Abstract
Increased body mass index (BMI) confers a survival advantage in maintenance hemodialysis (MHD) patients. Diabetic (DM) patients undergoing MHD have worse survival. There are limited studies examining the effect of obesity on the risk of death among MHD patients with diabetes.
Ninety-eight MHD patients were studied for median follow-up time of 78 months. Patients were classified according to the presence of obesity (BMI ≥ 30 kg/m2) or DM. Primary outcome was all-cause mortality. Cox regression was used to evaluate the effect of obesity on time to death. Effect modification and mediation analysis were also performed.
Mean age was 49 ± 13 years, 66% were male, 48% were obese and 34% were diabetic. Mortality rates (per 100 person years) were: 3.4 for non-diabetic obese, 8.6 for non-diabetic non-obese, 14.3 for diabetic non-obese and 18.1 for diabetic obese patients. Log-rank comparing diabetic obese versus non diabetic obese was significant (p=0.007). Diabetes was associated with an increased risk of mortality after adjustment for potential mediators. Effect modification of obesity in the mortality risk was different between patients with and without diabetes. With adjustment for adipokines, a greater effect modification by diabetes was observed whereas adjustment for inflammatory marker did not influence the effect modification.
Diabetic obese MHD patients have increased mortality risk compared to non-diabetic obese. Obesity does not offer survival benefits in Diabetic obese MHD patients and potentially may have detrimental effects. Larger studies evaluating the effect of adipokines and obesity in outcomes in the diabetic MHD population need to be undertaken.
Keywords: diabetes mellitus, obesity, hemodialysis, mortality, oxidative stress, inflammation
INTRODUCTION
Maintenance hemodialysis (MHD) patients have an increased death risk, which has not significantly improved over the last two decades.1 In contrast to general population, traditional cardiovascular risk factors display an inverse relationship with mortality in MHD patients. For example, while increased adiposity is associated with increased expression of pro-inflammatory cytokines, increased oxidative stress burden, adipocytokines imbalances and worse insulin resistance (IR) both in the general population and MHD patients2, 3, large observational studies have shown that increased body mass index (BMI) is associated with improved lifespan in MHD patients, even in very high ranges4-8. How obesity is associated with improved survival in MHD patients, as opposite to the general population, is still a matter of debate.
Amongst MHD patients, ones with diabetes mellitus (DM) are known to have the worst outcomes. The mechanisms leading to this increased death risk are not clearly elucidated but are proposed to be associated with the underlying metabolic disturbances, such as oxidative stress, systemic inflammation and the adipocytokine imbalance. Obesity is a well-known feature of DM, which could also worsen the underlying metabolic disturbances that would increase cardiovascular disease risk further. Studies examining the relationship and effect modification between DM, obesity and metabolic disturbances in ESRD are scarce. In this study, we hypothesized that presence of DM and obesity will lead to an exaggerated risk for metabolic disturbances and subsequently increased mortality risk in MHD patients.
MATERIAL-METHODS
Study Population
We conducted a retrospective analysis from 98 MHD patients whom had formerly participated in a variety of metabolic studies at Vanderbilt University Medical Center (VUMC) and the VA Tennessee Valley Health Care System (VA-TVHS) between 2003 and 2011 and had data available on body composition as well as on metabolic biomarkers. Follow up data was collected by chart review. Inclusion criteria included patients aged 18 years and older who were on MHD therapy for more than three months and were delivered an adequate dose of dialysis (single-pool Kt/V ≥ 1.2) on a thrice-weekly dialysis program using biocompatible hemodialysis membranes. Exclusion criteria included pregnancy, patients with severe unstable underlying disease who had clinical signs of overt infection, vasculitis or liver disease, and those hospitalized within one month prior to enrollment into the study.
Demographical and clinical data were obtained including age, sex, ethnicity, BMI and dialysis vintage. BMI was calculated as the weight in kilograms divided by the height in square meters. Patients were classified as obese and non-obese by BMI cut-off ≥ 30 kg/m2. The dialysis vintage was defined as the duration of time between the first day of the dialysis therapy and the day of the blood draws were obtained.
