Abstract
Gene transfer into hemopoietic stem cells could offer a lasting cure for a variety of congenital disorders. As a preclinical test for such a gene therapy, rhesus monkeys were transplanted with autologous bone-marrow cells infected with helper-free recombinant retroviruses carrying the human adenosine deaminase gene. The in vivo regenerative capacity of the infected bone marrow could be conserved, suggesting survival of repopulating hemopoietic stem cells. In the hemopoietic system of transplanted animals the foreign gene could be observed for as long as the animals were analyzed (in two monkeys greater than 1 yr after transplantation). Genetically modified cell types and tissues included peripheral blood mononuclear cells, granulocytes, bone-marrow cells of various densities, and spleen and lymph nodes. The presence of the provirus in the short-living granulocytes greater than 1 yr after bone-marrow transplantation provided evidence for the transduction of very primitive hemopoietic progenitors. Moreover, the gene transfer resulted in sustained production of functional human adenosine deaminase enzyme in peripheral blood mononuclear cells. These results demonstrate the feasibility of bone-marrow gene-therapy approaches, in particular for treating adenosine deaminase deficiency.
Full text
PDF

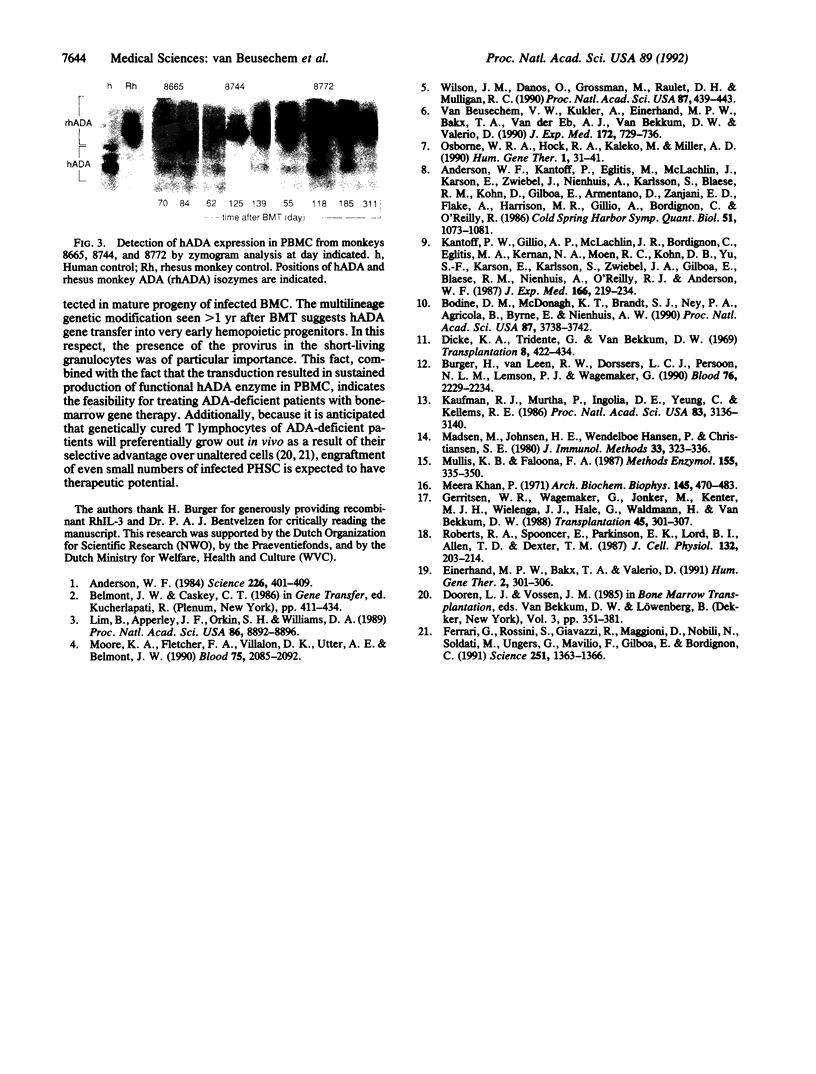
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F., Kantoff P., Eglitis M., McLachlin J., Karson E., Zwiebel J., Nienhuis A., Karlsson S., Blaese R. M., Kohn D. Gene transfer and expression in nonhuman primates using retroviral vectors. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):1073–1081. doi: 10.1101/sqb.1986.051.01.125. [DOI] [PubMed] [Google Scholar]
- Anderson W. F. Prospects for human gene therapy. Science. 1984 Oct 26;226(4673):401–409. doi: 10.1126/science.6093246. [DOI] [PubMed] [Google Scholar]
- Bodine D. M., McDonagh K. T., Brandt S. J., Ney P. A., Agricola B., Byrne E., Nienhuis A. W. Development of a high-titer retrovirus producer cell line capable of gene transfer into rhesus monkey hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990 May;87(10):3738–3742. doi: 10.1073/pnas.87.10.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger H., van Leen R. W., Dorssers L. C., Persoon N. L., Lemson P. J., Wagemaker G. Species specificity of human interleukin-3 demonstrated by cloning and expression of the homologous rhesus monkey (Macaca mulatta) gene. Blood. 1990 Dec 1;76(11):2229–2234. [PubMed] [Google Scholar]
- Dicke K. A., Tridente G., van Bekkum D. W. The selective elimination of immunologically competent cells from bone marrow and lymphocyte cell mixtures. 3. In vitro test for detection of immunocompetent cells in fractionated mouse spleen cell suspensions and primate bone marrow suspensions. Transplantation. 1969 Oct;8(4):422–434. doi: 10.1097/00007890-196910000-00014. [DOI] [PubMed] [Google Scholar]
- Einerhand M. P., Bakx T. A., Valerio D. IL-6 production by retrovirus packaging cells and cultured bone marrow cells. Hum Gene Ther. 1991 Winter;2(4):301–306. doi: 10.1089/hum.1991.2.4-301. [DOI] [PubMed] [Google Scholar]
- Ferrari G., Rossini S., Giavazzi R., Maggioni D., Nobili N., Soldati M., Ungers G., Mavilio F., Gilboa E., Bordignon C. An in vivo model of somatic cell gene therapy for human severe combined immunodeficiency. Science. 1991 Mar 15;251(4999):1363–1366. doi: 10.1126/science.1848369. [DOI] [PubMed] [Google Scholar]
