Abstract
Idiopathic pleuroparenchymal fibroelastosis (PPFE) is a rare subset of idiopathic interstitial pneumonias (IIPs). Here we present two patients with PPFE in which the histology was confirmed with transbronchial lung biopsy (TBLB). The 25-year-old and 64-year-old men were both slender with a long history of pulmonary upper lobe fibrosis and a marked restrictive impairment. Although the imaging findings supported the diagnosis of PPFE, surgical lung biopsy (SLB) seemed to be needed to identify fibroelastosis for the definite diagnosis. However, we selected TBLB instead of SLB because of their general condition and the risk such as prolonged pneumothorax after TBLB. TBLB specimens in both patients showed aggregates of elastic fibers in the submucosa that were essential clues for the histological diagnosis of PPFE. TBLB may be an alternative tool for the histological diagnosis of PPFE, although a multidisciplinary discussion is necessary for the final diagnosis of PPFE as a clinicopathological entity.
Keywords: Pleuroparenchymal fibroelastosis, Transbronchial lung biopsy, Surgical lung biopsy, Idiopathic interstitial pneumonia
1. Introduction
Pleuroparenchymal fibroelastosis (PPFE) has been identified as a distinct clinicopathological entity in recent years [1], [2]. This clinical feature is characterized by pulmonary fibrosis involving predominantly the upper lung fields. The diagnosis of PPFE is based mainly on surgical lung biopsy (SLB) results, but the vast majority of patients have markedly impaired lung function, which makes it impossible to perform SLB. We report on two patients with PPFE for whom transbronchial lung biopsy (TBLB) demonstrated fibroelastosis, a crucial histological finding for the diagnosis of PPFE.
2. Case report
2.1. Case 1
A 25-year-old man was referred to our hospital with a 10-year history of bilateral upper lung fibrosis. He had had asthma since the age of 2 years. He never smoked and had no occupational or environmental exposure. At the age of 19 years, he underwent a fiber-optic bronchoscopy examination at another hospital. However, at that time, TBLB specimens stained with hematoxylin and eosin (HE) did not allow any specific diagnosis. Since then, he has visited the hospital once a year for a medical check-up. Although he was asymptomatic, the lung fibrosis progressed gradually. He was then referred to our hospital.
On physical examination, he was slender with a body mass index (BMI) of 15.1 kg/m2 and had no clubbed fingers. Respiratory sounds were normal. A chest radiograph at the age of 15 years showed upper lung-dominant fibrotic opacities with elevated hilar opacities. Ten years later, upper lung fibrosis had worsened with markedly increased hilar opacities (Fig. 1a). A chest computed tomography (CT) taken at our hospital showed a flattened chest cage and subpleural consolidations with traction bronchiectasis located in the bilateral upper lung fields (Fig. 1b). By contrast, the lower lung fields were almost normal. Spirometry showed markedly restrictive impairment: forced vital capacity (FVC) was 1820 mL (43.5% pred.). Serum Krebs von den Lungen-6 (KL-6) concentration was near the upper limit of the normal range (470 U/mL), and Surfactant protein-D (SP-D) concentration was markedly increased (531 ng/mL).
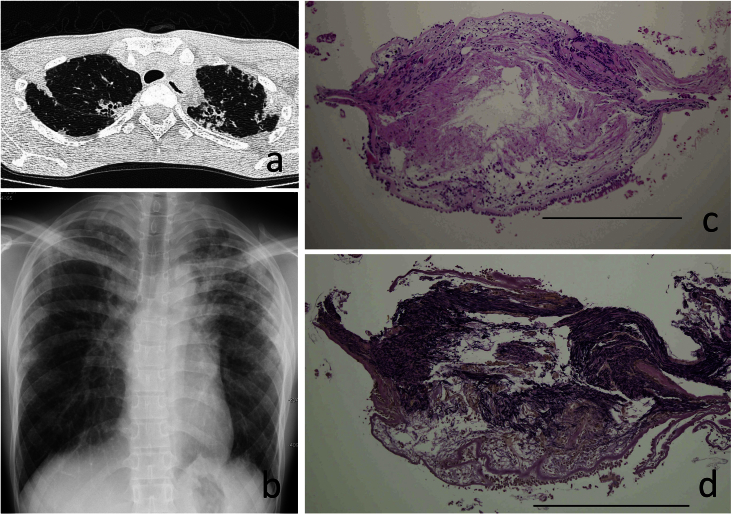
Fig. 1.
a. Chest radiograph of case 1. b. Upper lung fields of chest CT in case 1. c. TBLB specimen stained with hematoxylin and eosin (HE) in case 1, Scale bar = 500μ. d. TBLB specimen stained with Elastica van Gieson (EVG) in case 1, Scale bar = 500μ.