Clinical diagnosis of DM was made according to the American Diabetes Association (ADA) Clinical Practice Guidelines or presence of history of DM in medical records. The dates of death or other censoring events were obtained for all patients until February 1, 2012. Patient deaths were determined from VUMC and VA-TVHS medical records. Deaths at outside hospitals were screened from United States Death Index record system. Subjects were censored if they received kidney transplantation or moved to another dialysis unit and no survival data were available. The Institutional Review Board for each facility approved each study and written informed consent was obtained from all study participants.
Measurement of Body Composition
Assessment of body composition was performed by Dual Energy X-Ray Absorptiometry (DEXA) 3, 9 , which offers a rapid, noninvasive three-compartment evaluation that quantifies fat mass (FM), lean body mass (LBM), and bone mineral (BM) content with minimal radiation exposure. All DEXA measurements were done on non-dialysis day by using a Lunar Prodigy iDEXA machine, v.11.40.004 (software versions 2003 to 2011, General Electric, Madison, WI).
Laboratory Analysis
All blood sampling was performed at the General Clinical Research Center and analyzed at VUMC central laboratories. After blood drawn was performed, samples were transported on ice and centrifuged at 3000 rpm for 15 minutes before kept frozen at −80 °C. Plasma fasting glucose concentrations were analyzed by using the glucose oxidase method (Glucose analyzer 2; Beckman Coulter, Brea, CA). Concentrations of serum albumin, prealbumin, bicarbonate and intact parathyroid hormone (iPTH) were measured using standard methods at VUMC central laboratories. Serum leptin levels were performed at Diabetes Research Training Center (DRTC) hormone laboratory by using certified methods. High sensitivity C-reactive protein (hs-CRP) concentrations were measured by high-sensitivity particle-enhanced turbidimetric UniCel DxI Immunoassay system (Beckman Coulter). Plasma IL-6 levels were determined using cytometric bead arrays (Becton Dickinson, San Jose, CA). Plasma protein thiol groups were analyzed according to the procedure which previously published by Ellman 10et al and as modified by Hu 11 et al.
Statistical Analyses
Normally distributed variables were expressed as mean ± SD, and non-normally distributed variables were presented as median and interquartile range (25th- 75th percentiles). Categorical data were presented as percentage values and compared as Mann-Whitney U or χ2 tests when appropriate. Correlation analysis was performed by Spearman correlation coefficient. Comparisons among four groups of patients defined by obesity and DM were performed by nonparametric analysis using Kruskall-Wallis tests or χ2 tests.
The primary outcome was all-cause mortality. Kaplan-Meier survival curves with log-rank test are presented to compare mortality by BMI status and the presence or absence of DM. Event rates (mortality rates) were calculated as events per 100 person year of follow-up. Cox proportional hazard model was used to quantify the relationship between all-cause mortality and study variables, with and without adjustment for covariates. The variables selected for multivariate cox model were a priori selected within an allowable number of variables determined by 10 events per-variable rule to prevent over-fitting. The effect modification of obesity to the mortality risk associated with DM was assessed in a multivariable regression with including a cross product term between BMI and DM. Mediation analysis was planned to explore the potential mechanisms through which obesity modified the mortality risk of DM. This was indirectly assessed by introducing the proposed mediator in the multivariable model with the effect of interaction12-14. Statistical significance for all analysis was assesses at the 2-sided 95% confidence interval. Analyses were performed using SPSS 19 for Windows (Chicago, IL).
RESULTS
Characteristics of the study subjects
The mean age of study participants was 49 ± 13 years with a median follow up period of 78 (range, 1, 101) months. The study subjects were predominantly African-American (75%), 66 % were male, and 34 % had DM according to ADA criteria or medical history. The median duration of dialysis was 44 (range, 19, 105) months. The median BMI was 29 kg/m2 (interquartile range: 24.2, 36.3) and 48% of participants had obesity. Table 1 depicts the clinical characteristics of the study participants.
Table 1.
