I. Introduction
“Receptors recognize a distinct chemical entity and translate information from that entity into a form that the cell can read to alter its state” (Kenakin et al., 1992). Even though the receptors are often pharmacologically defined on the basis of synthetic compounds, they are assumed to have developed to respond to endogenous molecules. Therefore, receptors are generally named on the basis of their natural ligands. Hence, it is appropriate to very briefly summarize the evidence that purine nucleotides and nucleosides are natural ligands for a wide class of receptors.
In a seminal paper, Drury and Szent-Györgyi (1929) showed that adenosine exerted a large number of biological effects, including bradycardia and vasodilation. A wider interest in the role of adenosine followed from the demonstration in 1963 that adenosine can be produced by the hypoxic heart. Two groups independently formulated the hypothesis that adenosine may be involved in the metabolic regulation of coronary blood flow (Berne, 1963; Gerlach et al., 1963). The observation by de Gubareff and Sleator (1965) that the actions of adenosine in heart tissue could be blocked by caffeine suggested the existence of an adenosine receptor. The potent cardiovascular effects of adenosine led to an interest in the synthesis of new adenosine analogs, and careful dose-response studies with a number of these drugs (Cobbin et al., 1974) strongly suggested the presence of a receptor for adenosine-like compounds. Sattin and Rall (1970) reported that adenosine increased cyclic AMP accumulation in slices of rodent brain and that this adenosine-induced second-messenger response was blocked by methylxanthines. Their findings suggested that adenosine receptors exist in the central nervous system. The essentially simultaneous findings by Mcilwain (1972), that such brain slices actually elaborate adenosine in concentrations that would be sufficient to elevate cyclic AMP, provided support that these putative receptors were physiologically occupied by adenosine. Thus, in the 1970s there was good evidence that there were receptors for adenosine at which methylxanthines acted as antagonists. Biochemical evidence for the existence of multiple adenosine receptors was subsequently provided by the demonstration that adenosine analogs increased cyclic AMP production in some preparations and decreased it in others. Because the relative agonist potency for a variety of adenosine analogs was different for these two types of effects, the presence of two classes of receptors, called A1 and A2 (van Calker et al., 1979) or Ri and Ra (Londos et al., 1980), was proposed. The A1/A2 nomenclature is now generally used.
The presence of receptors for ADP, particularly on blood platelets, was also recognized several decades ago. Studies of the factors in blood that induce platelet aggregation led to the identification of ADP as an active component present in red blood cell extracts (Gaarder et al., 1961). The evidence that ADP and adenosine (presumably A2) receptors exist on platelets was summarized by Haslam and Cusack (1981).
Four decades ago, ATP was shown to produce important cardiovascular effects (Green and Stoner, 1950) and to be released from sensory nerves (Holton and Holton, 1954; Holton, 1959), hinting at a role in neural transmission. In his landmark review of purinergic nerves, Burnstock (1972) postulated the existence of specific ATP receptors. Although evidence in support of this idea was not overwhelming at the time, many subsequent studies have supported the existence of receptors for extracellular ATP (Burnstock and Brown, 1981; Gordon, 1986; O’Connor et al., 1991). Similarly, the evidence is now compelling that ATP plays important physiological and/ or pathophysiological roles in a variety of biological systems, including that of a neurotransmitter in peripheral and central neurons. Finally, diadenosinetetraphosphate is a dinucleotide stored in synaptic vesicles and chromaffin granules (Flodgaard and Klenow, 1982; Rodriguez del Castillo et al., 1988) and released therefrom (Pintor et al., 1991a, 1992). The purine dinucleotide also binds with subnanomolar affinity to receptors (Pintor et al., 1991b, 1993) and exerts biological effects (Pintor et al., 1993), indicating that it is an endogenous purinoceptor ligand.
Thus, strong evidence for the presence of receptors for the endogenous ligands adenosine, ADP, ATP, and dia-denosinetetraphosphate had accumulated. This group of receptors is called the purinoceptors. If at some future time there is compelling evidence that UTP, or another pyrimidine nucleotide, is an endogenous ligand at receptors that respond poorly or not at all to ATP, then this terminology may need revision.
II. General Considerations concerning the Classification of Purinoceptors
Classification of receptors should preferably be based on combined structural and pharmacological information (Kenakin et al., 1992). We are not yet in this ideal situation in the area of purinoceptors, and any proposed classification scheme by necessity must be tentative. In 1978 Burnstock made the important suggestion that there exists a family of receptors called purinergic receptors that can be subgrouped into two subclasses, P1 and P2 (Burnstock, 1978). A somewhat extended version of this classification scheme (Burnstock, 1980) is shown in table 1. The scheme has been extremely influential and was adopted by numerous authors in the field. It should, however, be borne in mind that the original criteria have been continuously updated and modified with the availability of new information (see footnotes to table 1). The current criteria for the subclassification are summarized table 2.
TABLE 1.
Original criteria for distinguishing two types of purinoceptors
| Antagonists | Agonist preferences | Changes in cyclic AMP |
Induction of prostaglandin synthesis |
|---|---|---|---|
| P1 receptors | |||
| Methylxanthines* | ADO > AMP > ADP > ATP† | Yes‡ | No§ |
| P2 receptors | |||
| Quinidine* | |||
| Imidazolines | No‡ | Yes§ | |
| 2,2′-Pyridylisatogen | ATP > ADP > AMP > ADO† | ||
| Apamin |
We still lack good antagonists at P2 receptors (see section III.B and Table 4). There are apparently adenosine receptors (A3 receptors, see below) where the classical methylxanthines are very poor antagonists.
Adenosine (ADO) and AMP do not activate P2 receptors. Adenine nucleotides may or may not be agonists at adenosine receptors.
Not all adenosine receptors (P1 purinoceptors) affect cyclic AMP formation. Conversely, adenine nucleotides may affect cyclic AMP formation.
Not all effects of ATP or ADP are mediated through changes in prostaglandin formation. Adenosine effects on Ca2+ may be associated with prostaglandin formation.
TABLE 2.
Subdivision of purinoceptors into P1 and P2 types. Current recommendations
| Receptor class | P1 purinoceptors | P2 purinoceptors |
|---|---|---|
| Effector system | G-protein coupled | G-protein coupled Intrinsic ion channel Nonselective pore |
| Natural ligand(s) so far identified |
Adenosine | ATP, ADP, diadeno- sinetetraphos- phate, (UTP?)* |
Whereas UTP is an endogenous compound and a ligand at some P2 purinoceptors, it remains to be shown that endogenous UTP acts via such receptors in vivo.
Structural information is now available for several subtypes of receptors for adenosine (Maenhaut et al., 1990; Libert et al., 1991; Mahan et al., 1991; Fink et al., 1992; Stehle et al., 1992; Salvatore et al., 1992; Zhou et al., 1992). The same is true for some P2 receptors (Lustig et al., 1993; Webb et al., 1993; for comparisons between several recombinant P2 receptors, see Barnard et al., 1994). Often, but not always, the information from the cloned receptors has supported earlier attempts at classification based on pharmacological criteria. Only limited structural information is presently available for receptors for adenine nucleotides. Thus, the current recommendations about the classification of receptors for adenosine are firmer than those regarding the adenine nucleotides. The pharmacological evidence is also more substantial in the case of the adenosine receptors than in the case of receptors for adenine nucleotides.
It has been strongly emphasized that antagonists, rather than agonists, are the preferred tools for pharmacological classification (Kenakin et al., 1992). The reason for this is that apparent agonist potency depends strongly not only on agonist binding to the receptor but also on the entire signal transduction machinery. Unfortunately, much of the pharmacological classification in the purinoceptor field rests on relative agonist potencies. Antagonists at adenosine receptors in many instances do not show the degree of selectivity that is ideal. Binding assays can be used for some of the receptors, but ligands are lacking for others. Thus, comparisons between receptors often have been based on different types of assays. In the case of receptors for ADP and ATP, classification is even more problematic in that currently available antagonists have not been unequivocally shown to be specific and selective.
One final consideration is of particular importance in the area of purinoceptors. It has been emphasized that extreme care must be taken to ensure that assays of biological activity are carried out under equilibrium conditions and that complications due to sites of loss or the influence of endogenous ligands are avoided (Kenakin et al., 1992). Because the purine nucleosides and nucleotides are extremely important metabolically, cells have elaborated very efficient systems for their degradation and/or uptake into cells. Such removal mechanisms are present on virtually all cells; this is in contrast to the situation for many neurotransmitters, for which sites of loss are particularly abundant in the nerves that use them but virtually absent elsewhere. Adenosine and several adenosine analogs are rapidly taken up and/or metabolized by transporters and enzymes. The efficiency of this process is extraordinary; the half-life of adenosine injected into the blood stream is on the order of 1 second (Möser et al., 1989). Nucleotides are also very rapidly degraded, and the degradation products are taken up by cells. Some of the nucleotide analogs and their breakdown products interfere not only with the receptors but also with the removal systems. Conversely, the available agents that may reduce the breakdown of the nucleotides have the possibility of interacting with the receptors. Finally, it is often difficult to achieve a temporal equilibrium in the case of at least some adenine nucleotide receptors, which desensitize very rapidly. Consequently, the evidence for subtypes of adenine nucleotide receptors that is based on relative agonist potency in complex biological systems must be regarded as tentative rather than definitive.
The IUPHAR receptor nomenclature committee also has suggested that classification-neutral labels such as 1–2–3 are to be preferred to other labels. In the case of purinoceptors, Burnstock (1978, 1980) proposed a P1 and P2 nomenclature for adenosine and adenine nucleotide (ATP, ADP) receptors, respectively. A classification into A1 and A2 adenosine receptors was proposed in 1979 (van Calker et al., 1979) and is now generally accepted, along with the A2a and A2b nomenclature. The basis for the latter is that the two cloned receptors show considerable sequence homology and essentially similar signal transduction mechanisms and can be readily distinguished based on pharmacological criteria. The P1 receptor designation is particularly used to contrast it with P2 receptors. A series of letters has been allocated in a rather random manner (P2X, P2Y, P2T, P2S, P2N, P2U, P2Z, P2D) for the adenine nucleotide receptors.
III. Proposed Receptor Classification
A. Adenosine (P1) Receptors
The terms adenosine receptor or P1 purinoceptor are used to designate this family of receptors. The term P1 is useful in situations in which comparison is made between P1 and P2 purinoceptors. The subtypes of adenosine/P1 purinoceptors are designated as A1, A2, A3,… receptors and can be further divided, e.g., into A2a, A2b (table 3); this is in agreement with the above-mentioned general recommendation for subtype numbering of the IUPHAR committee on receptor nomenclature. Additions of receptors into this scheme will be made if, and only if, both structural and pharmacological evidence indicate a specific subtype. Such criteria involve evidence that the structure is different from that of already established members of the adenosine family in the same species. This information must be supplemented by the demonstration that either the receptor has a unique distribution among cells and tissues or the cloned receptor exhibits a unique pharmacology. Because all adenosine receptors as yet characterized are G-protein coupled (see below), unique pharmacology should be demonstrated in an expression system in which the receptor couples to relevant G-proteins.
TABLE 3.
