ITS3 and ITS4. Amplicons were detected in the PCR-EIA format with a previously described C. albicans-specific DNA probe (16, 19, 59) and the A. fumigatus probe described in the present study (Table 3). The C. albicans-specific probe detected DNA only in tissue from C. albicans-infected and not A. fumigatus-infected rabbits (mean A650 for DNA from 70 and 10 mg, respectively, of kidney tissue from a C. albicans-infected rabbit was 1.980 and 1.600 versus 0.000 and 0.000 for DNA from the same amount of kidney tissue from an A. fumigatus-infected rabbit) or from C. albicans yeast cells (mean A650 for DNA from 104 CFU of C. albicans yeast cells in PBS was 1.550 versus 0.000 for DNA from an equal number of A. fumigatus conidia). In addition, the A. fumigatus probe detected DNA only in tissue from A. fumigatus-infected and not from C. albicans-infected rabbits (mean A650 for DNA from 70 and 10 mg, respectively, of kidney tissue from an A. fumigatus-infected rabbit was 1.360 and 0.130, versus 0.000 and 0.000 for DNA from the same amount of kidney tissue from a C. albicans-infected rabbit) or from A. fumigatus conidia (mean A650 for DNA from 104 CFU of A. fumigatus conidia in PBS was 2.110 versus 0.000 for DNA from an equal number of C. albicans yeast cells). The mean A650 values obtained for DNA extracted from 70 mg of tissue from rabbits infected with C. albicans was higher than that for 10 mg of tissue, but the values were not linear with respect to tissue mass. In contrast, the A650 value for DNA extracted from 10 mg of tissue from rabbits infected with A. fumigatus was approximately 10 times lower than that for DNA extracted from 70 mg of tissue. Such results may reflect differences in the total CFU per gram of tissue recovered from the C. albicans-infected compared with the A. fumigatus-infected rabbits. Tissues from the C. albicans-infected rabbits contained a mean of 1.5 × 107 CFU per g of tissue compared with a mean of 3.8 × 104 CFU per g of tissue from the A. fumigatus-infected rabbits. The efficiency of the PCR was apparently saturated with DNA from 10 mg of tissue from the C. albicans-infected rabbits (equivalent to 1.5 × 105 CFU of C. albicans cells), so that DNA from 70 mg of tissue did not give A650 values that were significantly higher than those for 10 mg of tissue. In contrast, DNA from 10 mg of tissue from A. fumigatus-infected rabbits (equivalent to 3.9 × 102 A. fumigatus conidia) did not saturate the efficiency of the PCR, and a 10-fold increase in the A650 signal was observed for DNA obtained from 70 mg of tissue (equivalent to 2.7 × 103 CFU).
Sensitivity of oligonucleotide probes.
To further explore the semiquantitative nature of the PCR-EIA system and to establish its limit of sensitivity, a known quantity of A. fumigatus DNA was 10-fold-serially diluted and PCR amplified using the ITS3 and ITS4 primer pair. Amplicons were then detected in the PCR-EIA format and by ethidium bromide staining after agarose gel electrophoresis. The limit of sensitivity of the PCR-EIA detection system was 0.5 pg of DNA target (approximately 1 to 10 conidia), compared to 5 pg of DNA detected by ethidium bromide staining after agarose gel electrophoresis. The A650 values for 5,000, 500, 50, 5, 0.5, 0.05, and 0.005 pg of A. fumigatus DNA after PCR amplification and detection in the PCR-EIA system were, respectively, 1.990, 1.393, 0.723, 0.211, 0.080, 0.007, and 0.000. As predicted, the A650 values of the PCR-EIA increased as the amount of target DNA was increased. Linearity was observed for DNA concentrations between 0.005 and 5 pg; however, linearity was only achieved for DNA concentrations between 5 and 5,000 pg (0.005 to 5 ng) when values were plotted on a semilogarithmic scale.