Distribution of Covariates Stratified by BMI and the Presence of Diabetes Mellitus
| Non-Diabetics (n=65) | Diabetics N=33 | Overall Comparison | Other Comparisons | |||
|---|---|---|---|---|---|---|
| Variable | Obese | Non-Obese | Obese | Non-Obese | P-value | P-value |
| Demographics | ||||||
| Age (years) | 44 ± 11 | 47 ± 13 | 55 ± 12 | 54 ± 12 | 0.02 | A |
| Males (%) | 63 | 82 | 43 | 67 | R | |
| Clinical Characteristics | ||||||
| KT/V | 1.5 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.3 | 1.6 ± 0.2 | 0.2 | |
| Albumin (g/dL) | 4 (3.9, 4.3) | 4 (3.8, 4.3) | 3.9 (3.6, 4) | 3.9 (3.6, 4.2) | 0.2 | A |
| Prealbumin (mg/dL) | 40 (33, 47) | 39 (30, 47) | 36 (30, 40) | 34 (27, 42) | 0.3 | |
| Creatinine (mg/dL) | 10.3 ± 2.9 | 8.8 ± 2.8 | 7.4 ± 2.6 | 8.2 ± 2.9 | 0.1 | A,C |
| Intact PTH (pg/mL) | 446 (272, 842) | 344 (191, 470) | 355 (260, 684) | 470 (240, 835) | 0.3 | |
| Bicarbonate (mg/dL) | 25 ± 4 | 25 ± 4 | 25 ± 4 | 27 ± 4 | 0.3 | |
| Metabolic Biomarkers | ||||||
| hsCRP (mg/dL) | 9.3 (5.7,14.8) | 3.9 (2.2, 8.6) | 13.2 (4.9, 17.65) | 6.75 (5.2, 18) | 0.01 | A,B,C |
| Interleukin-6 (pg/ml) | 6.2 (4.5, 16.3) | 5.4 (2.8, 13.7) | 14 (8.9, 18.5) | 9.8 (6.7, 16.9) | 0.08 | A, C |
| Protein thiols (μmol/mL) | 280 ± 59 | 254 ± 19 | 230 ± 36 | 196 ± 10* | 0.03 | A,C |
| Leptin (ng/ml) | 50.3 (16.4, 71.51) | 6.4 (2.68, 9.9) | 67.4 (20.9, 99.6) | 12.8 (6.3, 21.31) | 0.001 | B |
| Adiponectin (μg/ml) | 12 (8.3, 23.3) | 37.7 (23.7, 57.4) | 40.2 (18.8, 61.6) | 16.55 (9.8, 90.3) | 0.001 | B |
| Body Composition | ||||||
| BMI (kg/m2) | 36 ±4.8 | 24 ± 2.7 | 39 ± 7.0 | 27 ± 1.8 | 0.001 | A,B,C |
| Truncal Tissue Fat (%) | 46.3 ± 0.1 | 28.4 ± 11.1 | 45 ± 0.1 | 33.2 ± 0.1 | 0.001 | B |
| LBM (kg) | 56.5 ±12.1 | 48.7 ± 7.8 | 54.5 ± 13.8 | 50.1 ± 8.5 | 0.04 | B |
Statistically significant difference between diabetic vs. non diabetic patients
Statistically significant difference between obese vs. non-obese patients.
Statistically significant difference between diabetic obese vs. non-diabetic obese patients
• PTH, parathyroid hormone; BMI, Body Mass Index
There were only 3 patients with protein thiol measurements in the diabetic non obese patients
Body composition
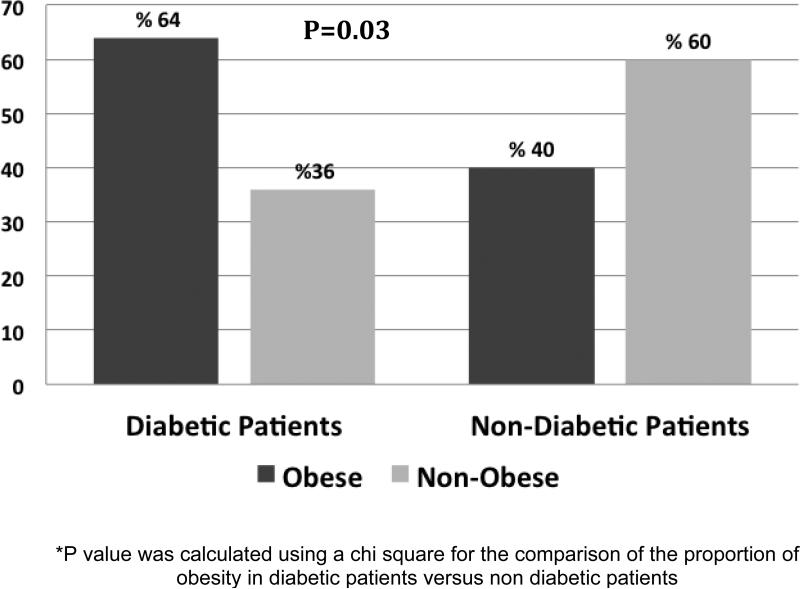
Obesity was most common in diabetic patients (64%) compared to the non-diabetics (40%) (P=0.03) (Figure 1) and diabetic patients had significantly higher BMI levels compared to the non-diabetic patients (34.8±8.3 kg/m2 versus 28.6±6.9 kg/m2; p<0.001; respectively). Diabetic MHD patients also had significantly higher truncal tissue fat percentage in general when compared to non-diabetic MHD patients (40.9±11 % versus 35.6±12%; p=0.05).