Classification of adenosine receptors (P1 purinoceptors)*
| Name | A1 | A2a | A2b | A3 |
|---|---|---|---|---|
| Structure | (Name of clone) | |||
| Deduced molecular mass† | ||||
| Amino acids | 326 | 410–412 | 332 | 320 |
| kDa | 37 | 45 | 36 | 36 |
| G-protein coupling | Gi(1–3) | Ga | Ga | Yes |
| Effectors‡ | ↓ cyclic AMP ↑IP3 ↑K+ ↓Ca2+ |
↑ cyclic AMP | ↑ cyclic AMP | ↓ cyclic AMP |
| Agonists§ |
High: (0.3–3 nM) CPA, CHA, R-PIA, ADAC |
High: (1–20 nM) NECA, CGS 21680, APEC, Ado |
High: (0.5–5 μm) NECA |
High (<10 nM) APNEA, N6-benzyl- NECA |
| Intermediate: (3–30 nM) |
Intermediate: (20–200 nM) |
Intermediate: (5–20 μM) |
Intermediate: (10–30 nM) |
|
| NECA, 2-Cl-Ado, Ado | 2-Cl-Ado, CV 1808, R-PIA, ADAC |
2-Cl-Ado, Ado, R-PIA | NECA, R-PIA | |
| Low: (30–350 nM) | Low: (200–500 nM) | Low: (20–100 μM) | Low: (100–1000 nM) | |
| S-PIA, DPMA | CPA, CHA, S-PIA | S-PIA | CGS 21680 | |
| Very low: (>350 nM) | Very low: (>100 μm) | Very low: (>1 μM) | ||
| CV 1808, CGS 21680, APEC |
CGS 21680, CV 1808 | Ado | ||
| Antagonists§ | High: (0.5–2 nM) | High: (20–100 nM) | High: (20–100 nM) | High: (1–20 nM) |
| CPX,║ XAC | XAC, CSC, KF 17837, CGS 15943 |
XAC, CPX,║ 8-PT, CGS 15943 |
BW-A 5221¶ | |
| Intermediate: (2–200 nM) | Intermediate: (0.2–2 μM) |
Intermediate: (0.5–10 μM) |
||
| CPT, 8-PT, CGS 15943 | CPT, CPX,,║ 8-PT | 8-pSPT | ||
| Low: (1–20 μm) | Low: (2–20 μm) | Low. (10–20 μm) | ||
| Theophylline., 8-pST, IBMX, KF 17387 |
DMPX, 8-pSPT, IBMX, theophylline |
Theophylline DMPX, IBMX |
||
| Very low: (>20 μm) | Very low: (>30 μm) | Very low: (>30 μM) | Very low: (>100 μM) | |
| Caffeine, DMPX, CSC | Caffeine | Caffeine, KF 17837 | 8-PT, XAC, IBMX | |
| Radioligands | CHA (agonist) | CGS 21680 (agonist) | None available | 125I-APNEA (agonist) |
| CPX║ (antagonist) | XAC (antagonist)# | 126I-AB-MECA** | ||
| Distribution†† | Brain (highest in cortex, hippocampus, cerebellum), testis, adipose tissue, heart, kidney |
Brain (highest in striatum, nucleus accumbens, tuberculum olfactorium) |
Wide. High in gas- trointestinal tract |
Testis. Wide in some species‡‡ |
Abbreviations: BW-A 522, 3-(3-iodo-4-ammobenzyl)-8-(4-oxyacetate)-1-propylxanthine; CPA, N6-cyclopentyladenosine; CHA, N6-cyclo-hexyladenosine; R-PIA, N6(R-phenylisopropyl)-adenosine; S-PIA, N6-(S-phenylisopropyl)-adenosine; ADAC, adenosine amine congener, NECA, 5′-N-ethyl-carboxamidoadenosine; CPA, N6-cyclopentyladenosine; CV 1808, 2-phenylaminoadenosine; CGS 21680, 2-[p-(2-carbonyl-ethyl)-phenylethylamino]-5′-N-ethylcarboxamidoadenosine; APEC, 2-[(2-ammoethylamino)carbonylethylphenylethylamino]-5′-N-ethylcarboxamidoadenosine; CPX (DPCPX), l,3-dipropyl-8-cyclopentylxanthine; XAC, xanthine amine congener; CPT, 8-cyclopentyltheophylline; 8-PT, 8-phenyltheophylline; CGS 15943, 9-chloro-2-(2-furanyl)-5,6-dihydro)-[1,2,4]-triazolo[1,5]quinazolin-5-imine monomethanesulfonate; 8-pSPT, 8-p-sulfophenyltheophylline; IBMX, 3-isobutyl-1-methylxanthine; KF 17387, 1,3-dipropyl-8-(3,4-dimethoxystyryl)-7-methylxanthine; DMPX, 1,3-dimethyl-7-propylxanthine; CSC, 8-(3-chlorostyryl)caffeine; APNEA, N6-2-(4-aminophenyl)ethyladenosine; AB-MECA, N6-(3-iodo-4-amino-benzyl(−5′-N-methyl-carboxamidoadenosine; 2-Cl-ado, 2-chloroadenosine; Ado, adenosine.
Additional binding sites for adenosine analogs have been reported: (a) Certain adenine derivatives (e.g., 2’,5′-dideoxyadenosine) are inhibitors of adenylyl cyclase via a so called P-site (Londos and Wolff, 1977). (b) A ubiquitous nonreceptor-binding site, adenotin, for NECA, has been well characterized from human placenta (Hutchison and Fox, 1989) and human platelets (Lohse et al, 1988). (c) A high-affinity binding site for NECA in the brain with a pharmacological profile that differs from reported adenosine receptors and from adenotin has been described (Lorenzen et al, 1992, 1993). (d) A binding site for CGS 21680 with properties that differ from those of A2a receptors has been reported (Johansson et al, 1993). (e) A binding site for CV 1808 with pharmacological properties different from known adenosine receptors has been reported and denoted “A4” by the authors (Cornfield et al., 1992). (f) Two further adenosine analogs which, when available, would be useful are 2-hexynyl-NECA (Cristalli et al., 1992) and 2-(p-methylphenyl)ethyl-Ado (Daly et al, 1993). The former has intermediate potency at A1 receptors and high potency at A2a receptors, whereas the latter has low potency at A1 receptors and high potency at A2a, receptors (as defined above). Both presumably will have very low potency at A2b receptors.
For further details, see Linden et al. (1993b).
This list is not complete. An attempt has been made to limit the presentation to effects that are probably a direct consequence of G-protein interaction. In addition, there are a number of consequences of phosphorylation events.
The agonists have been grouped into four categories according to potency: high, intermediate, low, and very low. In the case of A1 receptors, binding data are from rat brain, rat fat cells, and DDT1 MF-2 cells. Corresponding functional data exist for rat fat cells and DDT1 MF-2 cells. The potency figures are from the binding assays. In the case of A2a receptors, the binding data were obtained from rat striatum or PC12 cells. Corresponding functional assays are available in the same preparations (Hide et al., 1992). In the case of A2b receptors, no binding data are available. Most data were obtained from cyclic AMP accumulation in cells or brain slices. The data listed are based on adenylyl cyclase measurements (Bruns, 1981; Brackett and Daly, 1993). The data concerning A3 receptors is based on adenylyl cyclase in tranafected Chinese hamster ovary cells (Zhou et al., 1992).
CPX is also commonly referred to as DPCPX.
Whereas classical xanthines are poor ligands (at least in the rat), some 8-substituted compounds are quite active, at least on human and sheep A3 receptors (Linden et al., 1993a; Salvatore et al., 1993; Fozard and Hannon, 1993).
XAC has been used as an A2a. antagonist ligand, e.g., in human platelets (Ukena et al., 1986).
Linden et al (1993), Jacobson et al. (1993c).
Distribution information is based on Northern blots (all receptors), in situ hybridization (A1, A2a, and receptor autoradiography (A1, A2a).
The distribution is reported to be quite restricted in the rat (Zhou et al., 1992) but wide in, e.g., sheep (Linden et al., 1993a).
The predicted amino acid sequences of some of the recombinant adenosine receptors are shown in figure 1. There appear to be four major classes of these receptors. The A2a receptors are similar to the A2b receptors in the transmembrane parts but differ from the A2b (and other adenosine receptor types) in having a considerably larger COOH-terminal domain. There are differences in the primary structure of adenosine receptors of a single subtype cloned from different species, which may help to explain the differences that have been shown in binding studies (Ferkany et al., 1986; Stone et al., 1988).
Fig. 1.
Amino acid sequences of purinoceptors deduced from cloned DNAs, aligned for maximum homology. Note that the P2 receptor sequences belong to a completely different family than do the adenosine (P1) receptors. The adenosine receptors listed here are all from the rat: A1 (Mahan et al., 1991), 326 amino acids; A2a (Fink et al., 1992; Furlong et al., 1992), 410 amino acids; A2b, (Stehle et al., 1992), 332 amino acids; A3 (Meyerhof et al., 1991; Zhou et al., 1992), 319 amino acids. Other such recombinant sequences known are for the human (Salvatore et al., 1992; Libert et al., 1992; Townsend-Nicholson and Shine, 1992), canine (Libert et al., 1991), and bovine (Olah et al., 1992; Tucker et al., 1992) A1 receptors; the human (Salvatore et al., 1992) and canine (Maenhaut et al., 1990) A2a receptors; and the human (Salvatore et al., 1992) A2b receptor, each of these is extremely homologous to the corresponding rat receptor. The rabbit A2 receptor was also found to be highly homologous, and genomic cloning revealed that the receptor gene has an intron (Bhattacharya et al., 1993). The chicken P2yl receptor has 362 amino acids (Webb et al., 1993), and the mouse P2u receptor (Lustig et al., 1993) has 373 amino acids. The approximate start positions of the transmembrane helices, as designated on the basis of hydropathy plots, are shown by the symbols TM1 to TM7.
G-protein-coupled receptors show some structural similarities with bacteriorhodopsin, the structure of which has been determined with high-resolution electron cryomicroscopy (Henderson et al., 1990). Three-dimensional models of G-protein-coupled receptors may be constructed using this as a template (Hibert et al., 1991; Dudley et al., 1993), and attempts to model the ligand-receptor interaction have been made (van Galen et al., 1990; van der Wenden et al., 1992). Functional models of the adenosine receptors may serve as an aid in the synthesis of novel ligands (Ijzerman et al., 1992; van Galen et al., 1992; Jacobson et al., 1993b). A schematic representation of some aspects of these models is shown as figure 2.
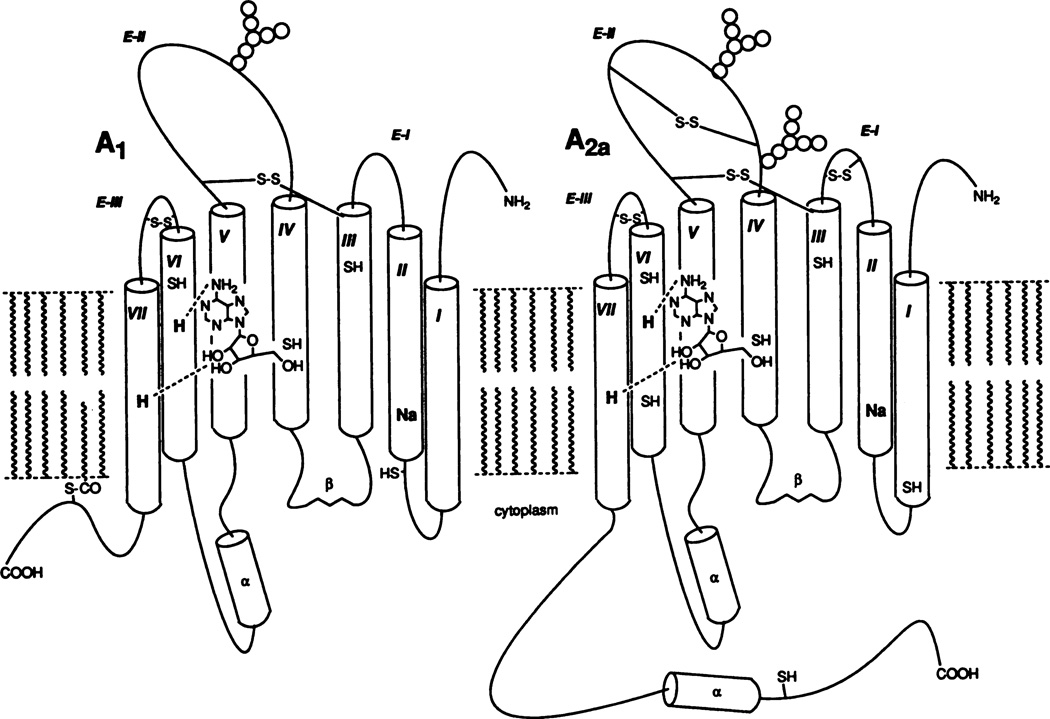
Fig. 2.