DISCUSSION
Our investigators previously demonstrated the usefulness of the PCR-EIA system for the identification and differentiation of medically important species of Candida (16, 19, 59) as well as for the identification of the dimorphic fungi (39). Here we describe the design of DNA probes, directed to the ITS2 region of rDNA, to differentiate seven of the most medically important species of Aspergillus from one another and from other opportunistic molds and yeasts by using the same PCR-EIA amplification and detection system. There are many advantages to the use of this system over those employed by others. First, the use of a universal, fungus-specific primer pair maximizes test utility by amplifying DNA from all fungal targets (68). The same primer pair that amplifies Candida species DNA (16, 19, 59) also amplifies Aspergillus species DNA and other mold and yeast DNA targets. Therefore, universal fungal primers have the potential to amplify fungal DNAs present in blood, body fluids, or tissues before an organism is isolated in pure culture and at a time when the identity of the infecting organism is unknown. Sensitivity is also increased by the use of rDNA amplification targets that, unlike single gene targets, are present in multiple copies (50 to 100 per haploid genome) (28, 68). Mitochondrial DNA has also been used as a multicopy gene target (5, 6, 12), but the variability of DNA sequences among different fungal strains in this target region may be a limiting factor (71). In contrast, the probes designed in the present study bound equally well to all strains of a given species, indicating little strain-to-strain variability in the probe binding region. Indeed, in the one case where the A. flavus probe did not bind to what was originally thought to be A. flavus DNA, comparative DNA sequence analysis revealed that the isolate was more similar to A. tamarii than A. flavus. Other researchers have also concluded that there is little strain-to-strain variability in the ITS2 rDNA region (24, 28, 72).
Second, the use of a two-step system, whereby products of the universal, fungus-specific primers are hybridized to DNA probes specific for each organism, allows for a greater degree of specificity than the use of primers alone. In addition, the PCR-EIA system employed in our laboratory uses two probes, a universal biotinylated capture probe to anchor amplicons to the wells of the streptavidin-coated microtitration plate and a digoxigenin-labeled detection probe. Both probes must bind in tandem to the target amplicon before a detection signal will be generated.
Five of the Aspergillus species probes designed as part of this study were found to be completely specific, could differentiate among the most medically important Aspergillus species, and discriminated aspergilli from other medically important opportunistic molds and yeasts tested. In two cases, some probe cross-reactivities were noted. In the first case, the A. nidulans probe cross-reacted with A. ustus DNA. However, the A. ustus probe did not cross-react with A. nidulans DNA. Therefore, a process of elimination could specifically identify both species. In the second case, the minor cross-reactivity of A. candidus DNA with the A. versicolor probe could be resolved based on its much-reduced (10-fold lower) A650 value compared to that for A. versicolor DNA. Discrimination of these species should be straightforward when known concentrations of DNA, such as those derived from pure cultures, are being analyzed in the PCR-EIA. In a diagnostic setting, where unknown quantities of fungal DNA would be present in body fluids or tissues, a positive result using the A. versicolor probe would most likely indicate the presence of A. versicolor, given the rarity of infections caused by A. candidus. However, if definitive identification of this or other less common species of aspergilli is required, a reference laboratory could perform DNA sequence analysis secondarily. Thus, the PCR-EIA probe matrix could be used in clinical laboratories as a rapid and simple screening test for the most commonly encountered fungi. DNA sequence analysis would only be required for less common fungi and could be conducted by reference laboratories with established sequencing capacity. As additional DNA sequence information is obtained, other probes could be designed. Direct DNA sequence analysis is not at present feasible in most clinical laboratories because of the initial cost to set up a sequencing facility, the cost and expertise required to maintain sequencing equipment, the cost of DNA sequencing reagents and capillaries, the time and labor required to obtain relatively large amounts of high-quality, pure DNA, and the expertise necessary to interpret and analyze sequencing results (28, 55, 71). Commercial systems have begun to appear (20) but need to become more cost-effective and user-friendly before they can be routinely used in most clinical laboratories. In addition: (i) public databases are not refereed and are often inaccurate; (ii) sequence information for many fungi is incomplete or not available; (iii) taxonomies of many fungi (particularly the molds) is in flux after the relatively recent introduction of molecular taxonomic methods; and (iv) controversy remains as to which gene or genes are best for DNA sequence analysis and fungal identification.
The ITS regions of rDNA have been proposed by several groups to be the most ideal regions for sequence-based discrimination of fungal species (10, 24, 28, 55, 72). Whereas the more-conserved 18S and 28S rDNA regions, which flank the ITS regions, have been used for the design of Aspergillus species probes, these probes were not species specific (15, 31, 36, 60, 65, 69, 70). Commercial systems using conserved rDNA regions have been found to be incomplete or to identify more than one organism as the same species (20). Nonetheless, sequence analysis of the more-conserved regions of rDNA may serve to provide a broader classification of Aspergillus species, whereas the ITS1 and ITS2 regions may allow for finer discrimination among species (24, 28, 55, 71). As the taxonomy of Aspergillus species has undergone several revisions since the initial organizational work of Raper and Fennell (9, 54), and as public DNA sequence databases and culture collections continue to be updated and corrected, molecular identification of Aspergillus species can only continue to improve.