Figure 1.
Presence of Obesity by Diabetes Status.
Clinical and Metabolic Markers
Table 1 depicts the clinical and laboratory differences over the four subgroups of interest. Serum creatinine levels were significantly lower in diabetic obese patients compared to non-diabetic obese patients (7.4 ±2.6 versus 10.3±2.9; p=0.03). Serum concentrations of IL-6 and hsCRP were significantly higher in diabetic obese compared to the non-diabetics obese (P=0.01 for both comparisons). Serum albumin concentrations and protein thiols levels were lower in the diabetic in general compared to the non-diabetics (p<0.02 and p=0.006 respectively). Leptin levels were higher in obese versus non-obese patients regardless of the diabetic status.
Mortality
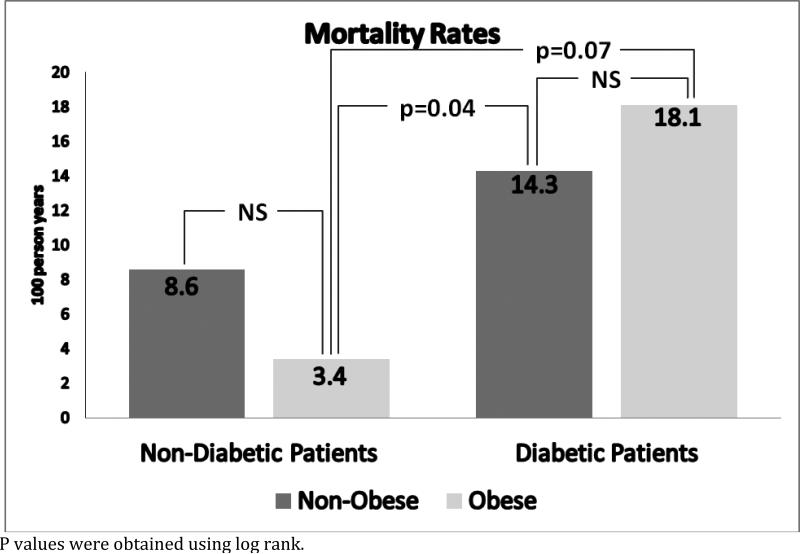
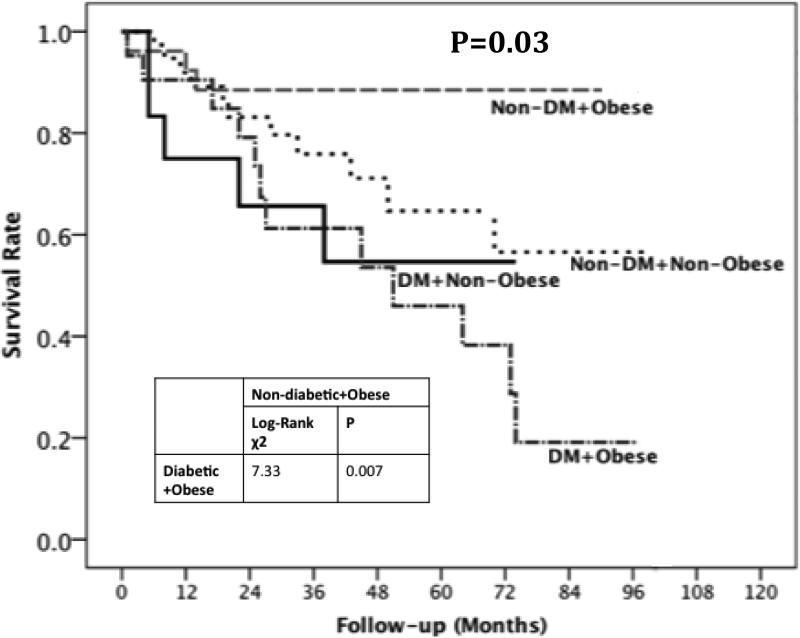
There were 31 deaths during a follow-up period of 318.3 person years of follow up with a mortality rate of 9.74 per 100-person year of follow up (Table 2). The mortality rates (per 100 person year) for the four subgroups were: 3.4 for non-diabetic obese, 8.6 for non-diabetic non-obese, 14.3 for diabetic non-obese and 18.1 for diabetic obese patients (Figure 2). KM survival curves according to the presence or absence of DM and obesity showed that non-diabetic obese patients had the best survival whereas diabetic obese patients had the worst survival (log rank p=0.007) (Figure 3). The unadjusted hazard ratio for the comparison diabetic obese versus non-diabetic obese was 4.9 (95% confidence interval 1.37, 17.4).