Proposed models of A1 and A2A adenosine receptor proteins based on sequence analysis (van Galen et al., 1992) and computer-assisted molecular modeling (Ijzerman et al., 1992). The seven transmembrane helices (I through VII) are arranged in a counterclockwise orientation (looking from the extracellular side) according to the experimentally determined structure of bacteriorhodopsin. Actually, I and VII are in proximity, forming a barrel shape, which surrounds the ligand-binding site. Histidyl residues (H) in the sixth and seventh helices are proposed to hydrogen bond to adenosine, through the purine N6- and ribose 2′,3′ positions, respectively. The locations of cysteinyl residues (SH) and hypothetical disulfide bridges (S-S, Jacobson et al., 1993d) are indicated. Glycosylation occurs on the second extracellular loop (E-II) in both receptors. In the A1 receptor, a potential palmitoylation site (S-CO) is shown as forming an additional anchor of the carboxy-terminal segment in the phospholipid bilayer. Cytoplasmic segments show a hypothetical secondary structure (α and β), predicted using computational algorithms (van Galen et al., 1992)
All of the recombinant adenosine receptors have the general structure that would place them in the rhodopsin-like group of the superfamily of G-protein-coupled receptors. The A2 receptors have been defined on the basis of their ability to stimulate adenylyl cyclase. Thus, they probably interact with the G-protein, Gs. It is not known whether there are other G-proteins that can interact with A2 receptors. Similarly, it is not known whether Gs activated by adenosine receptors can interact with effectors other than adenylyl cyclase (cf. the β-adrenoceptor in the heart which activates a Ca2+ channel via Gs), but experience from other signal transduction cascades suggests that this is a distinct possibility.
The A1 receptor has been shown to couple with Gi-1, Gi-2, Gi-3, and Go but not with Gs or Gz (Freissmuth et al., 1991; Munshi et al., 1991). In practically all instances (but see Fredholm et al., 1989; Thompson et al., 1992), responses to adenosine A1 receptor activation are blocked by pertussis toxin, which is compatible with an involvement of the Gi/Go family of G-proteins. In agreement with this, adenosine A1 receptors can induce a variety of different cellular responses, including inhibition of adenylyl cyclase (van Calker et al., 1978; Londos et al., 1980), stimulation of K+ conductance (Trussell and Jackson, 1985), inhibition of a Ca2+ conductance, probably through an N-type channel (Scholz and Miller, 1991), stimulation of phospholipase C, and generation of a Ca2+ and protein kinase C signal (Gerwins and Fredholm, 1992, 1994). Other reported effects include inhibition of inositol phospholipid hydrolysis (Kendall and Hill, 1988; Delahunty and Linden, 1988) and inhibition of transmitter release by a mechanism that does not involve a change in membrane K+ and Ca2+ conductances (Scholz and Miller, 1991). Based on evidence from other G-protein-coupled receptors it seems likely that some of these responses are mediated by the α-subunits of the G-proteins, whereas other effects may be due to the β,γ-subunits (Birnbaumer, 1992). Probably the degree of activation of the G-protein, and hence of the receptor, may differ by orders of magnitude depending on which subunit(s) mediates the response (Birnbaumer, 1992). The broad range of signaling responses emphasizes that it is not fruitful to attempt to subclassify adenosine receptors solely on the basis of whether the effects are mediated via cyclic AMP or not (Ribeiro and Sebastiao, 1986; Fredholm and Dunwiddie, 1988). Furthermore, the absolute potency of agonists in producing an effect cannot be used to classify receptors. Regarding the newly identified A3 receptor (Zhou et al., 1992; Meyerhof et al., 1991), little is so far known about its G-protein coupling. Because A3 receptors mediate inhibition of adenylyl cyclase, a Gi-like protein is a probable partner.
A list of drugs that appear to be useful to classify adenosine receptors is given in figure 3. The battery of pharmacological tools currently available for the classification of adenosine receptors is far from optimal, especially for use in a functional context, and some points need to be emphasized.
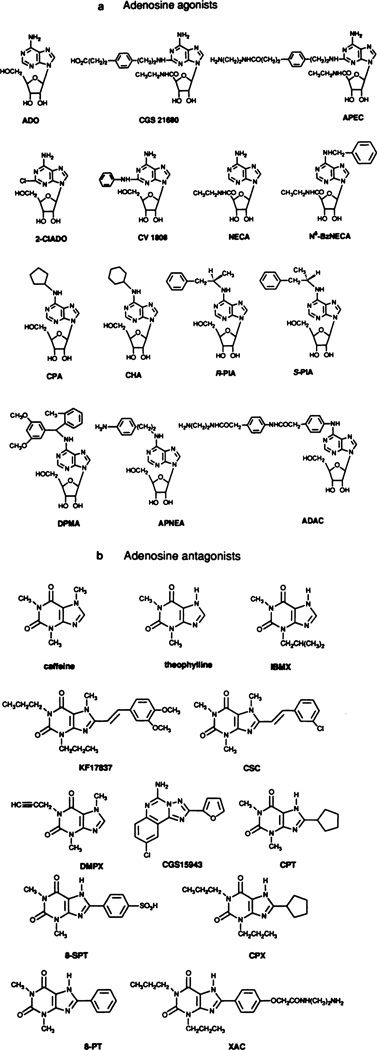
Fig. 3.
Ligands used to classify adenosine receptors. Adenosine agonists (a) include those that are selective for A1 receptors [N6-cyclopentyladenosine (CPA) > N6-cyclohexyladenosine (CHA) > N6(R-phenylisopropyl)-adenosine (R-PIA)], A2a receptors (2-[p-(2-car-bonylethyl)-phenylethylamino]-5′N-ethylcarboxamidoadenosine (CG21680)>2-[(2-aminoethylamino)carbonylethylphenylethylamino]-5′N-ethylcarboxamidoadenosine (APEC) > 2-phenylaminoadenosine (CV1808)), and A3 receptors [N6-benzyl-5′-N-ethyl-carboxamidoadenosine (NECA)]. NECA and 2-chloroadenosine (2-C1ADO) are essentially nonselective. Adenosine antagonists (b) include such that are A1 selective {l,3-dipropyl-8-cyclopentylxanthine (CPX, or sometimes DPCPX); 8-cyclopentyltheophylline (CPT) [and xanthine amine congener (XAC)] |, thosethatareA2a,selective[8-(3-chlorostryl)caffeine(CSC);1,3-dipropyl-8-(3,4-dimethoxystyryl)-7-methylxanthine (KF 17837)], moderately A2 selective [1,3-dimethyl-7-propylxanthine (DMPX) and 9-chloro-2-(2-furanyl)-5,6-dihydro-[1,2,4]-triazolo[1,5]quinazolin-5-imine monome-thanesulfonate(CGS 15943)}. Caffeine, theophylline, 3-isobutyl-1-methylxanthine (IBMX), and 8p-sulfophenyl theophylline are essentially nonselective. The latter compound penetrates the blood-brain barrier poorly. (For other abbreviations, see footnote to table 3).
First, sensitivity to methylxanthines cannot be taken as a universal sign of adenosine receptor involvement. Methylxanthine-induced blockade of a response is still highly suggestive of an involvement of adenosine receptors, but a lack of inhibition can no longer be taken as conclusive evidence against an adenosine receptor being involved. Because the methylxanthine-insensitive A3 receptor (table 3) may be present in significant quantities outside the testis, several older reports of methylxanthine-insensitive adenosine effects may have to be reinterpreted. Indeed, in a recent report it was shown that N6-2-(4-aminophenyl)ethyladenosine which shows a high affinity for the A3 receptors (Zhou et al., 1992) produces xanthine-insensitive hypotension in pithed rats (Fozard and Carruthers, 1993). The A3 receptor also appears to mediate xanthine-resistant adenosine actions on mast cells (Ramkumar et al., 1993). It was recently found that N6-benzyl-5′-N-ethyl-carboxamidoadenosine may be an A3-selective agonist (14-fold vs. A1 and A2a receptors; van Galen et al., 1993). Some 8-phenyl-substituted xanthines are potent antagonists, BW-A 522 being the most potent, with nanomolar affinities at least at ovine and human A3 receptors (Linden et al., 1993a; Salvatore et al., 1993; Fozard and Hannon, 1993).
Second, antagonists that are both selective and easily soluble are established for the A1 receptor type. However, both absolute and relative potencies for these selective antagonists at A1 receptors may differ among species. The more selective antagonists at A2 receptors (Sarges et al., 1990; Shimada et al., 1992) have usually not been examined in well-characterized functional systems, and information derives mainly from binding assays. Recent data indicate that l,3-dipropyl-8-(3,4-dimethoxystyryl)-7-methylxanthine is a selective A2a antagonist in functional assays (Shimada et al., 1992; Fredholm et al., unpublished observations). A related xanthine, 8-(3-chlorostyryl)caffeine, was recently found to be a very selective A2a antagonist (vs. A1), also in vivo (Jacobson et al., 1993b). A whole family of 8-styrylxanthines with A2a selectivity and varying physicochemical properties has been described (Jacobson et al., 1993a). These compounds are not yet readily available, which limits their usefulness in defining criteria for receptor classification.
Third, CGS 21680 and other 2-substituted adenosine analogs play an important role in pharmacological sub-classification of A2 receptors. These compounds discriminate well between A2a and A2b receptors. Cellular responses to CGS 21680 (and related compounds) may in addition depend critically on factors, such as receptor density and amounts and types of G-proteins and adenylyl cyclases, and not solely on the presence or absence of a specific adenosine A2a receptor subtype. However, recent data suggest that CGS 21680 may also bind to a structure, which may be a functional receptor, that is different from hitherto recognized adenosine receptors (Johansson et al., 1993; Cunha, Johansson, and Fred-holm, unpublished data).
