Highlights
-
•
FAP is initially managed with genetic testing, followed by yearly colonoscopy from 10 to 40 years of age.
-
•
Once diagnosed, total proctocolectomy with ileal-J pouch to anal anastomosis is recommended.
-
•
The ultimate treatment for FAP patients presenting with acute lower GI hemorrhage and hemodynamic compromise is unclear.
-
•
This is the first literature report of an emergency total proctocolectomy for lower GI hemorrhage in an uninsured patient with FAP in a community hospital.
-
•
It is essential to monitor the ileo-anal anastomosis with anoscopy.
Abstract
Introduction
Rectal bleeding is the most common symptom of Familial Adenomatous Polyposis (FAP). This case investigates the efficacy of emergency surgery for FAP with total proctocolectomy end ileostomy for recurrent lower gastrointestinal (GI) hemorrhage in an uninsured patient in a 266-bed community hospital. The optimal treatment for FAP with acute lower GI hemorrhage and hemodynamic compromise unresponsive to conservative management is unclear.
Presentation of case
A 41-year-old uninsured African American man with no past medical or family history presented to the emergency department with hematochezia lasting three days. A clinical diagnosis of FAP made on colonoscopy with biopsies revealed villous and tubulovillous adenomas without dysplasia. After blood products resuscitation, an emergency total proctocolectomy with end ileostomy was performed. A staged ileal J pouch to anal anastomosis and creation of protective loop ileostomy was performed months later after securing state funding. A final loop ileostomy reversal occurred six weeks later. His self reported quality of life is improved.
Discussion
Lower GI hemorrhage from FAP unresponsive to blood products may require emergency total proctocolectomy and end ileostomy with a staged ileal J pouch to anal anastomosis, which can be done in a community acute care hospital for an uninsured patient.
Conclusion
A total proctocolectomy is feasible in the emergency setting in an uninsured patient with lower GI bleeding and FAP. A staged ileal J pouch-anal anastomosis is easier to justify to the hospital compared to a staged completion colectomy with proctectomy. It is essential to monitor the ileo-anal anastomosis with anoscopy.
1. Introduction
FAP is the result of a tumor suppressor mutation of the APC gene (Adenomatous Polyposis Coli, 5q21–q22), which causes multiple (classically over 100) colorectal adenomatous polyps. By 45 years of age, untreated Classic FAP will cause colorectal cancer in appraoximately 100% of cases. While most patients are asymptomatic, the most common clinical presentation of colon cancer and FAP is rectal bleeding, which occurs in 58% and 37% of patients, respectively [1], [2]. The initial clinical presentation in patients with FAP under 20 years old is hematochezia with diarrhea, occurring in 28% and 13% of them, respectively [2]. The reason is that most cases are asymptomatic until the polyps are large enough to cause GI bleeding and anemia [3].
It is recommended to manage FAP with initial genetic screening, followed by yearly colonoscopy from 10 to 40 years old. Once FAP is diagnosed, colectomy or total proctocolectomy with ileal J pouch creation is recommended [4], [5]. This unique case corresponds to a 41 year old African American man with FAP and without medical insurance, in need of emergency surgical intervention that would address his lower GI hemorrhage and offer a definitive colorectal resection.