Table 2.
Number of events and follow time in obese and non-obese patients by Diabetes Status
| Non- Diabetics | Diabetics | Overall | |||
|---|---|---|---|---|---|
| Non-Obese | Obese | Non-Obese | Obese | ||
| Participants | 39 | 26 | 12 | 21 | 98 |
| Events | 11 | 3 | 5 | 12 | 31 |
| Censored | 28 | 23 | 7 | 9 | 67 |
| Percent of events | 28.2 | 11.5 | 41.7 | 57.1 | 31.6 |
| Percent Censored | 71.8 | 88.5 | 58.3 | 42.9 | 68.4 |
| Person years of follow up | 128.33 | 88.92 | 34.92 | 66.17 | 318.33 |
| Mortality rates Per 100 person years. | 8.6 | 3.4 | 14.3 | 18.1 | 9.74 |
Figure 2.
Mortality Rates per 100 person years of follow up in obese and non obese patients by Diabetes Status
Figure 3.
Kaplan Meier curve according to the presence of obesity and diabetes status
Predictors of Overall Survival
In unadjusted analyses, presence of diabetes [HR (CI 95%), 2.54 (1.25, 5.15) p=0.01] and older age [HR 1.05 (1.02, 1.09), p=0.001] were associated with higher risk of death (Table 3). Higher serum albumin [0.314 (0.141, 0.701), p=0.005] and prealbumin [HR 0.93 (0.89, 0.980, p=0.003] were associated with decreased risk of death. BMI [HR 0.99 (95%CI 0.94, 1.03)], lean body mass [HR 0.65 (0.03, 14.2)], truncal fat mass [HR0.955 (0.44, 2.07)] were not associated with mortality. None of the metabolic markers included in this study (i.e., hs-CRP, IL-6, HOMA-IR, adiponectin, leptin and protein thiols) were associated with mortality in the unadjusted analysis.
Table 3.