The goal of the present study, however, was not to define taxonomic boundaries for the aspergilli but rather to develop a practical method to accurately and reproducibly identify medically important Aspergillus species in a rapid and simple manner easily adapted for use in a clinical laboratory. The PCR-EIA uses a colorimetric detection matrix to provide sensitive, rapid, and objective amplicon detection. Unlike detection formats which use agarose gel electrophoresis, restriction fragment length polymorphism, single-strand conformational polymorphism analysis, Southern blotting, or DNA sequence analysis, the PCR-EIA is easy to perform and gives an objective, spectrophotometric absorbance reading. Adaptation of the PCR-EIA probes to a real-time, quantitative PCR system should be feasible, as we have previously demonstrated conversion of five of our Candida species probes from a PCR-EIA detection format to a real-time format (58). Use of a real-time PCR assay would provide quantitation of PCR products and reduce postamplification manipulation steps. Certain modifications of the probe sequences may be required, however, as demonstrated for the adaptation of our Candida species probes to real-time quantitation (58). Ideally, a microarray format, whereby all medically important fungal genera and species could be identified in a single, automated assay, would be desirable.
Standardization of DNA extraction for all Candida species was facilitated in the present study by using liquid growth cultures and a commercial DNA extraction kit (PureGene). A commercially available cell disruption and DNA extraction kit was also used to process DNA from infected rabbit tissues (Qbiogene). This latter system has been shown to be effective for obtaining sufficient quantities of DNA from pure cultures of yeasts and molds for PCR amplification (50) and may be considered as an easy method to obtain DNA from pure cultures for use in the PCR-EIA system. Cumbersome methods for DNA extraction from pure cultures of molds were used in the present study only because large quantities of high-quality DNA were required for DNA sequencing analysis and probe design. The limit of sensitivity of the PCR-EIA was 0.5 pg of DNA (1 to 10 conidia) when pure cultures were used as the source of target DNA. These data indicate that less-elaborate DNA isolation methods might provide sufficient quantities of DNA for the PCR-EIA system when pure cultures are used. Clinical specimens may require additional steps to remove potential inhibitors of the PCR assay (15, 19), but the 0.5-pg limit of test sensitivity is encouraging. Several commercial kits are now available which have been shown to be effective for obtaining sufficient amounts of DNA for PCR amplification purposes directly from blood and body fluids (40, 43). Indeed, automated systems for the extraction of DNA from blood have been developed and can be modified for the extraction of A. fumigatus DNA from blood (40). Commercial real-time PCR systems to detect fungal DNA once extracted in this manner are also emerging (LightCycler Candida kit; Roche).
Because filamentous fungi are difficult to differentiate from one another by morphology in histopathological tissue sections (25, 32, 47), an additional goal of the present study was to determine if probes could be designed to identify fungi using DNA extracted from tissues. Although obtaining tissue biopsies from thrombocytopenic patients is not generally recommended, in the absence of positive cultures and characteristic radiographic findings, biopsies are often the only diagnostic alternative. Tissue burden in the animal models of infection used in this study were quite high, and it remains to be determined if this method will be useful for the identification of fungi in tissues from patients with less-severe fungal infections. Nonetheless, the equivalent of 390 CFU of A. fumigatus could be easily detected by this method, and lower detection limits may be possible. Therefore, this system has the potential to be clinically useful for the detection of fungal DNA from tissue specimens. Investigations are under way to determine if probes can also be used to detect fungal DNA extracted from paraffin-embedded tissue sections or by direct in situ hybridization, as has been reported by other researchers for the detection of A. fumigatus and other fungi (3, 22, 46, 53).
The A650 values of the PCR-EIA have been previously shown to increase with increasing amounts of target DNA (19, 39, 59). As observed in the present study, at higher DNA concentrations (>105 cells or >2 ng per reaction mixture), the A650 values plateau and become saturated (39, 59); semiquantitative values can be obtained for DNA concentrations below these values (39, 59), and conversion to a real-time detection system should provide direct quantitation (41). Given that probe reactivity was highly specific and sensitive in the above experiments, these probes hold promise for the differentiation of fungi in tissues from infected patients as well as for the identification of organisms in body fluids and in pure culture.