B. P2/ATP Purinoceptors
The first overview of the functional effects of various ATP analogs was given by Burnstock and Kennedy (1985). By analyzing the nature of the responses to ATP and related compounds in a number of different biological systems, they discriminated between two major classes of receptors that were named P2X and P2Y purinoceptors, respectively. The two postulated P2 receptors were discriminated on the basis of response profiles to a number of ATP analogs: αβ-MeATP > βγ-MeATP > ATP = 2-MeSATP = ADP for the P2X subtype; 2-MeSATP > ATP >> αβ-MeATP = βγ-MeATP for the P2Y subtype. P2X receptors were also proposed to be quickly desensitized by αβ-MeATP. The agonist response profiles in the guinea pig isolated bladder and taenia coli were considered to represent prototypical P2X and P2Y receptors (Burnstock, 1991; Cusack, 1993). These studies were extended to other smooth muscle preparations, and P2X and P2Y purinoceptor activation was correlated with contraction and relaxation, respectively, but exceptions have been noted (Bailey and Hourani, 1992). The classification (table 4) rests very much on the potency of phosphothioate ATP derivatives, which were originally thought to be “nonhydrolyzable,” but many have more recently been shown to be degraded by ectonucleotidases (Cusack, 1993). Among the analogs 2-MeSATP, αβ-MeATP, l-βγ-MeATP may be particularly useful in discriminating between P2X and P2Y receptors (Hourani et al., 1985). Desensitization by αβ-MeATP and ANAPP3 (table 4) has so far not been reported to occur except at P2X receptors. Novel selective agonists for P2X and P2Y receptors have been introduced (Fischer et al., 1993; Burnstock et al., 1994), and studies with these ligands suggest that the receptors may exist in several subtypes.
TABLE 4.
Reported P2 purinoceptors*
| Name | P2x | P2Y | P2U | P2T | P2Z | P2D |
|---|---|---|---|---|---|---|
| Structure (known) | No | Yes† | Yes‡ | No | No | No |
| Molecular mass | ||||||
| (aa) | 373‡ | |||||
| (kDa) | 42† | 42 | 43§ | |||
| Type | Intrinsic ion channel ║ | G-protein-coupled ¶ | G-protein-coupled# | G-protein-coupled | Nonselective pore | G-protein coupled? |
| Effectors | Na+, K+, Ca2+ | ↑IP3/Ca2+/DAG; ↓cAMP, ↑phos- pholipase A2(↓K+-conduct- ance†† |
IP3/Ca2+/DAG; Ca2+, CI, and K+ currents |
IP3/Ca2+/DAG; cAMP |
Na+, K+, Ca2+ | ↑Ca2+** |
| Agonist | αβ-MeATP ≥ βγ- MeATP > ATP ≥ ADP > 2MeSATP >> UTP‡‡ |
2-MeSATP > ATP = ADP >> αβ MeATP >> UTP§§ |
UTP ≥ ATP = ATPγS >> 2- MeSATP = αβ- MeATP║║ |
2-substituted ADP > ADP¶¶ |
ATP4− | AP4A > ADPβS > AMP-PNP> Ap5A > αβ MeATP >> 2- MeSATP |
| Antagonist | Desensitization## by αβ-MeATP ANAPP3 Suramin*** (pKB = 5.0) PPADS†† |
Suramin (pA2 5.0)‡‡‡ |
None known§§§ | ATP (pA2 = 4.6)║║║ Suramin (pA2 = 4.6)¶¶¶, ### FPL 66096 (pKB = 8.7)### |
None known | |
| Radioligand | [3H]D-αβ-MeATP (PKH = 9.0;pKL = 7.0**** |
[35S]ADPβS (af- finity: pKd = 8.0)†††† |
β[32P]2-MeSADP (pKd = 8.0)‡‡‡‡ [35S]ATPαS (pKd = 8.5)§§§§ |
[3H]AP4A (1 × 10−10 M; 0.6 MM)║║║║ |
||
| Distribution | Smooth muscles, brain, heart, spleen¶¶¶¶ |
Wide distribu- tion#### |
Wide. Found in many cultured cells and in vas- cular muscle |
Platelets | Mast cells,***** macrophages, vas defer- ens††††† |
Chromaffin cells, rat brain synap- tosomes |
Abbreviations: IP3, inositol triphosphate; DAG, diacylglycerol; Me, methyl; cAMP, cyclic AMP; PNP, AP4A, diadenosine tetraphosphate; Ap5A, diadenosine pentaphosphate; ANAPP3, arylazido aminopropionyl ATP.
Determined by photoaffinity labeling using 2-(p-axidophenyl)-ethylthioadenosine 5′-diphosphate (Cristalli and Mills, 1993).
Responses to P2Y receptors are often, but not always, blocked by pertussis toxin indicating involvement of Gi/Go proteins. Often (Bruner and Murphy, 1993) pertussis toxin affords a partial blockade indicating involvement of also Gq/G11 proteins. In some instances (e.g., C6-2B glioma cells) the response (↓ cyclic AMP) is fully pertussis toxin sensitive.
P2U receptor-mediated responses are often only partially blocked by pertussis toxin, suggesting that they are mediated by both Gi/Go and by Gq/G11 proteins (Gerwins and Fredholm, 1992).
The increase in [Ca2+]i appears to be via mobilization of intracellular stores (Castro et al., 1992).
There are some minor differences between the potency order as determined in functional assays (e.g., rabbit ear artery, O’Connor et al., 1990) and in binding assays (Bo and Burnstock, 1992). The channel activity studied by patch-clamp is somewhat different in that ATP is equipotent with αβ-MeATP and βγ-MeATP (Bean, 1992).
Important differences have been noted. For relaxation of rat pulmonary vessels the potency order (relative to that of the most potent compound in the series) was 2-MeSATP (1) > ATP (0.02) = ADP (0.02) > βγ-MeATP (0.01) > αβ-MeATP (0.006) (Liu et al., 1989); responses to activation of the cloned P2Y1 receptor showed the order 2-MeSATP (1) = ATP (1) > ADP (0.05) >> αβ-MeATP, whereas binding to turkey erythrocytes shows the order 2-MeSATP (1) > ATP (0.1) ≥ ADP (0.07) > αβ-MeATP (0.002) > βγ-MeATP (0.0006) (Cooper et al., 1989).
UTP is sometimes considerably more potent than ATP (van Rhee et al., 1993).
Cusack and Hourani (1981; 1982a), MacFarlane (1983), Greco et al. (1991).
Desensitization is often rapid and may be irreversible, especially with photoactivated ANAPP3 (Hogaboom et al., 1980; Kasakov and Burnstock, 1983).
There are reports that suramin is (van der Zee et al., 1992) and is not (Wilkinson et al., 1993) an antagonist.
2-Propylthio-d-β,γ-difluoromethylene ATP (Humphries et al., 1993).
High-affinity sites represent less than half the total binding sites (Pintor et al., 1993). The second set of figures within parentheses are Ki values for a low affinity site.
Contractile responses in smooth muscle have been well characterized, e.g., in rabbit mesenteric artery (Burnstock and Warland, 1987) and rabbit ear artery (O’Connor et al., 1990). Binding has been studied, e.g., in rat bladder, brain, heart, vas deferens, and spleen (Bo and Burnstock, 1990; Michel et al., 1993). ATP-induced channel activity has been studied, e.g., in PC12 cells, but this probably is a secondary event (Nakazawa et al., 1991), sensory neurons (Krishtal et al., 1983; Bean et al., 1990) and ear artery muscle (Benham and Tsien, 1987).
Functional responses have been studied, e.g., in guinea pig aorta (Dainty et al., 1992) and in turkey erythrocytes (Berrie et al., 1989). Binding has been studied in turkey erythrocytes (Cooper et al., 1989).
Most recent evidence suggests that the P2X purinoceptor family represents an intrinsic ion channel permeable to Na+, K+, and Ca2+ (Bean, 1992). P2Y purinoceptors constitute G-protein-linked receptors, often coupled to stimulation of phospholipase C activity and, hence, to inositol trisphosphate formation (O’Connor et al., 1991), but additional transduction mechanisms, including modulation of cyclic AMP generation (Okajima et al., 1989; Yamada et al., 1992; Boyer et al., 1993) and arachidonic acid mobilization (Bruner and Murphy, 1990, 1993) have also been demonstrated.
After the 1985 Burnstock and Kennedy proposal, Gordon (1986) further subdivided the P2 purinoceptors by assigning the name P2T to the receptor for ADP on blood platelets (Humphries et al., 1993) and P2Z for the “receptor” that mediates responses to ATP4− in mast cells (Dahlqvist and Diamant, 1974) and macrophages (Steinberg and Silverstein, 1987), which appears to represent the opening of a fairly nonselective type of pore. There is now good evidence that there are receptors that respond to UTP, ATP, and ATPγS, but not to 2-MeSATP or αβ-MeATP, which has led to the definition of the so-called “P2U” or “nucleotide” or “pyrimidine” receptor (table 4; O’Connor et al., 1991; Dubyak, 1991). There also appears to be a receptor for diadenosinetetraphosphate, which was called a P2D subtype (Hilderman et al., 1991; Castro et al., 1992). Some characteristics of the P2 purinoceptors are summarized in table 4.
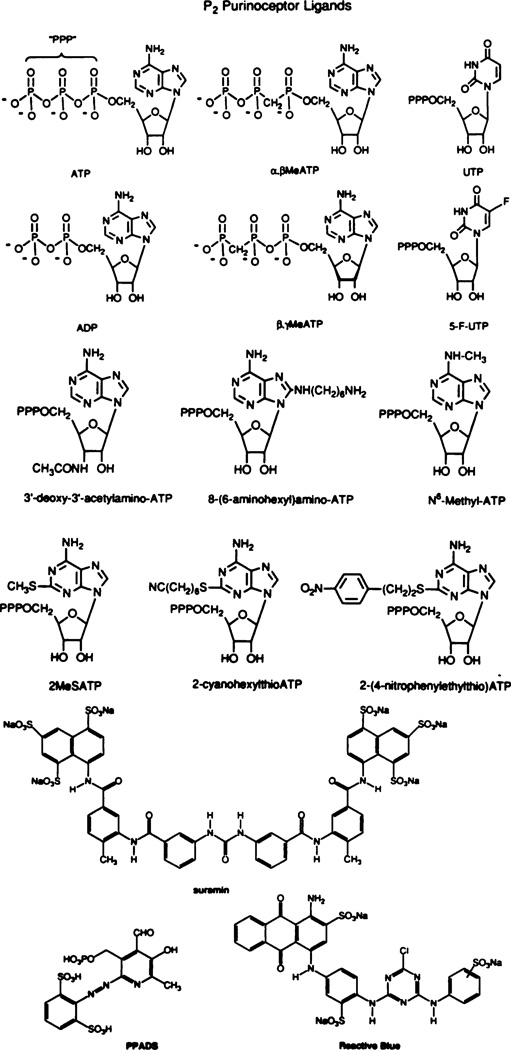
A problem that has always hampered research in the P2 purinoceptor field is the lack of selective antagonists. The trypanoside suramin (Dunn and Blakeley, 1988; Voogd et al., 1993) sometimes behaves as a competitive antagonist but does not appear to distinguish between the P2X and P2Y subtypes (Hoyle et al., 1990). Suramin is also very effective in inhibiting the actions of certain growth factors (Betsholtz et al., 1986; Peng et al., 1991), presumably secondarily to interactions with the corresponding receptors (Eriksson et al., 1991). The potency of suramin against basic fibroblast growth factor (Peng et al., 1991; IC50 is in the low micromolar range) is at least as high as against P2 receptors. Suramin is also an inhibitor of several enzymes, including 5′-nucleotidase (Hourani and Chown, 1989), something that is of particular concern when the compound is used to discriminate between P1 and P2 actions. 2–2′-Pyridylisatogen was reported to be a weakly selective antagonist of the relaxant effects of ATP in smooth muscle (Spedding et al., 1975) but did not antagonize the effects of adenosine (Spedding and Weetman, 1976). Pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid, synthesized by Lambrecht and co-workers (1992), is a novel type of P2 antagonist with a potency at P2X receptors in the nanomolar range (Ziganshin et al., 1993). The displacement of [3H]αβMeATP was biphasic, suggesting multiple affinity sites or multiple receptor subtypes (Ziganshin et al., 1993). Reactive Blue 2 has been reported to selectively antagonize ATP actions at the P2Y subtype, although concentration and time of exposure are critical. The structures of a number of compounds useful for the study of P2 purinoceptors are shown in figure 4.