Un-adjusted Hazard Ratios of Mortality by Covariates
| Variables | HR (95% CI) | P |
|---|---|---|
| Demographics | ||
| Age (years) | 1.05 (1.02, 1.09) | 0.001 |
| Clinical Characteristics | ||
| Diabetes | 2.54 (1.25, 5.15) | 0.01 |
| Albumin (g/dL) | 0.31 (0.14, 0.70) | 0.005 |
| Prealbumin (mg/dL) | 0.93 (0.89, 0.98) | 0.003 |
| Metabolic Biomarkers | ||
| hsCRP (mg/dL) | 1.57 (0.83, 2.97) | 0.1 |
| IL-6 (pg/ml) | 1.07 (0.99, 1.14) | 0.08 |
| Protein Thiols (μmol/mL) | 0.97 (0.92, 1.02) | 0.2 |
| Leptin (ng/ml) | 0.98 (0.95, 1.01) | 0.2 |
| Adiponectin (μg/ml) | 1.63 (0.82, 3.24) | 0.2 |
| HOMA IR | 0.95 (0.52, 1.76) | 0.9 |
| Body Composition | ||
| BMI (kg/m2) | 0.99 (0.94, 1.03) | 0.6 |
| Truncal Tissue Fat % | 0.96 (0.44, 2.07) | 0.9 |
| Lean Body Mass, gm | 0.65 (0.03, 14.2) | 0.8 |
Multivariate statistical models incorporating several covariates are presented in Table 4. DM remained an independent risk factor for overall mortality after adjustment for BMI, age, IL-6 or leptin. However, DM was no longer associated with mortality after adjusting for adiponectin. We performed an interaction analysis of the effect modification of BMI in the risk of death conferred by diabetes. Five units of BMI increase had a protective effect with a risk reduction in mortality of 46% in the non-diabetic group (p=0.03), while in the diabetic group the beneficial effect conferred by increasing BMI was negligible at 7% (p=0.61). The interaction term was considered significant (p=0.09). When adiponectin was added to the model to assess mediation, we found that hazard ratio for mortality increased to 1.31, though it did not reach statistical significance (p=0.47).
Table 4.
Hazard Ratios for Death by Multivariable Cox Regression Analyses
| HR (95 %CI) | P | |
|---|---|---|
| Model / £ | ||
| Diabetes Mellitus | 3.39 (1.57, 7.33) | 0.02 |
| BMI (kg/m2) | 0.96 (0.91, 1.01) | 0.09 |
| Model II | ||
| Diabetes Mellitus | 2.30 (1.00, 5.30) | 0.05 |
| BMI(kg/m2) | 0.96 (0.91, 1.01) | 0.15 |
| Age | 1.04 (1.01, 1.08) | 0.01 |
| Model III | ||
| Diabetes Mellitus | 2.14 (0.82, 1.59) | 0.1 |
| HOMA-IR | 0.85 (0.45, 5.63) | 0.6 |
| Model IV | ||
| Diabetes Mellitus | 3.37 (1.55, 7.35) | 0.002 |
| BMI (kg/m2) | 0.58 (0.31, 1.08) | 0.08 |
| IL-6 (pg/ml) | 1.12 (0.67, 1.89) | 0.7 |
| Model V | ||
| Diabetes Mellitus | 3.13 (0.93, 10.55) | 0.06 |
| BMI (kg/m2) | 1.02 (0.89, 1.16) | 0.8 |
| Leptin (ng/ml) | 0.59 (0.32, 1.09) | 0.09 |
| Model VI | ||
| Diabetes Mellitus | 2.45 (0.80, 7.52) | 0.1 |
| BMI(kg/m2) | 0.96 (0.86, 1.05) | 0.4 |
| Adiponectin (μg/ml) | 1.37 (0.66, 2.84) | 0.4 |
BMI, body mass index; IL-6, interleukin 6
£ Interaction of DM*BMI was significant at a p=0.09
The number of variables per model was limited to 3 to prevent over-fitting.
DISCUSSION
In this study, we evaluated the interaction between DM, obesity, and mortality amongst a well-phenotyped cohort of MHD patients. Consistent with our initial hypothesis, co-existence of DM and obesity lead to a higher risk of death, while non-diabetic obese MHD patients had the best survival. In our study an obese patient with diabetes had 4.9-fold higher mortality risk compared to an obese patient without diabetes. These data indicate that DM modifies the association between obesity and survival advantage in MHD patients and that in these patients the recommendations for weight management (i.e. loss or gain) should be individualized.
The mechanisms explaining the adverse effects of obesity in the diabetic MHD patient compared to the non-diabetic obese MHD patients are unclear. The adipose tissue is a recognized active endocrine organ that produces important adipocytokines, including IL-6, TNF-alpha, adiponectin and leptin along with other metabolically active proteins15. In addition to the systemic inflammatory response related to obesity, adiposity also increases oxidative stress via the NADPH oxidase activation and other pathways16. However, in MHD patients, these deleterious effects seem to be only uncovered under specific circumstances, such as the presence of diabetes as we observed in our current study.