2. Case presentation
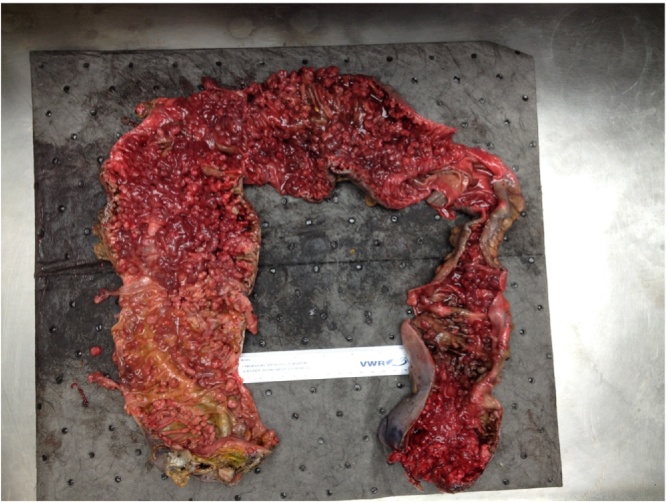
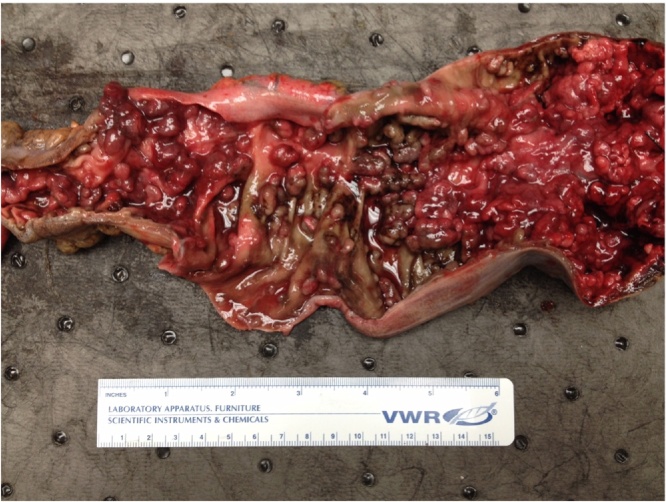
A 41-year-old uninsured African American man with no past medical or family history of cancer presented to the emergency department with hematochezia lasting three days. Initial vitals were a blood pressure of 134/66, temperature of 36.8C, heart rate of 92, and respiratory rate of 18. Initial hemoglobin was 5.2 gm/dL. He was transfused 6 units of packed red blood cells. A colonoscopy performed on a prior hospitalization when the Surgery service was not consulted and due to acute blood loss anemia from lower GI bleeding showed hundreds of pedunculated/sessile polyps. Pathology demonstrated tubulovillous and villous adenomas without high-grade dysplasia or invasive carcinoma. After blood product resuscitation for 2 h after surgical consultation on hospital day 2, a decision was made to perform a total proctocolectomy with end ileostomy immediately following the transfusion. There was a superficial surgical site infection opened at the bedside on post operative day 4, and therefore the Clavien-Dindo Classification of surgical complications is grade I. His discharge hemoglobin was 9.7 gm/dL. The final pathology correlated with the colonoscopy biopsies and showed over 100 adenomatous polyps without dysplasia or neoplasia (Fig. 1, Fig. 2, Fig. 3 ). The pathology department classified this case as FAP.
Fig. 1.
Total proctocolectomy specimen with more than 100 adenomatous polyps in a patient with FAP and acute lower GI hemorrhage.
Fig. 2.
Ascending colon with numerous adenomatous polyps, without atypia or neoplasia.
Fig. 3.
Descending colon with numerous adenomatous polyps, without atypia or neoplasia.
Three months later, he recovered after this surgery and underwent resection of 30 cm of distal ileum due to multiple polyps in that segment, creation of an ileal J pouch to anal anastomosis with protective loop ileostomy. The Clavien-Dindo Classification for this second surgery is grade II due to post operative ileus for 7 days that required a short course of TPN. His loop ileostomy remained functional and he did not develop a parastomal hernia. However, he visited the emergency department twice due to mild dehydration from high stoma output which required a brief hospitalization for two days. The third staged surgery was a loop ileostomy reversal six weeks later. At this time, intraoperative anoscopy revealed 4 cm of retained distal rectum containing several polyps, which was not obvious during the second operation. The Clavien-Dindo Classification does not apply to the third surgery, since there were no complications. He follows up in clinic every 3 months and his self reported quality of life is good, with fewer than five bowel movements per day.
3. Discussion
The challenge of this case was the emergency situation that warranted oncologic resection in a patient who had already been hospitalized with complications from lower GI bleeding due to FAP and with a lack of follow up due to his insurance status. The first procedure was an emergency total proctocolectomy with end ileostomy creation. We opted for this approach versus a subtotal colectomy because at the time of presentation it was impossible to determine which of the hundreds of polyps in his colon and rectum were bleeding. Performing a subtotal colectomy would not guarantee that the remaining multiple polyps in the rest of the colon and rectum would not bleed and cause symptoms in the future. An ileal J pouch to anal anastomosis was not created at this time due to the risky nature of that procedure. Instead, we chose to operate at a later date to create this anastomosis with the resection of the end ileostomy, and creation of a protective loop ileostomy.
It has been established that in patients with these criteria, a total proctocolectomy with ileal pouch anal anastomosis or an ileostomy should be recommended: colon cancer on initial presentation, over 30 rectal polyps at that time, APC mutations between codons 1250 and 1464, or over 1000 colonic adenomas. They are not appropriate candidates for rectal preservation [6].
To our knowledge, no one, at least in the English language, has reported an emergency total proctocolectomy in a case with FAP. The closest procedure we found in the literature was a laparoscopic total abdominal colectomy and “open” mucosal proctectomy with ileoanal pouch anastomosis and diverting loop ileostomy, which was performed on 3 patients with FAP [7]. However, it was not entirely clear if these three cases were actually done in the acute setting.