Fig. 4.
Ligands used to characterize P2 purinoceptors. The structures shown are of the d-isomers. Some studies have also been done on l-isomers. α,β-methylene (Me) ATP is a potent and selective P2X agonist. 8-(6-aminohexylamino)ATP is selective for P2Y vs. P2X receptors and may discriminate between subforms (Burnstock et al., 1994). UTP and 5-F-UTP are active at P2U but not at P2Y receptors. 2-MethylthioATP is active at P2Y but not at P2U receptors. None of these are very active at P2X receptors. The long-chain functionalized congeners (Fischer et al., 1993) 2-(p-nitrophenylthio)ATP and 2-(6-cyanhexylthio)ATP maintain or increase potency at P2Yreceptors. The nitro derivative may discriminate between forms of P2X receptors. N6-methyl ATP may discriminate between forms of P2Y receptors. The structures of some compounds—suramin, pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), and Reactive Blue—that have antagonistic properties are also shown.
Molecular information concerning P2 purinoceptors is becoming available. Xenopus oocytes injected with mRNA from embryonic guinea pig brain (Fournier et al., 1990; Honoré et al., 1991), promyelocytic leukemia cells (HL60; Murphy and Tiffany, 1990), J774 murine macrophage-like cells (Hickman et al., 1993; Nuttle et al., 1993), or guinea pig vas deferens (Russell et al., 1993) were conferred with the ability to respond to ATP. The pharmacology corresponded to that of several of the proposed P2 receptor subforms.
P2Y (Webb et al., 1993) and P2U purinoceptors (Lustig et al., 1993) have recently been cloned. As shown from figure 1, these receptors are more similar to each other than they are to adenosine receptors. Interestingly, they are not closer to the adenosine receptor than to other G-protein-coupled receptors. Based on such a comparison of these sequences with a more recently cloned ADP receptor, it has been proposed that the G-protein-coupled P2 purinoceptors will constitute a distinct family within the superfamily of G-protein-coupled receptors (Barnard et al., 1994).
P2Y purinoceptor-mediated responses show different agonist pharmacology in a variety of tissues and preparations (Burnstock, 1991; Fischer et al., 1993), suggesting a subclassification of the “classic” P2Y purinoceptor. Other data suggest that the currently designated “P2U,” “P2T,” and “P2D” purinoceptor subtypes may have to be reclassified.
On these grounds it must be emphasized that the current classification of the P2 series must be considered unsatisfactory for the long term. When additional structural information is obtained and truly selective antagonists become available, a revised nomenclature will be established. Even now it is clear that there is a basis for distinguishing two major families of P2 purinoceptors, one coupled to intrinsic ion channels and the other coupled to G-proteins. For the transition period the IUPHAR Committee on Receptor Nomenclature and Drug Classification, in keeping with the proposal by Abbracchio and Burnstock (1994), recommends that any new subtypes of G-protein-coupled receptor be termed P2Y1, P2Y2, P2Y3,… purinoceptors and any new subtypes of intrinsic ion channel be termed P2X1, P2X2, P2X3,… purinoceptors. The 2X and 2Y are not subscripted, to avoid confusion with previous usage and to facilitate the use of lower case, as in p2y1, p2y2,… to refer to cloned receptors whose correspondence to a pharmacologically defined subtype has not been firmly established. Lower case is being used in this way in other IUPHAR nomenclatures, e.g., adrenoceptors, muscarinic cholinoceptors, and 5-hydroxytryptamine receptors. The term P2Z purinoceptor should be reserved for novel receptor structures that do not correspond to the P2X and P2Y purinoceptor structure. A possible example could be the mast cell P2 purinoceptor, if this is established to be a nonselective ion pore opened by ATP. Although not an ideal system of classification, it does allow consecutive numbering and obviates the need for arbitrary designation of letters when new subtypes are identified.
Footnotes
The classification of purinoceptors described in this review has been sanctioned by the IUPHAR Committee on Receptor Nomenclature and Drug Classification. As stated in the review, the nomenclature may have to be revised when more information becomes available. IUPHAR Purinioceptor Classification Subcommittee: Prof. Bertil B. Fredholm (Chairman), Section of Molecular Neuropharmacology, Department of Physiology and Pharmacology, Karolinska Institutet S.-171 77 Stockholm, Sweden (phone: Int + 46-8-728 79 39, fax: Int + 46-8-33 16 53); Dr. Alison Abbott, Nature, Macmillan Magazines Ltd., Sandstraβe 41, D.-8000 München 2, Germany (phone: Int + 49-89-52 70 36, fax: Int + 49-89-523 22 22); Prof. Geoffrey Burnstock, University College London, Gower Street, London WC1E 6BT, United Kingdom (phone: Int + 44-71-387 70 50, fax: Int + 44-71-380 73 49); Dr. John W. Daly, Ph.D., Chief, Laboratory of Bioorganic Chemistry, NIDDK, National Institutes of Health, Building 8, Room 1A-15, Bethesda, Maryland 20892, USA (phone: Int + 1-301-496 40 24, fax: Int + 1-301-402 00 08); Dr. Paul Left, Manager of Pharmacology, Fisons Pharmaceuticals, Research and Development Labs, Pharmaceutical Division, Bakewell Road, Loughborough, Leistershire LE11 0RH, United Kingdom (phone: Int + 44-509-61 10 11, fax: Int + 44-509-21 04 50); Prof. T. Kendall Harden, Department of Pharmacology, University of North Carolina, School of Medicine, Chapel Hill, North Carolina 24999, USA (phone: Int + 1-919-966-3744, fax: Int + 1-919-966-5640); Prof. Dr. med. Ulrich Schwabe, Pharmakologisches Institut der Universität Heidelberg, Im Neuenheimer Feld 366, D.-6900 Heidelberg 1, Germany (phone: Int + 49-6221-56 39 02, fax: Int + 49-6221-56 39 44); Dr. Michael Williams, Divisional Vice President, Neuroscience Research, Abbott Laboratories, D.-464, AP10, Abbott Park, Illinois 60064-3500, USA (phone: Int + 1-708-937 81 86, fax: Int + 1-708-937-9195)
REFERENCES
- Abbracchio M, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol. Ther. doi: 10.1016/0163-7258(94)00048-4. in press. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Hourani SMO. Effects of purines on the longitudinal muscle of rat colon. Br. J. Phamacol. 1992;105:885–892. doi: 10.1111/j.1476-5381.1992.tb09073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard EA, Burnstock G, Webb TE. G-protein coupled receptors for ATP and other nucleotides: a new receptor family. Trends Pharmacol. Sci. 1994;15:67–70. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Bean BP. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol. Sci. 1992;13:87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Bean BP, Williams CA, Ceelen PW. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J. Neurosci. 1990;10:11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham CD, Tsien RW. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature (Lond.) 1987;238:275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am. J. Physiol. 1963;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- Berrie CP, Hawkins PT, Stephens LR, Harden TK, Downes CP. Phosphatidylinositol 4,5-bisphosphate hydrolysis in turkey erythrocytes is regulated by P2Y-purinoceptors. Mol. Pharmacol. 1989;35:526–532. [PubMed] [Google Scholar]
- Betsholtz C, Johnsson A, Heldin C-H, Westermark B. Efficient reversion of simian sarcoma virus-transformation and inhibition of growth factor-induced mitogenesis by suramin. Proc. Natl. Acad. Sci. USA. 1986;83:6440–6444. doi: 10.1073/pnas.83.17.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Dewitt DL, Burnatowska-Hledin M, Smith WL, Spielman WS. Cloning of an adenosine A1 receptor-encoding gene from rabbit. Gene. 1993;128:285–288. doi: 10.1016/0378-1119(93)90576-o. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for β/γdimers as well as α subunits. Cell. 1992;71:1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Bo X, Burnstock G. Species differences in characteristics and distribution of [3H]α,β-methylene ATP binding sites in urinary bladder and urethra of rat, guinea-pig and rabbit. Eur. J. Pharmacol. 1992;216:59–66. doi: 10.1016/0014-2999(92)90209-m. [DOI] [PubMed] [Google Scholar]
- Bo XN, Burnstock G. High- and low-affinity binding sites for [3H]-α,β-methylene ATP in rat urinary bladder membranes. Br. J. Pharmacol. 1990;101:291–296. doi: 10.1111/j.1476-5381.1990.tb12703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Lazarowski ER, Chen X-H, Harden TK. Identification of a P2Y-purinergic receptor that inhibits adenylyl cyclase. J. Pharmacol. Exp. Ther. 1993;267:1140–1146. [PubMed] [Google Scholar]
- Brackett LE, Daly JW. Functional characterization of the A2B, adenosine receptor in NIH 3T3 fibroblasts. Biochem. Pharmacol. 1993 doi: 10.1016/0006-2952(94)90480-4. in press. [DOI] [PubMed] [Google Scholar]
- Bruner G, Murphy S. ATP-evoked arachidonic acid mobilization in astrocytes is via a P2Y-purinergic receptor. J. Neurochem. 1990;55:1569–1575. doi: 10.1111/j.1471-4159.1990.tb04940.x. [DOI] [PubMed] [Google Scholar]
- Bruner G, Murphy S. Purinergic P2Y receptors on astrocytes are directly coupled to phospholipase A2. Glia. 1993;7:219–224. doi: 10.1002/glia.440070305. [DOI] [PubMed] [Google Scholar]
- Bruns RF. Adenosine receptor activation in human fibroblasts: nucleoside agonists and antagonists. Can. J. Physiol. Pharmacol. 1981;55:673–691. doi: 10.1139/y80-110. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol. Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Bolis L, Straub RW, editors. Cell Membrane Receptors for Drugs and Hormones. New York: Raven Press; 1978. pp. 107–118. [Google Scholar]
- Burnstock G. Purinergic nerves and receptors. Prog. Biochem. Pharmacol. 1980;16:141–154. [PubMed] [Google Scholar]
- Burnstock G. Overview (purinergic receptors) In: Imai S, Nakazawa M, editors. Role of Adenosine and Adenine Nucleotides in the Biological System. Amsterdam, The Netherlands: Elsevier; 1991. pp. 3–16. [Google Scholar]
- Burnstock G, Brown CM. An introduction to purinergic receptors. In: Burnstock G, editor. Purinergic Receptors. London, UK: Chapman and Hall; 1981. pp. 1–46. [Google Scholar]