Diabetes is a clinical syndrome characterized by excessive vascular inflammation and oxidative stress burden, both due to an increased production and ineffective scavenging of reactive oxygen species (ROS)17, 18. There are various mechanisms that contribute to the formation of reactive oxygen species (ROI) in diabetes, including the level of hyperglycemia. There is also evidence for the role of protein kinase C, advanced glycation end products (AGE) and activation of transcription factors, such as NF kappa B, but the exact signaling pathways remain a matter of discussion19. Our observations indicating that diabetes obese patients had the highests leveks of pro-inflammatory cytokines and lowest levels of protein thiols suggest that these pathways may be exaggerated in these patients
In contrast to the previous reports20, we observed that protein thiol levels were significantly decreased in the diabetic patients regardless of presence of obesity, while obese non-diabetic patients had higher levels of protein thiols. Quantitatively, plasma free protein thiols are a measure of endogenous antioxidant capacity21. These observations suggest that the combination of diabetes and obesity may contribute to the largest imbalance of a decreased antioxidant defense and exaggerated oxidative stress burden. While our study did not include other markers of oxidative stress burden, the results are highly relevant as antioxidant interventions may represent an important target to improve outcomes in ESRD patients, especially ones with DM.
Another intriguing finding in our mediation models is that when adjusting for adiponectin, the risk of death associated with diabetes increased, suggesting that adiponectin could be providing a beneficial effect in diabetic MHD patients. Adiponectin has also important antioxidant properties22. Animal models have shown that adiponectin specifically increases NO production by eNOS phosphorylation and decreases NO inactivation by blocking superoxide production 23, 24, and that it is vasculo-protective. It is important to highlight that, although not proven, it has been considered that an increase in adiponectin in the face of an insult may be a compensatory mechanism to reduce oxidative burden22. Our obese diabetic patients had the highest levels of adiponectin. These observations suggest that the depleted antioxidant defense may be a tipping point in this population.
In our study the non-diabetic obese had the best outcomes. An important observation in these patients was their higher lean body mass (LBM), which was not statistically significantly different, however may be reflective of other important nutritional factors that were not evaluated in our study that confounds the relationship of obesity with survival. The combination of high protein thiol levels along with higher LBM might be reflecting higher dietary protein intake, which is not evaluated in our study. In addition, higher LBM is associated with higher levels of physical activity, which is another important determinant of outcomes in MHD patients25, 26.
There are several limitations of our study. First, the small number of patients limited our ability to test important relationships such as oxidative stress burden and risk of mortality, however the trends observed in this study are informative and hypothesis generating. Second, a significant portion of our study subjects were African-American which is reflective of the racial distribution for dialysis patients in the Southeast region of the United States. The strengths of our study include relatively long follow-up period (i.e. median follow-up of 78 months), availability of detailed metabolic parameters, such as inflammatory markers, adipocytokines and markers of oxidative stress, and the use DEXA as a method to determine body composition.
In conclusion, we have shown that although obesity confers survival advantage in MHD patients, the presence of obesity in diabetic MHD patients not only eliminates this beneficial effect, but potentially increases mortality risk. There are several important metabolic pathways that may play a role in the poor outcomes observed in the diabetic MHD patients more so if obese, including adipokine pathways and imbalance between the oxidative stress defense and oxidative stress burden. Larger studies evaluating or targeting these pathways are needed in the future in order to improve survival in the diabetic MHD patients.
ACKNOWLEDGMENT
This article was presented orally during the International Society of Renal Nutrition annual conference on June 26-30, 2012 in Honolulu, Hawaii.
SUPPORT
This study is supported in part by Clinical Translational Science Award 1UL-1RR024975 from the National Center for Research Resources, Vanderbilt Diabetes Research and Training Center Grant DK20593, K24 DK62849 and R01 DK45604 from the National Institute of Diabetes and Digestive and Kidney Diseases, Veterans Administration Merit Award 1I01CX000414 and Satellite Health Norman Coplon Extramural Grant Program. S.M. Deger is supported by International Society of Nephrology/Turkish Society of Nephrology fellowship award. A. Hung work was supported by a Career Development Award (2-031-09S) from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Clinical Sciences Research. The sponsors had no influence on the design, execution, and analysis of the results of the study.