The uninsured patient presented a separate challenge. The Pathology department was unable to perform genetic testing for the APC gene because of the patient’s lack of resources and our inability to secure insurance for him after multiple attempts. Instead, a clinical diagnosis was made based on the presence of hundreds of colonic and rectal polyps. A limited fund for charity cases was secured with a state program and our hospital to allow the patient to undergo the subsequent staged operations that improved his quality of life. Schwartz and colleagues discussed this important topic and concluded that patients with limited resources to medical care and insurance have a higher risk for emergency presentation of diseases that are preventable and otherwise treatable with elective surgery [8]. This is attributable to the fact that these patients do not have access to screening programs. On the other hand, postoperative complications are more frequent in government coverage groups compared to the uninsured group [8]. Similarly, according to Shahan and colleagues, factors such as government coverage, elderly age, black race, and males were associated with increased mortality, higher complication rates, and failure to resuscitate in emergency general surgery cases [9]. This patient fit some of these criteria.
Other potential challenges for the future include complications related to the J pouch. These include pelvic sepsis, pouch anastomotic fistulae, and anastomotic dehiscence, which all increase the likelihood of pouch failure [5]. The most common complication, illustrated in one study of 84 patients, is pouch-anal anastomotic stricture [10]. The complication with the highest risk of morbidity is pouch-anal dehiscence [10]. Nevertheless, the majority of the patients (94%) found the pouch to be superior to a permanent ileostomy [10]. Of concern, there is a high incidence of low-grade dysplasia in the pouch for patients with FAP; however, higher grades of dysplasia and cancer are rare [11]. Lifelong pouch surveillance is recommended every 6–12 months [12].
The plan for the future is six-month surveillance with esophagogastroduodenoscopy, enteroscopy of the J pouch, completion proctectomy with transanal total mesorectal excision combined with J pouch takedown and creation of a true J pouch to anal anastomosis.
An important learning point is that anoscopy to inspect the ileal J pouch to anal anastomosis should have been performed during the second operation, which would have demonstrated 4 cm of retained distal rectum containing several polyps. The reason for not performing anoscopy after creating the anastomosis was the need to protect these delicate tissues from unnecessary injury from instrumentation given the expected edema when such a technically difficult anastomosis is created. However, this could have easily prevented having to return at a later time for a completion proctectomy with a transanal total mesorectal excision technique, which is planned for next year after the patient has fully recovered and state-based financial resources are secured again. He will undergo proctoscopy and enteroscopy every year if he decides to undergo surveillance instead of completion proctectomy, although the latter procedure has been recommended to him.
We will also administer the SF-36 assessment tool during his next clinic appointment since it has been shown to be useful in assessing postoperative recovery for colorectal surgery [13]. The patient’s 21-year-old daughter has been offered the option to undergo a screening colonoscopy at this time.
4. Conclusion
A total proctocolectomy is a feasible option in the emergency setting in a patient with FAP in critical condition due to severe lower GI bleeding and who does not have resources or insurance. A staged ileal J pouch to anal anastomosis is much easier to justify to the hospital in an uninsured patient compared to a staged completion colectomy with proctectomy. It is essential to monitor the ileo-anal anastomosis with anoscopy. This is the first report in the literature of an emergency total proctocolectomy for FAP for lower GI hemorrhage in an uninsured patient.
Conflict if interest
No conflicts of interest.
Funding
No funding.
Ethical approval
Ethical approval was given by the hospital’s IRB committee. Since it is not a cohort series or retrospective review or RTC, there was no additional permit required.
Consent
We confirm that consent has been obtained.
Author contribution
Rodolfo J. Oviedo, MD, FACS: design, writing, editing.
Bruce M. Dixon, BA: writing, data collection.
Chase W. Sofiak, BS: data collection.
Guarantor
Guarantor: Rodolfo J. Oviedo, MD, FACS.
Contributor Information
Rodolfo J. Oviedo, Email: rodolfo.j.oviedo@gmail.com.
Bruce M. Dixon, Email: dixonbm@acomedu.org.
Chase W. Sofiak, Email: sofiakcw@acomedu.org.