- Burnstock G, Fischer B, Maillard M, Ziganshin AU, Ralevic V, Knight G, Brizzolara A, Von Isakovics A, Boyer JL, Harden KA, Jacobson KA. Structure-activity relationships for derivatives of adenosine-5 -triphosphate as agonists at P2Y purinoceptors: heterogeneity within P2X and P2Y-subtypes. Drug Dev. Res. 1994 doi: 10.1002/ddr.430310308. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Kennedy C. Is there a basis for distinguishing two types of Ps-purinoceptor? Gen. Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Warland JJI. P2-purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2Y but not the P2X-purinoceptor. Br. J. Pharmacol. 1987;90:383–391. doi: 10.1111/j.1476-5381.1987.tb08968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro E, Pintor J, Miras-Portugal MT. Ca2+-stores mobilization by diadenosine tetraphosphate, AD4A, through a putative P2Y purinoceptor in adrenal chromaffin cells. Br. J. Pharmacol. 1992;106:833–837. doi: 10.1111/j.1476-5381.1992.tb14421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbin LB, Einstein R, McGuire MH. Studies on the coronary dilator actions of some adenosine analogues. Br. J. Pharmacol. 1974;50:25–33. doi: 10.1111/j.1476-5381.1974.tb09589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CL, Morris AJ, Harden TK. Guanine nucleotide-sensitive interaction of a radiolabeled agonist with a phospholipase C-linked P2Y-purinergic receptor. J. Biol. Chem. 1989;264:6202–6206. [PubMed] [Google Scholar]
- Cornfield LJ, Su S, Sills MA. The novel binding site labeled by [3H]CV 1808 is associated with potassium channel activation. FASEB J. 1992;6:1008A. [Google Scholar]
- Cristalli G, Eleuteri A, Vittori S, Volpini R, Lohse MJ, Klotz KN. 2-Alkynyl derivatives of adenosine and adenosine-5′-N-ethyluronamide as selective agonists at A2 adenosine receptors. J. Med. Chem. 1992;35:2363–2368. doi: 10.1021/jm00091a003. [DOI] [PubMed] [Google Scholar]
- Cristalli G, Mills DCB. Identification of a receptor for ADP on blood platelets by photoaffinity labelling. Biochem. J. 1993;291:875–881. doi: 10.1042/bj2910875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack NJ. P1 receptors: subclassification and structure-activity relationships. Drug Dev. Res. 1993;28:244–252. [Google Scholar]
- Cusack NJ, Hourani SMO. Partial agonist behaviour of adenosine 5′-O-(2-thiodiphosphate) on human platelets. Br. J. Pharmacol. 1981;73:405–408. doi: 10.1111/j.1476-5381.1981.tb10436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack NJ, Hourani SMO. Adenosine 5′ -diphosphate antagonists and human platelets: no evidence that aggregation and inhibition of stimulated adenylate cyclase are mediated by different receptors. Br. J. Pharmacol. 1982a;776:221–227. doi: 10.1111/j.1476-5381.1982.tb09210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack NJ, Hourani SMO. Competitive inhibition by adenosine 5′-triphosphate of the actions on human platelets of 2-chloroadenosine 5′-diphosphate, 2-azido-adenosine 5′-diphosphate and 2-methylthioadenosine 5′-diphosphate. Br. J. Pharmacol. 1982b;77:329–333. doi: 10.1111/j.1476-5381.1982.tb09302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist R, Diamant B. Interactions of ATP and calcium on the rat mast cell: effects on histamine release. Acta Physiol. Scand. 1974;34:368–384. doi: 10.1111/j.1600-0773.1974.tb03533.x. [DOI] [PubMed] [Google Scholar]
- Dainty IA, Leff P, Mckechnie K, O’Connor EA. Endothelium-dependent relaxations to ATP in guinea-pig but not rat aorta are mediated predominantly through P2Y-purinocepton. Ink J. Purines Pyrimidines. 1992;3:70. [Google Scholar]
- Daly JW, Padgett WL, Secunda SI, Thompson RD, Olsson RA. Structure-activity relationships for 2-subetituted adenosines at A1 and A2 adenosine receptors. Pharmacology. 1993;46:91–100. doi: 10.1159/000139033. [DOI] [PubMed] [Google Scholar]
- De Gubareff T, Slbator W., Jr Effects of caffeine on mammalian atrial muscle, and its interaction with adenosine and calcium. J. Pharmacol. Exp. Ther. 1965;148:202–214. [PubMed] [Google Scholar]
- Delahunty TM, Linden J. Adenosine inhibits TRH-stimulated phos-phoinositide hydrolysis and reduces inositol phosphate accumulation in GH3 cells. FASEB J. 1988;2:A1132. [Google Scholar]
- Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J. Physiol (Lond.) 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak GR. Signal transduction by P2-purinergic receptors for extracellular ATP. Am. J. Respir. Cell Mol. Biol. 1991;4:295–300. doi: 10.1165/ajrcmb/4.4.295. [DOI] [PubMed] [Google Scholar]
- Dudley MW, Peet NP, Demeter DA, Weintraub HJR, Ijzerman AP, Nordvall G, Van Galen PJM, Jacobson KA. Adenosine A1, receptor and ligand molecular modeling. Drug Dev. Res. 1993;28:237–243. doi: 10.1002/ddr.430280309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn PM, Blakeley AGH. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br. J. Pharmacol. 1988;93:243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson AE, Cousens LS, Weaver LH, Matthews BW. Three-dimensional structure of human basic fibroblast growth factor. Proc. Natl Acad. Sci. USA. 1991;88:3441–3445. doi: 10.1073/pnas.88.8.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedan JS, Dagirmanjian JP, Attfield MD, Chideckel EW. Evidence that the P2X purinoceptor of the smooth muscle of the guinea pig vas deferens is an ATP4− receptor. J. Pharmacol. Exp. Ther. 1990;255:46–51. [PubMed] [Google Scholar]
- Ferkany JW, Valentine HL, Stone GA, Williams M. Adenosine A1 receptors in mammalian brain: species differences in their interactions with agonists and antagonists. Drug Dev. Res. 1986;9:85–93. [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM. Molecular cloning of the rat A2 adenosine receptor selective co-expression with D2 dopamine receptors in rat striatum. MoL Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Fischer B, Boyer JL, Hoyle CHV, Ziganshin AU, Brizzarola AL, Knight GE, Zimmet J, Burnstock G, Harden TK, Jacobson KA. Identification of potent, selective P2Y-purinoceptor agonists: structure activity relationships for 2-thioether derivatives of adenosine-5′-triphosphate. J. Med. Chem. 1993;36:3937–3946. doi: 10.1021/jm00076a023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodgaard H, Klenow H. Abundant amounts of diadenosine 5′,5‴-p1,p4-tetraphosphate are present and releasable, but metabolically inactive in human platelets. Biochem. J. 1982;208:737–742. doi: 10.1042/bj2080737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier F, Honoré E, Collin T, Guilbault P. Ins(1,4,5)P3 formation and fluctuating chloride current response induced by external ATP in Xenopus oocytes injected with embryonic brain mRNA. FEBS Lett. 1990;277:205–208. doi: 10.1016/0014-5793(90)80845-a. [DOI] [PubMed] [Google Scholar]
- Fozard JR, Carruthers AM. Adenosine A3 receptors mediate hypotension in the angiotensin II-supported circulation of the pithed rat. Br. J. Pharmacol. 1993;109:3–5. doi: 10.1111/j.1476-5381.1993.tb13522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozard JR, Hannon JP. BW-A 522 blocks adenosine A3 receptor-mediated hypotensive responses in the rat. Eur. J. Pharmacol. 1994 doi: 10.1016/0014-2999(94)90604-1. in press. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Dunwiddie TV. How does adenosine inhibit transmitter release? Trends Pharmacol. Sci. 1988;9:130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Proctor W, Van Der Ploeg I, Dunwiddie TV. In vivo pertussis toxin treatment attenuates some, but not all, adenosine A1 effects in slices of the rat hippocampus. Eur. J. Pharmacol. 1989;172:249–262. doi: 10.1016/0922-4106(89)90055-2. [DOI] [PubMed] [Google Scholar]
- Freissmuth M, Schutz W, Linder ME. Interactions of the bovine brain A1-adenosine receptor with recombinant G protein α-subunits. Selectivity for rGia–3. J. Biol. Chem. 1991;266:17778–17783. [PubMed] [Google Scholar]
- Furlong TJ, Pierce KD, Selbie LA, Shine J. Molecular characterization of a human brain adenosine A2 receptor. Mol. Brain Res. 1992;15:62–66. doi: 10.1016/0169-328x(92)90152-2. [DOI] [PubMed] [Google Scholar]
- Gaarder A, Jonsen J, Laland S, Hellem A, Owren PA. Adenosine diphosphate in red cells as a factor in the adhesiveness of human blood platelets. Nature (Lond.) 1961;192:531–532. doi: 10.1038/192531a0. [DOI] [PubMed] [Google Scholar]
- Gerlach E, Deuticke B, Dreisbach RH. Der Nucleotid-Abbau im Herzmuskel bei Sauerstoffmangel und seine mögliche Bedeutung für die Coronardurchblutung. Naturwissenschaften. 1963;50:228–229. [Google Scholar]
- Gerwins P, Fredholm BB. ATP and its metabolite adenosine act synergistically to mobilize intracellular calcium via the formation of inositol 1,4,5-trisphosphate in a smooth muscle cell line. J. Biol. Chem. 1992;267:16081–16087. [PubMed] [Google Scholar]
- Gerwins P, Fredholm BB. Adenosine A1, agonists stimulate protein kinase C in a smooth muscle cell line. J. Biol. Chem. 1994 revised version submitted. [Google Scholar]
- Gordon JL. Extracellular ATP: effects, sources and fate. Biochem. J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco NJ, Yamamoto N, Jackson BW, Tandon NN, Moos JRM, Jamieson GA. Identification of a nucleotide-binding site on glycoprotein IIb. J. Biol. Chem. 1991;266:13627–13633. [PubMed] [Google Scholar]
- Green HN, Stoner HB. Biological Actions of the Adenine Nucleotides. London, UK: H. K. Lewis & Co. Ltd; 1950. pp. 1–221. [Google Scholar]
- Haslam RJ, Cusack NJ. Blood platelet receptors for ADP and for adenosine. In: Burnstock G, editor. Purinergic Receptors. London, UK: Chapman and Hall; 1981. pp. 221–285. [Google Scholar]
- Henderson R, Baldwin JM, Ceska TA, Zemlin F, Beckmann E, Downing KH. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J. Mol. Biol. 1990;213:899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Hibert MF, Trumpp-Kallmeyer S, Bruinvels A, Hoflack J. Three-dimensional models of neurotransmitter G-binding protein-coupled receptors. Mol. Pharmacol. 1991;40:8–15. [PubMed] [Google Scholar]
- Hickman SE, Semnad CE, Field M, Silverstein SC. Expression of macrophage ATP and UTP receptors. FASEB J. 1993;7:A712. [Google Scholar]
- Hide I, Padgett WL, Jacobson KA, Daly JW. A2a adenosine receptors from rat striatum and rat pheochromocytoma PC12 cells: characterization with radioligand binding and by activation of adenylate cyclase. Mol. Pharmacol. 1992;41:352–359. [PMC free article] [PubMed] [Google Scholar]
- Hilderman RH, Martin M, Zimmerman JK, Pivorun EB. Identification of a unique membrane receptor for adenosine 5′,5‴-P1,P4-tetraphosphate. J. Biol. Chem. 1991;266:6915–6918. [PubMed] [Google Scholar]