The slender constitution with flattened chest cage [3] and the long history of upper lung lesions with restrictive impairment raised the possibility that the disease was PPFE. We obtained the previous biopsy specimens stained with HE and the paraffin blocks, and additional histological specimens stained with Elastica van Gieson (EVG) were prepared to identify elastofibrosis. The EVG specimen revealed dense elastosis just beneath the subepithelial reticular basement membrane of the peripheral airway (Fig. 1c, d). Another specimen showed that alveoli were filled with collagen in association with septal elastosis. All of these histological findings are consistent with those of PPFE.
2.2. Case 2
A 64-year-old man was referred to our hospital with a diagnosis of pulmonary upper lobe fibrosis. He had experienced repeated pneumothorax since the age of 18 years. In his 30s, he noticed cough and mild fever. He received antituberculous medical therapy under the diagnosis of suspected pulmonary tuberculosis, but the cough remained. A few years ago, he noticed exertional dyspnea. Since then, cough and dyspnea have been gradually worsening and he has experienced repeated episodes of fever. He had smoked 20 to 30 cigarettes a day for about 20 years and had quit smoking in his late 30s. His mother had rheumatoid arthritis and systemic sclerosis.
On admission, he was slender with a BMI of 17.9 kg/m2 and had no clubbed fingers. Respiratory sounds were normal. A chest radiograph showed upper lobe-dominant fibrotic opacities with elevated hilar opacities (Fig. 2a). Chest CT showed subpleural consolidations with traction bronchiectasis and bilateral apical cysts (Fig. 2b). However, the lower lung fields were almost free from fibrotic lesions. FVC and total lung capacity (TLC) were decreased: 2220 mL (59.4% pred.) and 4970 mL (87.0% pred.), respectively. However, residual volume (RV) was increased (2850 mL, 142.5% pred.), which resulted in an increased RV/TLC ratio (154.6% pred.).
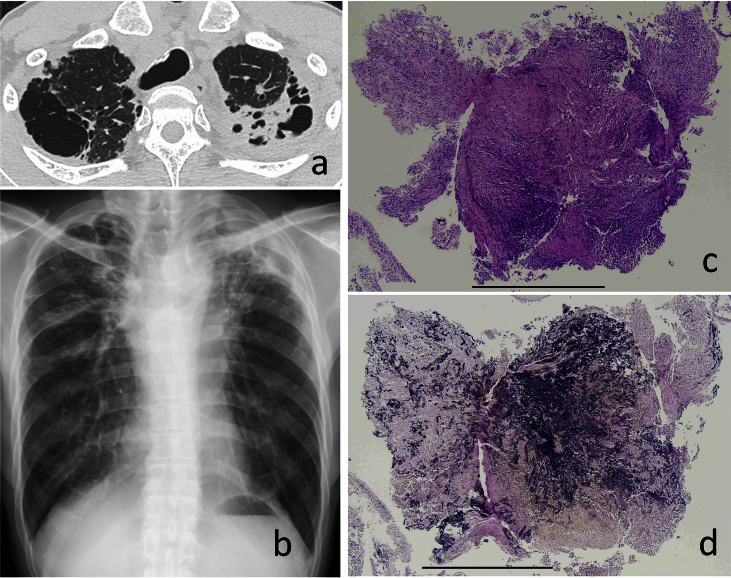
Fig. 2.
a. Chest radiograph of case 2. b. Upper lung fields of chest CT in case 2. c. TBLB specimen (HE) in case 2, Scale bar = 1mm. d. TBLB specimen (EVG) in case 2, Scale bar = 1mm.
He underwent fiber-optic bronchoscopy examination. TBLB specimens obtained from the left upper lobe showed fibrosis, but there was no specific diagnosis. However, the histological specimen stained with EVG revealed dense aggregates of elastic fibers that were associated with intra-alveolar fibrosis (Fig. 2c, d). These histological findings are consistent with those of PPFE.