It remains to be determined whether there are environmental or other Aspergillus species not yet tested in the PCR-EIA probe matrix that will cross-react with our probes or whether other genes or other rDNA regions may give better discrimination among the less common species of aspergilli involved in human disease. BLAST searches using Aspergillus species probe sequences indicate that some cross-reactivities with rare or nonpathogenic fungi may occur. It is unlikely that such cross-reactivities will be diagnostically significant. It must be remembered, however, that the GenBank database is not complete for all fungi or all ITS2 rDNA sequences, and it is possible that DNA from fungi that we did not test may cross-react with our probes. This may be particularly the case for Aspergillus species that are from the same taxonomic groups or sections where molecular siblings may occur (34). A full examination of clinical isolates, including those obtained from sterile and nonsterile body sites, is currently under way. Environmental contamination has not yet been a problem in our studies, as appropriate controls have been instituted (19, 35). Contaminated commercial reagents have not been encountered to date, although such instances have been reported by others to occur (6, 42).
In conclusion, specific probes were designed to differentiate seven of the most medically important Aspergillus species from one another and from other medically important opportunistic molds and yeasts in a PCR-EIA format. Studies are currently under way to determine the sensitivity and specificity of the Aspergillus species probes in the PCR-EIA and real-time PCR formats for the diagnosis of IA in hematopoietic stem cell transplant patients and in patients with hematological malignancies.
Acknowledgments
We thank Joshua Westerman, Hetal Vaishnav, and Catherine Wolfe for valuable technical assistance.
REFERENCES
- 1.Ascioglu, S. J., H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, T. J. Walsh, et al. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 2.Baddley, J. W., P. G. Pappas, A. C. Smith, and S. A. Moser. 2003. Epidemiology of Aspergillus terreus at a university hospital. J. Clin. Microbiol. 41:5525-5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bialek, R., A. Feucht, C. Aepinus, G. Just-Nübling, V. J. Robertson, J. Knobloch, and R. Hohle. 2002. Evaluation of two nested PCR assays for detection of Histoplasma capsulatum DNA in human tissue. J. Clin. Microbiol. 40:1644-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blum, U., M. Windfuhr, C. Buitrago-Tellez, G. Sigmund, E. W. Herbst, and M. Langer. 1994. Invasive pulmonary aspergillosis: MRI, CT, and plain radiographic findings and their contribution for early diagnosis. Chest 106:1156-1161. [DOI] [PubMed] [Google Scholar]
- 5.Bretagne, S., J. M. Costa, E. Bart-Delabesse, N. Dhedin, C. Rieux, and C. Cordonnier. 1998. Comparison of serum galactomannan antigen detection and competitive polymerase chain reaction for diagnosing invasive aspergillosis. Clin. Infect. Dis. 26:1407-1412. [DOI] [PubMed] [Google Scholar]
- 6.Bretagne, S., J. M. Costa, A. Marmorat-Khuong, F. Poron, C. Cordonnier, M. Vidaud, and J. Fleury-Feith. 1995. Detection of Aspergillus species DNA in bronchoalveolar lavage samples by competitive PCR. J. Clin. Microbiol. 33:1164-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caillot, D., O. Cassanovas, A. Bernard, J. F. Couaillier, C. Durand, B. Cuisenier, E. Solary, F. Piard, T. Petrella, A. Bonnin, G. Couaillault, M. Dumas, and H. Guy. 1997. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J. Clin. Oncol. 15:139-147. [DOI] [PubMed] [Google Scholar]
- 8.Catten, M., A. H. Murr, J. A. Goldstein, A. N. Mhatre, and A. K. Lalwani. 2001. Detection of fungi in the nasal mucosa using polymerase chain reaction. Laryngoscope 111:399-403. [DOI] [PubMed] [Google Scholar]
- 9.Chang, J.-M., H. Oyaizu, and J. Sugiyama. 1991. Phylogenetic relationships among eleven selected species of Aspergillus and associated teleomorphic genera estimated from 18S ribosomal RNA partial sequences. J. Gen. Appl. Microbiol. 37:289-308. [Google Scholar]