- Hogaboom GK, O’Donnell JP, Fedan JS. Purinergic receptors: photoaffinity analog of adenosine triphosphate is a specific adenosine triphosphate antagonist. Science (Washington DC) 1980;208:1273–1276. doi: 10.1126/science.6103581. [DOI] [PubMed] [Google Scholar]
- Holton FA, Holton P. The capillary dilator substances in dry powders of spinal roots: a possible role of ATP in chemical transmission from nerve endings. J. Physiol (Lond.) 1954;126:124–140. doi: 10.1113/jphysiol.1954.sp005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton P. The liberation of ATP on antidromic stimulation of sensory nerves. J. Physiol. (Lond.) 1959;145:494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E, Fournier F, Collin T, Nargeot J, Guilbault P. Functional expression of P2Y purinoceptors in Xenopus oocytes injected with brain mRNA. Pflugers Arch. 1991;418:447–452. doi: 10.1007/BF00497772. [DOI] [PubMed] [Google Scholar]
- Hourani SMO, Chown JA. The effects of some possible inhibitors of ectonucleotidases on the breakdown and pharmacological effects of ATP in the guinea-pig urinary bladder. Gen. Pharmacol. 1989;20:413. doi: 10.1016/0306-3623(89)90188-2. [DOI] [PubMed] [Google Scholar]
- Hourani SMO, Cusack NJ, Welfors LA. l-AMP-PCP, an ATP receptor agonist in guinea pig bladder, is inactive on taenia coli. Eur. J. Pharmacol. 1985;108:197–200. doi: 10.1016/0014-2999(85)90726-5. [DOI] [PubMed] [Google Scholar]
- Hourani SMO, Hall DA, Nieman CJ. Effects of the P2-purinoceptor antagonist, suramin, on human platelet aggregation induced by adenosine 5′-diphosphate. Br. J. Pharmacol. 1992;105:453–457. doi: 10.1111/j.1476-5381.1992.tb14274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle CH, Knight GE, Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br. J. Pharmacol. 1990;99:617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries RG, Tomlinson W, Ingall AH, Kindon ND, Leff P. FPL66096: a novel, highly potent and selective antagonist at human platelet P2T-purinoceptors. Br. J. Pharmacol. 1993;110(Suppl.) doi: 10.1111/j.1476-5381.1994.tb17100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KA, Fox IH. Purification and characterization of the adenosine A2-like binding site from human placental membrane. J. Biol. Chem. 1989;264:19898–19903. [PubMed] [Google Scholar]
- Ijzerman AP, Van Galen PJM, Jacobson KA. Molecular modeling of adenosine receptors. I. The ligand binding site of the A1 receptor. Drug Des. Discover. 1992;9:49–67. [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Gallo-Rodriguez C, Melman N, Fischer B, Maillard M, Van Bergen A, Van Galen PJ, Karton Y. Structure-activity relationships of 8-styrylxanthines as A2-selective adenosine antagonists. J. Med. Chem. 1993a;36:1333–1342. doi: 10.1021/jm00062a005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Nikodijevic O, Padgett W, Gallo-Rodriguez C, Maillard M, Daly JW. 8-(3-Chlorostyryl)caffeine is a selective A2-adenosine antagonist in vitro and in vivo. FEBS Lett. 1993b;323:141–144. doi: 10.1016/0014-5793(93)81466-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Nikodijevic O, Shi D, Gallo-Rodriguez C, Olah ME, Stiles GL, Daly JW. A role for central A3-adenosine receptors. Mediation of behavioral depressant effects. FEBS Lett. 1993c;336:57–60. doi: 10.1016/0014-5793(93)81608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Van Galen PJM, JI XO, Ramkumar V, Olah M, Stiles M. Molecular characterization of A1 and A2a receptors. Drug. Dev. Res. 1993d;28:226–231. doi: 10.1002/ddr.430280307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B, Georgiev V, Parkinson FE, Fredholm BB. The binding of the adenosine A2 selective agonist [3H]CGS 21680 to rat cortex differs from its binding to rat striatum. Eur. J. Pharmacol. 1993;247:103–110. doi: 10.1016/0922-4106(93)90066-i. [DOI] [PubMed] [Google Scholar]
- Kasakov L, Burnstock G. The use of the slowly degradable analogue, α,β-methylene ATP, to produce desensitisation of the P2-purinoceptor effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur. J. Pharmacol. 1983;86:291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Kenakin TP, Bond RA, Bonner TI. Definition of pharmacological receptors. Pharmacol. Rev. 1992;44:351–361. [PubMed] [Google Scholar]
- Kendall DA, Hill SJ. Adenosine inhibition of histamine-stimulated inositol phospholipid hydrolysis in mouse cerebral cortex. J. Neurochem. 1988;50:497–502. doi: 10.1111/j.1471-4159.1988.tb02939.x. [DOI] [PubMed] [Google Scholar]
- Krishtal OA, Marchenko SM, Pidoplichko VI. Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci. Lett. 1983;35:41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- Lambrecht G, Friebe T, Grimm U, Windscheif U, Bungart E, Hildebrandt C, Bäumert HG, Spatz-Kümbel G, Mutschler E. PPADS, a novel functionally selective antagonist of P2 purinoceptor-mediated responses. Eur. J. Pharmacol. 1992;217:217–219. doi: 10.1016/0014-2999(92)90877-7. [DOI] [PubMed] [Google Scholar]
- Leff P, Wood BE, O’Connor SE. Suramin is a slowly-equilibrating but competitive antagonist at P2X receptors in the rabbit isolated ear artery. Br. J. Pharmacol. 1990;101:645–649. doi: 10.1111/j.1476-5381.1990.tb14134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert F, Schiffmann SN, Lefort A, Parmentier M, Gérard C, Dumont JE, Vanderhaeghen J-J, Vassart G. The orphan receptor cDNA RDC7 encodes an A1 adenosine receptor. EMBO J. 1991;10:1677–1682. doi: 10.1002/j.1460-2075.1991.tb07691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert F, Van Sande J, Lefort A, Czernilofsky A, Dumont JE, Vassart G, Ensinger HA, Mendla KD. Cloning and functional characterization of a human A1 adenosine receptor. Biochem. Biophys. Res. Commun. 1992;187:919–926. doi: 10.1016/0006-291x(92)91285-x. [DOI] [PubMed] [Google Scholar]
- Linden J, Taylor HE, Robeva AS, Tucker AL, Stehle JH, Rivkees SA, Fink JS, Reppert SM. Molecular cloning and functional expression of a sheep A3 adenosine receptor with widespread tissue distribution. Mol. Pharmacol. 1993a;44:524–532. [PubMed] [Google Scholar]
- Linden J, Tucker AL, Robeva AS, Graber SG, Munshi R. Properties of recombinant adenosine receptors. Drug Dev. Res. 1993b;28:232–236. [Google Scholar]
- Liu SF, McCormack DG, Evans TW, Barnes PJ. Characterization and distribution of P2-purinoceptor subtypes in rat pulmonary vessels. J. Pharmacol Exp. Ther. 1989;251:1204–1210. [PubMed] [Google Scholar]
- Lohse MJ, Elger B, Lindenborn-Fotinos J, Klotz KN, Schwabe U. Separation of solubilized A, adenosine receptors of human platelets from non-receptor (3H]NECA binding sites by gel filtration. Naunyn Schmiedebergs Arch. Pharmacol. 1988;337:64–68. doi: 10.1007/BF00169478. [DOI] [PubMed] [Google Scholar]
- Londos C, Cooper DMF, Wolff J. Subclasses of external adenosine receptors. Proc. Natl Acad. Sri. USA. 1980;77:2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C, Wolff J. Two distinct adenosine-sensitive sites on adenylate cyclase. Proc. Natl Acad. Sci. USA. 1977;74:5482–5486. doi: 10.1073/pnas.74.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen A, Grün S, Vogt H, Schwabe U. Identification of novel high affinity adenosine binding protein from bovine striatum. Naunyn Schmiedebergs Arch. Pharmacol. 1992;346:63–68. doi: 10.1007/BF00167572. [DOI] [PubMed] [Google Scholar]
- Lorenzen A, Nitsch-Kirsch M, Vogt H, Schwabe U. Characterization of membrane-bound and solubilized high-affinity binding sites for 5′-N-ethylcarboxamido[3H]adenosine from bovine cerebral cortex. J. Neurochem. 1993;60:745–751. doi: 10.1111/j.1471-4159.1993.tb03210.x. [DOI] [PubMed] [Google Scholar]
- Lustig KD, Shiau AK, Brake AJ, Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc. Natl. Acad. Sci. USA. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane DE, Srivastaa PC, Mills DCB. 2-Methylthioadenosine [β-32P]diphosphate: an agonist and radioligand for the receptor that inhibits the accumulation of cyclic AMP in platelets. J. Clin. Invest. 1983;71:420–428. doi: 10.1172/JCI110786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenhaut C, Van Sande J, Libert F, Abramowicz M, Parmentier M, Vanderhaegen JJ, Dumont JE, Vassart G, Schiffmann S. RDC8 codes for an adenosine A2 receptor with physiological constitutive activity. Biochem. Biophys. Res. Commun. 1990;173:1169–1178. doi: 10.1016/s0006-291x(05)80909-x. [DOI] [PubMed] [Google Scholar]
- Mahan LC, Mcvrttie LD, Smyk-Randall EM, Nakata H, Monsma FJ, Jr, Gerfen CR, Sibley DR. Cloning and expression of an A1 adenosine receptor from rat brain. Mol. Pharmacol. 1991;40:1–7. [PubMed] [Google Scholar]
- McIlwain H. Regulatory significance of the release and action of adenine derivatives in cerebral systems. Biochem. Soc. Symp. 1972;36:69–85. [PubMed] [Google Scholar]
- Meyerhof W, Müller-Brechlin R, Richter D. Molecular cloning of a novel putative G-protein coupled receptor expressed during rat spermiogenesis. FEBS Lett. 1991;284:155–160. doi: 10.1016/0014-5793(91)80674-r. [DOI] [PubMed] [Google Scholar]
- Michel AD, Brown HC, Sewter C, Kennedy I, Humphrey PPA. Identification of [3H]-α,β-methylene-ATP binding sites in rat brain and peripheral tissues. Br. J. Pharmacol. 1993;108(Suppl.) [Google Scholar]
- Möser GH, Scharder J, Deussen A. Turnover of adenosine in plasma of human and dog blood. Am. J. Physiol. 1989;256:C799–C806. doi: 10.1152/ajpcell.1989.256.4.C799. [DOI] [PubMed] [Google Scholar]
- Munshi R, Pang I-H, Sternweis PC, Linden J. A1 adenosine receptors of bovine brain couple to guanine nucleotide-binding proteins G11, G12 and G0. J. Biol Chem. 1991;266:22285–22289. [PubMed] [Google Scholar]
- Murphy PM, Tiffany HL. Characterization of phagocyte P2 nucleotide receptors expressed in Xenopus oocytes. J. Biol. Chem. 1990;265:11615–11621. [PubMed] [Google Scholar]
- Nakazawa K, Fujimori K, Takanaka A, Inoue K. Comparison of adenosine triphosphate-activated and nicotine-activated inward currents in rat phaeochromocytoma cells. J. Physiol. (Lond.) 1991;434:647–660. doi: 10.1113/jphysiol.1991.sp018491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttle LC, El-Moatassim C, Dubyak GR. Expression of the pore-forming P2z purinoreceptor in Xenopus oocytes injected with poly(A)+ RNA from murine macrophages. Mol. Pharmacol. 1993;44:93–101. [PubMed] [Google Scholar]
- O’Connor SE, Wood BE, Leff P. Characterisation of P2X-receptors in rabbit isolated ear artery. Br. J. Pharmacol. 1990;101:640–644. doi: 10.1111/j.1476-5381.1990.tb14133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor SE, Dainty IA, Leff P. Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol Sci. 1991;12:137–141. doi: 10.1016/0165-6147(91)90530-6. [DOI] [PubMed] [Google Scholar]