- Gerritsen W. R., Wagemaker G., Jonker M., Kenter M. J., Wielenga J. J., Hale G., Waldmann H., van Bekkum D. W. The repopulation capacity of bone marrow grafts following pretreatment with monoclonal antibodies against T lymphocytes in rhesus monkeys. Transplantation. 1988 Feb;45(2):301–307. doi: 10.1097/00007890-198802000-00010. [DOI] [PubMed] [Google Scholar]
- Kantoff P. W., Gillio A. P., McLachlin J. R., Bordignon C., Eglitis M. A., Kernan N. A., Moen R. C., Kohn D. B., Yu S. F., Karson E. Expression of human adenosine deaminase in nonhuman primates after retrovirus-mediated gene transfer. J Exp Med. 1987 Jul 1;166(1):219–234. doi: 10.1084/jem.166.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Murtha P., Ingolia D. E., Yeung C. Y., Kellems R. E. Selection and amplification of heterologous genes encoding adenosine deaminase in mammalian cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3136–3140. doi: 10.1073/pnas.83.10.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B., Apperley J. F., Orkin S. H., Williams D. A. Long-term expression of human adenosine deaminase in mice transplanted with retrovirus-infected hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8892–8896. doi: 10.1073/pnas.86.22.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen M., Johnsen H. E., Hansen P. W., Christiansen S. E. Isolation of human T and B lymphocytes by E-rosette gradient centrifugation. Characterization of the isolated subpopulations. J Immunol Methods. 1980;33(4):323–336. doi: 10.1016/0022-1759(80)90003-4. [DOI] [PubMed] [Google Scholar]
- Meera Khan P. Enzyme electrophoresis on cellulose acetate gel: zymogram patterns in mgh-mouse and man--Chinese hamster somatic cell hybrids. Arch Biochem Biophys. 1971 Aug;145(2):470–483. doi: 10.1016/s0003-9861(71)80007-3. [DOI] [PubMed] [Google Scholar]
- Moore K. A., Fletcher F. A., Villalon D. K., Utter A. E., Belmont J. W. Human adenosine deaminase expression in mice. Blood. 1990 May 15;75(10):2085–2092. [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Osborne W. R., Hock R. A., Kaleko M., Miller A. D. Long-term expression of human adenosine deaminase in mice after transplantation of bone marrow infected with amphotropic retroviral vectors. Hum Gene Ther. 1990 Spring;1(1):31–41. doi: 10.1089/hum.1990.1.1-31. [DOI] [PubMed] [Google Scholar]
- Roberts R. A., Spooncer E., Parkinson E. K., Lord B. I., Allen T. D., Dexter T. M. Metabolically inactive 3T3 cells can substitute for marrow stromal cells to promote the proliferation and development of multipotent haemopoietic stem cells. J Cell Physiol. 1987 Aug;132(2):203–214. doi: 10.1002/jcp.1041320204. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Danos O., Grossman M., Raulet D. H., Mulligan R. C. Expression of human adenosine deaminase in mice reconstituted with retrovirus-transduced hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990 Jan;87(1):439–443. doi: 10.1073/pnas.87.1.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beusechem V. W., Kukler A., Einerhand M. P., Bakx T. A., van der Eb A. J., van Bekkum D. W., Valerio D. Expression of human adenosine deaminase in mice transplanted with hemopoietic stem cells infected with amphotropic retroviruses. J Exp Med. 1990 Sep 1;172(3):729–736. doi: 10.1084/jem.172.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]