- 10.Chen, S. C. A., C. L. Halliday, and W. Meyer. 2002. A review of nucleic acid-based diagnostic tests for systemic mycoses with an emphasis on polymerase chain reaction-based assays. Med. Mycol. 40:333-357. [DOI] [PubMed] [Google Scholar]
- 11.Cornet, M., L. Fleury, C. Maslo, J. F. Bernard, G. Brucker, and the Invasive Aspergillosis Surveillance Network of the Assistance Publique-Hopitaux de Paris. 2002. Epidemiology of invasive aspergillosis in France: a six-year multicentric survey in the Greater Paris area. J. Hosp. Infect. 51:288-296. [DOI] [PubMed] [Google Scholar]
- 12.Costa, C., D. Vidaud, M. Olivi, E. Bart-Delabesse, M. Vidaud, and S. Bretagne. 2001. Development of two real-time quantitative TaqMan PCR assays to detect circulating Aspergillus fumigatus DNA in serum. J. Microbiol. Methods 44:263-269. [DOI] [PubMed] [Google Scholar]
- 13.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 14.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608-615. [DOI] [PubMed] [Google Scholar]
- 15.Einsele, H., H. Hebart, G. Roller, J. Loeffler, I. Rothenhofer, C. A. Muller, R. A. Bowden, J.-A. van Burik, D. Engelhard, L. Kanz, and U. Schumacher. 1997. Detection and identification of fungal pathogens in blood by using molecular probes. J. Clin. Microbiol. 35:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elie, C. M., T. J. Lott, E. Reiss, and C. J. Morrison. 1998. Rapid identification of Candida species with species-specific DNA probes. J. Clin. Microbiol. 36:3260-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellepola, A. N. B., S. F. Hurst, C. M. Elie, and C. J. Morrison. 2003. Rapid and unequivocal differentiation of Candida dubliniensis from other Candida species using species-specific DNA probes: comparison with phenotypic identification methods. Oral Microbiol. Immunol. 18:379-388. [DOI] [PubMed] [Google Scholar]
- 18.Fortun, J., P. Martin-Davila, A. Sanchez-Souza, C. Quereda, E. Navas, R. Barcena, E. Vicente, A. Candelas, A. Honrubia, J. Nuno, V. Pintado, S. Moreno, et al. 2001. Aspergillus antigenemia sandwich-enzyme immunoassay test as a serodiagnostic method for invasive aspergillosis in liver transplant recipients. Transplantation 71:145-149. [DOI] [PubMed] [Google Scholar]
- 19.Fujita, S.-I., B. A. Lasker, T. J. Lott, E. Reiss, and C. J. Morrison. 1995. Microtitration plate enzyme immunoassay to detect PCR-amplified DNA from Candida species in blood. J. Clin. Microbiol. 33:962-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall, L., S. Wohlfiel, and G. D. Roberts. 2003. Experience with the MicroSeq D2 large-subunit ribosomal DNA sequencing kit for the identification of commonly encountered, clinically important yeast species. J. Clin. Microbiol. 41:5099-5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto, A., Y. Yamakami, P. Kamberi, E. Yamagata, R. Karashima, H. Nagaoka, and M. Nasu. 1998. Comparison of PCR, (1-3)-beta-glucan, and galactomannan assays in sera of rats with experimental invasive aspergillosis. J. Clin. Lab. Anal. 12:257-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayden, R. T., P. A. Isotalo, T. Parrett, D. M. Wolk, X. Qian, G. D. Roberts, and R. V. Lloyd. 2003. In situ hybridization for the differentiation of Aspergillus, Fusarium, and Pseudallescheria species in tissue section. Diagn. Mol. Pathol. 12:21-26. [DOI] [PubMed] [Google Scholar]
- 23.Hayette, M.-P., D. Vaira, F. Susin, P. Boland, G. Christiaens, P. Melin, and P. De Mol. 2001. Detection of Aspergillus species DNA by PCR in bronchoalveolar lavage fluid. J. Clin. Microbiol. 39:2338-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry, T., P. C. Iwen, and S. H. Hinrichs. 2000. Identification of Aspergillus species using internal transcribed spacer regions 1 and 2. J. Clin. Microbiol. 38:1510-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung, C. C., S. C. Chang, P. C. Yang, and W. C. Hseigh. 1994. Invasive pulmonary pseudallescheriasis with direct invasion of the thoracic spine in an immunocompromised patient. Eur. J. Clin. Infect. Dis. 13:749-751. [DOI] [PubMed] [Google Scholar]