- Okajima F, Sato K, Nazarea M, Sho K, Kondo Y. A permissive role of pertussis toxin substrate G-protein in P2-purinergic stimulation of phosphoinositide turnover and arachidonate release in FRTL-5 thyroid cells. J. Biol. Chem. 1989;264:13029–13037. [PubMed] [Google Scholar]
- Olah ME, Ren H, Ostrowski J, Jacobson KA, Stiles GL. Cloning, expression, and characterization of the unique bovine A1 adenosine receptor. Studies on the ligand binding site by site-directed mutagenesis. J. Biol. Chem. 1992;267:10764–10770. [PMC free article] [PubMed] [Google Scholar]
- Peng HB, Baker LP, Chen Q. Induction of synaptic development in cultured muscle cells by basic fibroblast growth factor. Neuron. 1991;6:237–246. doi: 10.1016/0896-6273(91)90359-8. [DOI] [PubMed] [Google Scholar]
- Pintor J, Diaz-Rey MA, Miras-Portugal MT. Ap4A and ADP-β-S binding to P2 purinoceptors present on rat brain synaptic terminals. Br. J. Pharmacol. 1993;108:1094–1099. doi: 10.1111/j.1476-5381.1993.tb13510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintor J, Diaz-Rey MA, Torres M, Miras-Portugal MT. Presence of diadenosine polyphosphates—Ap4A and Ap4A—in rat brain synaptic terminals. Ca2+ dependent release evoked by 4-aminopyridine and veratridine. Neurosci. Lett. 1992;136:141–144. doi: 10.1016/0304-3940(92)90034-5. [DOI] [PubMed] [Google Scholar]
- Pintor J, Torres M, Castro E, Miras-Portugal MT. Characterization of diadenosine tetraphosphate (Ap4A) binding sites in cultured chromaffin cells: evidence for a P2Y site. Br. J. Pharmacol. 1991a;103:1980–1984. doi: 10.1111/j.1476-5381.1991.tb12363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintor J, Torres M, Miras-Portugal MT. Carbacbol induced release of diadenosine polyphosphates—Ap4A and Ap5A—from perfused bovine adrenal medulla and isolated chromaffin cells. Life Sci. 1991b;48:2317–2324. doi: 10.1016/0024-3205(91)90268-g. [DOI] [PubMed] [Google Scholar]
- Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J. Biol Chem. 1993;268:16887–16890. [PubMed] [Google Scholar]
- Ribeiro JA, Sebastiao AM. Adenosine receptors and calcium: basis for proposing a third (A3) adenosine receptor. Prog. Neurobiol. 1986;26:179–209. doi: 10.1016/0301-0082(86)90015-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez del Castillo A, Torres M, Delicado EG, Miras-Portugal MT. Subcellular distribution studies of diadenosine polyphosphates—Ap4A and Ap3A—in bovine adrenal medulla: presence in chromaffin granules. J. Neurochem. 1988;51:1696–1703. doi: 10.1111/j.1471-4159.1988.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Russell SN, Horner MA, Westfall DP, Buxton ILO, Horowitz B. Characterization and functional expression of ATP receptor in vas deferens smooth muscle. Biophys. J. 1993;84:A84. [Google Scholar]
- Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. Molecular cloning and characterization of the human A2 adenosine receptor. Proc. Natl Acad. Sci. USA. 1993;90:10365–10369. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore CA, Luneau CJ, Johnson RG, Jacobson MA. Molecular cloning and characterization of human A1, and A2 adenosine receptors. Int. J. Purine Pyrimidine Res. 1992;3:82. [Google Scholar]
- Sarges R, Howard HR, Browne RG, Lebel LA, Seymour PA, Koe BK. 4-Amino: 1,2,4:triazolo:4,3-a:quinoxalines. A novel class of potent adenosine receptor antagonists and potential rapid-onset antidepressants. J. Med. Chem. 1990;33:2240–2254. doi: 10.1021/jm00170a031. [DOI] [PubMed] [Google Scholar]
- Sattin A, Rall TW. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3′,5′-monophosphate content of guinea pig cerebral cortex slices. Mol. Pharmacol. 1970;6:13–23. [PubMed] [Google Scholar]
- Scholz KP, Miller RJ. Analysis of adenosine actions on Ca2+ currents and synaptic transmission in cultured rat hippocampal pyramidal neurones. J. Physiol (Lond) 1991;435:373–393. doi: 10.1113/jphysiol.1991.sp018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KZ, North RA. Excitation of rat locus coeruleus neurons by adenosine 5′-triphosphate: ionic mechanism and receptor characterization. J. Neurosci. 1993;13:894–899. doi: 10.1523/JNEUROSCI.13-03-00894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada J, Suzuki F, Nonaka H, Ishii A, Ichikawa S. (E)-1,3-dialkyl-7-methyl-8-(3,4,5-trimethoxystyryl)xanthines: potent and selective adenosine A2 antagonists. J. Med. Chem. 1992;35:2342–2345. doi: 10.1021/jm00090a027. [DOI] [PubMed] [Google Scholar]
- Spedding M, Sweetman AJ, Weetman DF. Antagonism of adenosine 5′-triphosphate-induced relaxation by 2–2′-pyridylisatogen in the taenia of guinea-pig caecum. Br. J. Pharmacol. 1975;53:575–583. doi: 10.1111/j.1476-5381.1975.tb07397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spedding M, Weetman DF. Identification of separate receptors for adenosine and adenosine 5′-triphosphate in causing relaxations of the isolated taenia of the guinea-pig caecum. Br. J. Pharmacol. 1976;57:305–310. doi: 10.1111/j.1476-5381.1976.tb07480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle JH, Rivkees SA, Lee JJ, Weaver DR, Deeds JD, Reppert SM. Molecular cloning and expression of the cDNA for a novel A2-adenosine receptor subtype. Mol Endocrinol. 1992;6:384–393. doi: 10.1210/mend.6.3.1584214. [DOI] [PubMed] [Google Scholar]
- Steinberg TH, Silverstein SC. Extracellular ATP4− promotes cation fluxes in the J774 mouse macrophage cell line. J. Biol Chem. 1987;262:3118–3122. [PubMed] [Google Scholar]
- Stone GA, Jarvis MF, Sills MA, Weeks B, Snowhill EW, Williams M. Species differences in high-affinity adenosine A2 binding sites in striatal membranes from mammalian brain. Drug Dev. Res. 1988;15:31–46. [Google Scholar]
- Thompson SM, Haas HL, Gähwiler BH. Comparison of the actions of adenosine at pre- and postsynaptic receptors in the rat hippocampus in vitro. J. Physiol (Lond.) 1992;451:347–363. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend-Nicholson A, Shine J. Molecular cloning and characterisation of a human brain A1 adenosine receptor cDNA. Mol. Brain Res. 16:365–370. 1992. doi: 10.1016/0169-328x(92)90248-a. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Jackson MB. Adenosine-activated potassium conductance in cultured striatal neurons. Proc. Natl. Acad. Sci. USA. 1985;82:4857–4861. doi: 10.1073/pnas.82.14.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AL, Linden J, Robeva AS, D’Angelo DD, Lynch KR. Cloning and expression of a bovine adenosine A1 receptor cDNA. FEBS Lett. 1992;297:107–111. doi: 10.1016/0014-5793(92)80338-h. [DOI] [PubMed] [Google Scholar]
- Ukena D, Daly JW, Kirk KL, Jacobson KA. Functionalized congeners of 1,3-dipropyl-8-phenylxanthine: potent antagonists for adenosine receptors that modulate membrane adenylate cyclase in pheochromocytoma cells, platelets and fat cells. Life Sci. 1986;38:797–807. doi: 10.1016/0024-3205(86)90596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Calker D, Muller M, Hamprecht B. Adenosine inhibits the accumulation of cyclic AMP in cultured brain cells. Nature (Lond.) 1978;276:839–841. doi: 10.1038/276839a0. [DOI] [PubMed] [Google Scholar]
- Van Calker D, Muller M, Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J. Neurochem. 1979;33:999–1006. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- Van Der Wenden EM, Ijzerman AP, Soudijn W. A steric and electrostatic comparison of three models for the agonist/antagonist binding site on the adenosine A1 receptor. J. Med. Chem. 1992;35:629–635. doi: 10.1021/jm00082a003. [DOI] [PubMed] [Google Scholar]
- Van Der Zee L, Nelemans A, Den Hertog A. Nucleotide receptors on DDT1 MF-2 vas deferens cells. Eur. J. Pharmacol. 1992;215:317–320. doi: 10.1016/0014-2999(92)90048-9. [DOI] [PubMed] [Google Scholar]
- Van Galen PJ, Stiles GL, Michaels G, Jacobson KA. Adenosine A1 and A2 receptors: structure-function relationships. Med. Res. Rev. 1992;12:423–471. doi: 10.1002/med.2610120502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Galen PJM, Van Bergen AH, Gallo-Rodrigubz C, Melman N, Olah M, Ijzerman AP, Stiles GL, Jacobson KA. A binding site model and structure activity relationships for the rat A3 adenosine receptor. Mol. Pharmacol. 1993 submitted. [PMC free article] [PubMed] [Google Scholar]
- Van Galen PJM, Van Vlijmen HWT, Ijzerman AP, Soudijn W. A model for the antagonist binding site on the adenosine A1 receptor, based on steric, electrostatic, and hydrophobic properties. J. Med. Chem. 1990;33:1708–1713. doi: 10.1021/jm00168a027. [DOI] [PubMed] [Google Scholar]
- Van Rhee AM, Van Winden CE, Nagelkerke JF, De Bont HJ, Ijzerman AP, Soudijn W. Binding of the radioligand [36S]adenosine 5′-O-(2-thiodiphosphate) and intracellular calcium response in rat liver parenchymal cells. Biochem. Pharmacol. 1993;45:801–807. doi: 10.1016/0006-2952(93)90162-p. [DOI] [PubMed] [Google Scholar]
- Voogd TE, Vansterkenburg ELM, Wilting J, Janssen LHM. Recent research on the biological activity of suramin. Pharmacol. Rev. 1993;45:177–203. [PubMed] [Google Scholar]
- Webb TE, Simon J, Krishek BJ, Bateson AN, Smart TG, King BF, Burnstock G, Barnard EA. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993;324:219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- Wilkinson GF, Purkiss JR, Boarder MR. The regulation of aortic endothelial cells by purines and pyrimidines involves co-existing P2Y-purinoceptors and nucleotide receptors linked to phospholipase C. Br. J. Pharmacol. 1993;108:689–693. doi: 10.1111/j.1476-5381.1993.tb12862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Hamamori Y, Akita H, Yokoyama M. P2-purinoceptor activation stimulates phosphoinositide hydrolysis and inhibits accumulation of cAMP in cultured ventricular myocytes. Circ. Res. 1992;70:477–485. doi: 10.1161/01.res.70.3.477. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Li C, Olah ME, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of an adenosine receptor the A3 adenosine receptor. Proc. Natl Acad. Sci. USA. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziganshin AU, Hoyle CHV, Bo X, Lambrecht G, Mutschler E, Bäumert HG, Burnstock G. PPADS selectively antagonizes P2X-purinoceptor-mediated responses in the rabbit urinary bladder. Br. J. Pharmacol. 1993;110:1491–1495. doi: 10.1111/j.1476-5381.1993.tb13990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]