3. Discussion
SLB is believed to be mandatory for the precise diagnosis of PPFE. In clinical practice, however, attending doctors hesitate to perform SLB in patients with suspected PPFE because of the patient's physiological or general condition and the risk of postoperative complications such as prolonged air leak and pneumothorax. TBLB is not recommended as a tool for histological diagnosis of IIPs, including usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP), because TBLB specimens are too tiny to observe histological changes in the entire area of secondary lobules.
The essential histology of PPFE includes aggregates of elastic fibers, especially in the subpleural areas, and intra-alveolar collagenous fibrosis with septal elastosis with or without collagenous thickening of the visceral pleura [4], [5]. The anatomical distribution in the secondary lobules is not a crucial factor in the histological diagnosis of PPFE compared with that in UIP and NSIP. TBLB may reach the essential lesions of PPFE and will make the clinical diagnosis of PPFE more reliable. Although PPFE is predominantly found in a subpleural area, peribronchial distribution is also found [6]. For these reasons, TBLB may be a useful alternative for obtaining a histological diagnosis when SLB cannot be performed. In addition, re-evaluation of the previous TBLB samples may be important as in the case 1 that had a long history of pulmonary upper lobe fibrosis.
In recent years, transbronchial lung cryobiopsy (TBLC) has been developed as an alternative to SLB, a diagnostic tool of histology [7]. PPFE may be a suitable candidate for TBLC as well as TBLB. Larger samples may be obtained with TBLC than with TBLB. The utility of CT-guided transthoracic core lung biopsy (TTB) for PPFE has been also examined [8]. Fibroelastosis can be identified with less invasive TBLB when peribronchial aggregates of elastic fibers are the target of the biopsy. In that situation, larger samples may not necessarily be required. The diagnostic accuracy of TTB for PPFE might be similar to that of TBLB, but TBLB has the benefit of being able to collect bronchial samples for further cytology, bacteriology, and biochemistry analysis. However, multidisciplinary discussion is necessary to improve the diagnostic accuracy for PPFE as a clinicopathological entity.
In conclusion, TBLB may be a suitable alternative for SLB for obtaining histological diagnosis in patients with suspected PPFE when SLB is a probable risk for the patients' general condition.
Acknowledgements
The authors declare no conflict of interest.
This study is partially supported by the Practical Research Project for Rare Intractable Diseases from Japan Agency for Medical Research and Development, AMED. This study is also partially supported by a grant from the Ministry of Health, Labour and Welfare of Japan awarded to the Study Group on Diffuse Pulmonary Disorders, Scientific Research/Research on intractable diseases.
References
- 1.Travis W.D., Costabel U., Hansell D.M. An official American thoracic society/European respiratory society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frankel S.K., Cool C.D., Lynch D.A., Brown K.K. Idiopathic pleuroparenchymal fibroelastosis. Description of a novel clinicopathologic Entity. Chest. 2004;126:2007–2013. doi: 10.1378/chest.126.6.2007. [DOI] [PubMed] [Google Scholar]
- 3.Harada T., Yoshida Y., Kitasato Y. The thoracic cage becomes flattened in the progression of pleuroparenchymal fibroelastosis. Eur. Respir. Rev. 2014;23:260–263. doi: 10.1183/09059180.00006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy T.L., Tominaga M., Hansell D.M. Pleuroparenchymal fibroelastosis; a spectrum of histopathological and imaging phenotypes. Eur. Respir. J. 2012;40:377–385. doi: 10.1183/09031936.00165111. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe K., Nagata N., Kitasato Y. Rapid decrease in vital capacity in patients with idiopathic pulmonary upper lobe fibrosis. Respir. Investig. 2012;50:88–97. doi: 10.1016/j.resinv.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum J.N., Butt Y.M., Johnson K.A. Pleuroparenchymal fibroelastosis: a pattern of chronic lung injury. Hum. Pathol. 2015;46:137–146. doi: 10.1016/j.humpath.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Casoni G.L., Tomassetti S., Cavazza A. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLOS ONE. 2014;9:e86716. doi: 10.1371/journal.pone.0086716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esteves C., Costa F.R., Redondo M.T. Pleuroparenchymal fibroelastosis: role of high-resolution computed tomography (HRCT) and CT-guided transthoracic core lung biopsy. Insights Imaging. 2016;7:155–162. doi: 10.1007/s13244-015-0448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]