- 26.Hurst, S. F., G. H. Reyes, D. W. McLaughlin, E. Reiss, and C. J. Morrison. 2000. Comparative evaluation of commercial latex agglutination and sandwich enzyme immunoassays with a competitive binding inhibition enzyme immunoassay to detect antigenemia and antigenuria for the rapid diagnosis of invasive aspergillosis. Clin. Diagn. Lab. Immunol. 7:477-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwen, P. C., M. E. Rupp, A. N. Langnas, E. C. Reed, and S. H. Hinrichs. 1998. Invasive pulmonary aspergillosis due to Aspergillus terreus: 12-year experience and review of the literature. Clin. Infect. Dis. 26:1092-1097. [DOI] [PubMed] [Google Scholar]
- 28.Iwen, P. C., S. H. Hinrichs, and M. E. Rupp. 2002. Utilization of the internal transcribed spacer regions as molecular targets to detect and identify human fungal pathogens. Med. Mycol. 40:87-109. [DOI] [PubMed] [Google Scholar]
- 29.Kambouris, M. E., U. Reichard, N. J. Legakis, and A. Velegraki. 1999. Sequences from the aspergillopepsin PEP gene of Aspergillus fumigatus: evidence on their use in selective PCR identification of Aspergillus species in infected clinical samples. FEMS Immunol. Med. Microbiol. 25:255-264. [DOI] [PubMed] [Google Scholar]
- 30.Kami, M., T. Fukui, S. Ogawa, Y. Kazuyama, U. Machida, Y. Tanaka, Y. Kanda, T. Kashima, Y. Yamazaki, T. Hamaki, S.-I. Mori, H. Akiyama, Y. Mutou, H. Sakamaki, K. Osumi, S. Kimura, and H. Hirai. 2001. Use of real-time PCR on blood samples for diagnosis of invasive aspergillosis. Clin. Infect. Dis. 33:1504-1512. [DOI] [PubMed] [Google Scholar]
- 31.Kappe, R., C. N. Okeke, C. Fauser, M. Maiwald, and H. G. Sonntag. 1998. Molecular probes for the detection of pathogenic fungi in the presence of human tissue. J. Med. Microbiol. 47:811-820. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman, L., P. G. Standard, M. Jalbert, and D. E. Kraft. 1997. Immunohistologic identification of Aspergillus spp. and other hyaline fungi by using polyclonal fluorescent antibodies. J. Clin. Microbiol. 35:2206-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawamura, S., S. Maeski, T. Noda, Y. Hirakata, K. Tomono, T. Tashiro, and S. Kohno. 1999. Comparison between PCR and detection of antigen in sera for diagnosis of pulmonary aspergillosis. J. Clin. Microbiol. 37:218-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumeda, Y., and T. Asao. 1996. Single-strand conformational polymorphism analysis of PCR-amplified ribosomal DNA internal transcribed spacers to differentiate species of Aspergillus section Flavi. Appl. Environ. Microbiol. 62:2947-2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwok, S., and R. Higuichi. 1989. Avoiding false positives with PCR. Nature (London) 39:237-238. [DOI] [PubMed] [Google Scholar]
- 36.Lass-Florl, C., J. Aigner, E. Gunsilius, A. Petzer, D. Nachbaur, G. Gastl, H. Einsele, J. Loffler, M. P. Dierich, and R. Wurzner. 2001. Screening for Aspergillus spp. using polymerase chain reaction of whole blood samples from patients with hematological malignancies. Br. J. Haematol. 113:180-184. [DOI] [PubMed] [Google Scholar]
- 37.Lass-Florl, C., G. Kofler, G. Kropshofer, J. Hermans, A. Kreczy, M. P. Dierich, and D. Niederwieser. 1998. In vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J. Antimicrob. Chemother. 42:497-502. [DOI] [PubMed] [Google Scholar]
- 38.Lin, S.-J., J. Schranz, and S. M. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 39.Lindsley, M. D., S. F. Hurst, N. J. Iqbal, and C. J. Morrison. 2001. Rapid identification of dimorphic and yeast-like fungal pathogens using specific DNA probes. J. Clin. Microbiol. 39:3505-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loeffler, J., K. Schmidt, H. Hebart, U. Schumacher, and H. Einsele. 2002. Automated extraction of genomic DNA from medically important yeast species and filamentous fungi by using the MagNA Pure LC system. J. Clin. Microbiol. 40:2240-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loeffler, J., N. Henke, H. Hebart, D. Schmidt, L. Hagmeyer, U. Schumacher, and H. Einsele. 2000. Quantification of fungal DNA by using fluorescence resonance energy transfer and the light cycler system. J. Clin. Microbiol. 38:586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loeffler, J., H. Hebart, R. Bialek, L. Hagmeyer, D. Schmidt, F. P. Serey, M. Hartmann, J. Eucker, and H. Einsele. 