References
- 1.Majumdar S.R., Fletcher R.H., Evans A.T. How does colorectal cancer present? symptoms, duration, and clues to location. Am. J. Gastroenterol. 1999;94:3039–3045. doi: 10.1111/j.1572-0241.1999.01454.x. http://dx.doi.org/10.1111/j.1572-0241.1999.01454.x [DOI] [PubMed] [Google Scholar]
- 2.Kennedy R.D., Potter D.D., Moir C.R., El-Youssef M. The natural history of familial adenomatous polyposis syndrome: a 24 year review of a single center experience in screening, diagnosis, and outcomes. J. Pediatr. Surg. 2014;49:82–86. doi: 10.1016/j.jpedsurg.2013.09.033. http://dx.doi.org/10.1016/j.jpedsurg.2013.09.033 [DOI] [PubMed] [Google Scholar]
- 3.Half E., Bercovich D., Rozen P. Familial adenomatous polyposis. Orphanet J. Rare Dis. 2009;4:22. doi: 10.1186/1750-1172-4-22. http://dx.doi.org/10.1186/1750-1172-4-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefevre J.H., Bretagnol F., Ouaïssi M., Taleb P., Alves A., Panis Y. Total laparoscopic ileal pouch-anal anastomosis: prospective series of 82 patients. Surg. Endosc. 2008;23:166–173. doi: 10.1007/s00464-008-0121-8. http://dx.doi.org/10.1007/s00464-008-0121-8 [DOI] [PubMed] [Google Scholar]
- 5.Martin S.T., Tevlin R., Heeney A., Peirce C., Hyland J.M., Winter D.C. How I do it: the stapled ileal J pouch at restorative proctocolectomy. Tech. Coloproctol. 2011;15:451–454. doi: 10.1007/s10151-011-0757-6. http://dx.doi.org/10.1007/s10151-011-0757-6 [DOI] [PubMed] [Google Scholar]
- 6.Carmichael J.C., Mills S. Surgical management of familial adenomatous polyposis. Seminars in Colon and Rectal Surgery. 2011;22:108–111. http://dx.doi.org/10.1053/j.scrs.2010.12.010 [Google Scholar]
- 7.Marohn M., Hanly E., Mckenna K., Varin C. Laparoscopic total abdominal colectomy in the acute setting. J. Gastrointest. Surg. 2005;9:881–887. doi: 10.1016/j.gassur.2005.04.017. http://dx.doi.org/10.1016/j.gassur.2005.04.017 [DOI] [PubMed] [Google Scholar]
- 8.Schwartz D.A., Hui X., Schneider E.B., Ali M.T., Canner J.K., Leeper W.R. Worse outcomes among uninsured general surgery patients: does the need for an emergency operation explain these disparities? Surgery. 2014;156:345–351. doi: 10.1016/j.surg.2014.04.039. http://dx.doi.org/10.1016/j.surg.2014.04.039 [DOI] [PubMed] [Google Scholar]
- 9.Shahan C.P., Bell T., Paulus E., Zarzaur B.L. Emergency general surgery outcomes at safety net hospitals. J. Surg. Res. 2015;196:113–117. doi: 10.1016/j.jss.2015.02.044. http://dx.doi.org/10.1016/j.jss.2015.02.044 [DOI] [PubMed] [Google Scholar]
- 10.Skarsgard E.D., Atkinson K.G., Bell G.A., Pezim M.E., Seal A.M., Sharp F. Function and quality of life results after ileal pouch surgery for chronic ulcerative colitis and familial polyposis. Am. J. Surg. 1989;157:467–471. doi: 10.1016/0002-9610(89)90636-3. http://dx.doi.org/10.1016/0002-9610(89)90636-3 [DOI] [PubMed] [Google Scholar]
- 11.Boostrom S.Y., Mathis K.L., Pendlimari R., Cima R.R., Larson D.W., Dozois E.J. Risk of neoplastic change in ileal pouches in familial adenomatous polyposis. J. Gastrointest. Surg. 2013;17:1804–1808. doi: 10.1007/s11605-013-2319-x. http://dx.doi.org/10.1007/s11605-013-2319-x [DOI] [PubMed] [Google Scholar]
- 12.Zahid A., Kumar S., Koorey D., Young C. Pouch adenomas in Familial Adenomatous Polyposis after restorative proctocolectomy. Int. J. Surg. 2015;13:133–136. doi: 10.1016/j.ijsu.2014.11.048. http://dx.doi.org/10.1016/j.ijsu.2014.11.048 [DOI] [PubMed] [Google Scholar]
- 13.Antonescu I., Carli F., Mayo N.E., Feldman L.S. Validation of the SF-36 as a measure of postoperative recovery after colorectal surgery. Surg. Endosc. 2014;28:3168–3178. doi: 10.1007/s00464-014-3577-8. http://dx.doi.org/10.1007/s00464-014-3577-8 [DOI] [PubMed] [Google Scholar]