Footnotes
AUTHORS’ CONTRIBUTIONS
SMD conceived the study, and participated in its design, worked to draft of the manuscript, CDE helped to obtain and on the data helped to draft the manuscript, AB performed the statistical analysis, AS performed the statistical analysis, TAI conceived of the study and its design and draft of the manuscript and AM conceived of the study and participated in its design and draft of the manuscript. All authors read and approved the final manuscript.
DISCLOSURES
None
REFERENCES
- 1.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011;57:A8, e1–526. doi: 10.1053/j.ajkd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 3.Axelsson J, Rashid Qureshi A, Suliman ME, et al. Truncal fat mass as a contributor to inflammation in end-stage renal disease. Am J Clin Nutr. 2004;80:1222–9. doi: 10.1093/ajcn/80.5.1222. [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–54. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 6.Beddhu S, Pappas LM, Ramkumar N, Samore MH. Malnutrition and atherosclerosis in dialysis patients. J Am Soc Nephrol. 2004;15:733–42. doi: 10.1097/01.asn.0000113319.57131.28. [DOI] [PubMed] [Google Scholar]
- 7.Pifer TB, McCullough KP, Port FK, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int. 2002;62:2238–45. doi: 10.1046/j.1523-1755.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 8.Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. 1999;55:1560–7. doi: 10.1046/j.1523-1755.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- 9.Donadio C, Halim AB, Caprio F, Grassi G, Khedr B, Mazzantini M. Single- and multi-frequency bioelectrical impedance analyses to analyse body composition in maintenance haemodialysis patients: comparison with dual-energy x-ray absorptiometry. Physiol Meas. 2008;29:S517–24. doi: 10.1088/0967-3334/29/6/S43. [DOI] [PubMed] [Google Scholar]
- 10.Ellman G. Tissue sulfhydryl groups. Archieves of Biochemistry and Biophysics. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 11.Hu ML, Louie S, Cross CE, Motchnik P, Halliwell B. Antioxidant protection against hypochlorous acid in human plasma. The Journal of laboratory and clinical medicine. 1993;121:257–62. [PubMed] [Google Scholar]
- 12.Mackinnon DP, Fairchild AJ. Current Directions in Mediation Analysis. Curr Dir Psychol Sci. 2009;18:16. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 15.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 16.Higdon JV, Frei B. Obesity and oxidative stress: a direct link to CVD? Arterioscler Thromb Vasc Biol. 2003;23:365–7. doi: 10.1161/01.ATV.0000063608.43095.E2. [DOI] [PubMed] [Google Scholar]
- 17.Coughlan MT, Thorburn DR, Penfold SA, et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742–52. doi: 10.1681/ASN.2008050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 20.Lim PS, Chen SL, Wu MY, Hu CY, Wu TK. Association of plasma adiponectin levels with oxidative stress in hemodialysis patients. Blood Purif. 2007;25:362–9. doi: 10.1159/000107509. [DOI] [PubMed] [Google Scholar]
- 21.Himmelfarb J, McMenamin E, McMonagle E. Plasma aminothiol oxidation in chronic hemodialysis patients. Kidney Int. 2002;61:705–16. doi: 10.1046/j.1523-1755.2002.00151.x. [DOI] [PubMed] [Google Scholar]
- 22.Prior SL, Tang TS, Gill GV, Bain SC, Stephens JW. Adiponectin, total antioxidant status, and urine albumin excretion in the low-risk “Golden Years” type 1 diabetes mellitus cohort. Metabolism. 2011;60:173–9. doi: 10.1016/j.metabol.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, Tao L, Yuan Y, et al. Endothelial dysfunction in adiponectin deficiency and its mechanisms involved. J Mol Cell Cardiol. 2009;46:413–9. doi: 10.1016/j.yjmcc.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao L, Gao E, Jiao X, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–16. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 25.Painter P. Physical functioning in end-stage renal disease patients: update 2005. Hemodial Int. 2005;9:218–35. doi: 10.1111/j.1492-7535.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- 26.Ikizler TA, Wingard RL, Harvell J, Shyr Y, Hakim RM. Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: a prospective study. Kidney Int. 1999;55:1945–51. doi: 10.1046/j.1523-1755.1999.00410.x. [DOI] [PubMed] [Google Scholar]