1999. Contaminations occurring in fungal PCR assays. J. Clin. Microbiol. 37:1200-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loeffler, J., H. Hebart, U. Schumacher, H. Reitze, and H. Einsele. 1997. Comparison of different methods for extraction of DNA of fungal pathogens from cultures and blood. J. Clin. Microbiol. 35:3311-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maertens, J., J. Verhaegen, H. Demuynck, P. Brock, G. Verhoef, P. Vandenberghe, J. Van Eldere, L. Verbist, and M. Boogaerts. 1999. Autopsy-controlled prospective evaluation of serial screening for circulating galactomannan by a sandwich enzyme-linked immunosorbent assay for hematological patients at risk for invasive aspergillosis. J. Clin. Microbiol. 37:3223-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNeil, M. M., S. L. Nash, R. A. Hajjeh, M. A. Phelan, L. A. Conn, B. D. Plikaytis, and D. W. Warnock. 2001. Trends in mortality due to invasive mycotic diseases in the United States, 1980-1997. Clin. Infect. Dis. 33:641-647. [DOI] [PubMed] [Google Scholar]
- 46.Montone, K. T., and L. A. Litzky. 1995. Rapid method for detection of Aspergillus 5S ribosomal RNA using a genus-specific oligonucleotide probe. Am. J. Clin. Pathol. 103:48-51. [DOI] [PubMed] [Google Scholar]
- 47.Morrison, C. J. 2002. Laboratory diagnosis of opportunistic infections in the intensive care unit, p. 55-104. In R. A. Barnes and D. W. Warnock (ed.), Fungal infection in the intensive care unit. Kluwer Academic Publishers, Norwell, Mass.
- 48.Morrison, C. J., S. F. Hurst, S. L. Bragg, R. J. Kuykendall, H. Diaz, J. Pohl, and E. Reiss. 1993. Heterogeneity of the purified extracellular aspartyl proteinase from Candida albicans: characterization with monoclonal antibodies and N-terminus amino acid sequence analysis. Infect. Immun. 61:2030-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morrison, C. J., S. F. Hurst, and E. Reiss. 2003. Competitive binding inhibition enzyme-linked immunosorbent assay that uses the secreted aspartyl proteinase of Candida albicans as an antigenic marker for diagnosis of disseminated candidiasis. Clin. Diagn. Lab. Immunol. 10:835-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Müller, F. M., K. E. Werner, M. Kasai, A. Francesconi, S. J. Chanock, and T. J. Walsh. 1998. Rapid extraction of genomic DNA from medically important yeasts and filamentous fungi by high-speed cell disruption. J. Clin. Microbiol. 36:1625-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nolan, C. 1989. Appendix E: commonly used techniques in molecular cloning, p. E.1-E. 39. In J. Sambrook, E. F. Fritsch, and T. Maniatis (ed.), Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 52.O'Sullivan, C. E., M. Kasai, A. Francesconi, V. Petratis, R. Petraitiene, A. M. Kelaher, A. A. Sarafandi, and T. J. Walsh. 2003. Development and validation of a quantitative real-time PCR assay using fluorescence resonance energy transfer technology for detection of Aspergillus fumigatus in experimental invasive pulmonary aspergillosis. J. Clin. Microbiol. 41:5676-5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park, C. S., J. Kim, and K. T. Montone. 1997. Detection of Aspergillus ribosomal RNA using biotinylated oligonucleotide probes. Diagn. Mol. Pathol. 6:255-260. [DOI] [PubMed] [Google Scholar]
- 54.Peterson, S. W. 2000. Phylogenetic relationships in Aspergillus based on rDNA sequence analysis, p. 323-355. In R. A. Samson and J. I. Pitt (ed.), Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 55.Pryce, T. M., S. Pallidino, D. Kay, and G. W. Coombs. 2003. Rapid identification of fungi by sequencing the ITS1 and ITS2 regions using an automated capillary electrophoresis system. Med. Mycol. 41:369-381. [DOI] [PubMed] [Google Scholar]
- 56.Rath, P. M., and R. Ansorg. 2000. Identification of medically important Aspergillus species by single strand conformational polymorphism (SSCP) of the PCR-amplified intergenic spacer region. Mycoses 43:381-386. [PubMed] [Google Scholar]
- 57.Salonen, J., O. P. Lehtonen, M. R. Terasjarvi, and J. Nikoskelainen. 2000. Aspergillus antigen in serum, urine, and bronchoalveolar lavage specimens of neutropenic patients in relation to clinical outcome. Scand. J. Infect. Dis. 32:485-490. [DOI] [PubMed] [Google Scholar]
- 58.Shin, J.-H., F. S. Nolte, B. P. Holloway, and C. J. Morrison. 1999. Rapid identification of up to three Candida species in a single reaction tube by a 5′ exonuclease assay using fluorescent DNA probes. J. Clin. Microbiol. 37:165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin, J.-H., F. S. Nolte, and C. J. Morrison. 1997. Rapid identification of Candida species in blood cultures by a clinically useful PCR method. J. Clin. Microbiol. 35:1454-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skladny, H., D. Buchheidt, C. Baust, F. Krieg-Schneider, W. Seifarth, C. Leib-Mosch, and R. Hehlmann. 1999. Specific detection of Aspergillus species in blood and bronchoalveolar lavage samples of immunocompromised patients by two-step PCR. J. Clin. Microbiol. 37:3865-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stynen, D., J. Sarfati, A. Goris, M. C. Prevost, M. Lesourd, H. Kamphuis, V. Darras, and J. P. Latge. 1992. Rat monoclonal antibodies against Aspergillus galactomannan. Infect. Immun. 60:2237-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutton, D. A., S. E. Sanche, S. G. Revankar, A. W. Fothergill, and R. G. Rinaldi. 1999. In vitro amphotericin B resistance in clinical isolates of Aspergillus terreus, with head-to-tail comparison to voriconazole. J. Clin. Microbiol. 37:2343-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turenne, C. Y., S. E. Sanche, D. J. Hoban, J. A. Karlowsky, and A. M. Kabani. 1999. Rapid identification of fungi by using the ITS2 genetic region and an automated fluorescent capillary electrophoresis system. J. Clin. Microbiol. 37:1846-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verweij, P. E., D. Poulain, T. Obayashi, T. F. Patterson, D. W. Denning, and J. Ponton. 1998. Current trends in the detection of antigenemia, metabolites, and cell markers for the diagnosis and therapeutic monitoring of fungal infections. Med. Mycol. 36(Suppl. 1):146-155. [PubMed] [Google Scholar]
- 65.Verweij, P. E., J. P. Latge, A. J. Rijs, W. J. Melchers, B. E. De Pauw, J. A. Hoogkamp-Korstanje, and J. F. Meis. 1995. Comparison of antigen detection and PCR assay using bronchoalveolar lavage fluid for diagnosing invasive pulmonary aspergillosis in patients receiving treatment for hematological malignancies. J. Clin. Microbiol. 33:3150-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wald, A., W. Leisenring, J. van Burik, and R. A. Bowden. 1997. Natural history of aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J. Infect. Dis. 175:1459-1466. [DOI] [PubMed] [Google Scholar]
- 67.Walsh, T. J., A. Francesconi, M. Kasai, and S. J. Chanock. 1995. PCR and single-strand conformational polymorphism for recognition of medically important opportunistic fungi. J. Clin. Microbiol. 33:3216-3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White, T. J., T. D. Burns, S. B. Lee, and J. W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Helfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols. Academic Press, Inc., San Diego, Calif.
- 69.Wu, Z., Y. Tsumura, G. Blomquist, and X.-R. Wang. 2003. 18S rRNA gene variation among common airborne fungi, and development of specific oligonucleotide probes for the detection of fungal isolates. Appl. Environ. Microbiol. 69:5389-5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamakami, Y., A. Hashimoto, I. Tokimatsu, and M. Nasu. 1996. PCR detection of DNA specific for Aspergillus species in serum of patients with invasive aspergillosis. J. Clin. Microbiol. 34:2464-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yeo, S. F., and B. Wong. 2002. Current status of nonculture methods for diagnosis of invasive fungal infections. Clin. Microbiol. Rev. 15:465-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao, J., F. Kong, R. Li, X. Wang, Z. Wan, and D. Wang. 2001. Identification of Aspergillus fumigatus and related species by nested PCR targeting ribosomal DNA internal transcribed spacer regions. J. Clin. Microbiol. 39:2261-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]