Abstract
MPV17-related hepatocerebral mitochondrial DNA depletion syndrome (MDS) is a very rare condition, and only a few cases have been reported in East Asian countries. Here, we describe four Korean children affected by hepatocerebral MDS. The DGUOK, POLG1, and MPV17 genes were analyzed, and all patients had MPV17 mutations.
Abbreviations: MDS, mitochondrial DNA depletion syndrome; SD, standard deviation
Keywords: Hepatocerebral mitochondrial DNA depletion syndrome, MPV17, Navajo neurohepatopathy
1. Introduction
Mitochondrial DNA depletion syndrome (MDS) is a clinically heterogeneous group of diseases associated with a reduced copy number of mitochondrial DNA in affected tissues and organs. MDS is usually classified as myopathic, encephalomyopathic, hepatocerebral, or neurogastrointestinal [1]. Hepatocerebral MDS is characterized by early-onset hepatopathy and neurological manifestations. Four genes are known to be associated with hepatocerebral MDS: POLG1, DGUOK, C10orf2, and MPV17 [1], [2]. MPV17-related MDS is a very rare condition, for which only approximately 40 different mutations have been reported (www.hgmd.cf.ac.uk). In our current study, four Korean children with hepatocerebral MDS are described, and MPV17 mutations were identified in all these patients.
1.1. Case descriptions
We describe four patients from three non-consanguineous Korean families—three male and one female patient (Table 1). Two patients (Patients 2 and 3) were siblings. They were born after 36–39 wks of gestation with normal birth weights [3]. Their perinatal periods were uneventful. The presenting signs included persistent neonatal jaundice in Patients 1 to 3 at 2–4 mos of age and growth retardation with elevated hepatic enzymes in Patient 4 at 12 mos of age. Out of the four patients, two were underweight (weight < 2 SD below mean), and one had short stature (height < 2 SD below mean). The serum aspartate transferase, alanine transferase, gamma-glutamyl transferase and total and direct bilirubin levels were elevated in all patients (Table 1). The serum lactic acid level was elevated in all patients as well and the lactate/pyruvate ratio was 28–41 (Table 1).
Table 1.
Clinical and molecular findings of the 4 patients with MPV17-related hepatocerebral MDS.
| Patient1 |
Patient2 |
Patient3 |
Patient4 |
|
|---|---|---|---|---|
| Sex | Male | Male | Male | Female |
| Gestational period | 36 wks | 37 wks | 39 wks | 38 wks |
| Birth weight | 2955 g (− 0.9 SD) |
3000 g (− 0.8 SD) |
3400 g (− 0.1 SD) |
3220 g (− 0.4 SD) |
| Age at presentation | 4 mos | 2 mos | 2 mos | 12 mos |
| Hepaticmanifestations | Cholestasis | Cholestasis | Cholestasis | Cholestasis |
| Steatohepatitis | Hepatomegaly | Hepatomegaly | Hepatomegaly | |
| Liver failure | Steatohepatitis | Steatohepatitis | Steatohepatitis | |
| Liver failure | Liver failure | Liver failure | ||
| Neurologicalmanifestations | Developmental delays | Developmental delays | Developmental delays | Developmental delays |
| Hypotonia | Hypotonia | Hypotonia | Hypotonia | |
| Peripheral motor neuropathy | Horizontal nystagmus | |||
| Growth parameters | 11 mos | 2 mos | 4 mos | 24 mos |
| Height | 1.2 SD | 0.4 SD | − 1.8 SD | − 3.0 SD |
| Weight | − 1.0 SD | − 2.2 SD | − 1.9 SD | − 3.8 SD |
| Metabolic | Hypoglycemia | Hypoglycemia | Hypoglycemia | Lactic acidosis |
| Lactic acidosis | Lactic acidosis | Lactic acidosis | ||
| Others | Recurrent vomiting | Recurrent vomiting | Recurrent vomiting | Recurrent vomiting |
| Nephrocalcinosis | Retinal dystrophy | |||
| Aspartate transferase (normal range, < 40 IU/L)/alanine transferase (normal range, < 40 IU/L) | 370/164 | 113/55 | 133/57 | 202/93 |
| Plasma lactate (normal range, 0.2–1.2 mg/dL) | 41.4 | 69.3 | 53.1 | 45.0 |
| Lactate/pyruvate ratio | 27.6 | 38.5 | 40.8 | 37.8 |
| MPV17 mutations | c.[197T > A];[293C > T] (p.[Val66Glu];[Pro98Leu]) | c.[451dupC];[451dupC] (p.[Leu151Profs*39]; [Leu151Profs*39]) |
c.[451dupC];[451dupC] (p.[Leu151Profs*39]; [Leu151Profs*39]) |
c.[280G > C]; c.[293C > T](p.[Gly94Arg];[Pro98Leu]) |
| Brain MR | Normal | Normal | Myelination delays | Not done |
| Outcome (cause of death) | Death at 2 yrs and 2 mos (liver failure) | Death at 6 mos (liver failure) | Death at 6 mos (liver failure) | Death at 2 yrs and 4 mos (liver failure) |
SD, standard deviation; Bold character, a novel mutation.
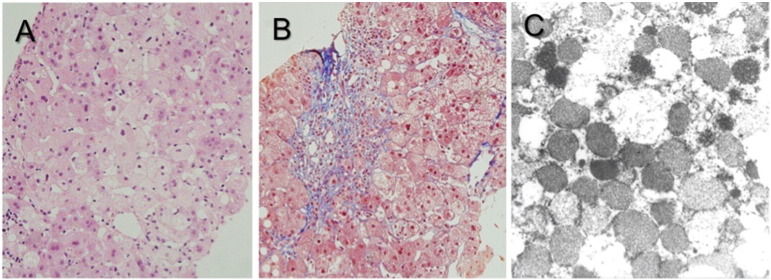
Abdominal ultrasound showed diffusely increased hepatic parenchymal echogenicity in all patients. Histological examination revealed fatty changes and the ballooning of hepatocytes with severe cholestasis and septal fibrosis with ductal damages. Electron microscopic examination revealed hepatocytes with densely packed mitochondria with disoriented or lost cristae (Fig. 1). Hypoglycemic episodes were recurrent in Patients 1–3. Patient1 had nephrocalcinosis. Seizures did not develop in any patient, but profound motor hypotonia and developmental delay were evident in all four cases. Patient 1 had peripheral motor neuropathy, and Patient 2 had horizontal nystagmus and retinal dystrophy. Brain imaging studies performed in all patients between 3 and 26 mos of age were unremarkable, except for delayed myelination in Patient3. Genetic testing was performed on all patients for POLG1, DGUOK, and MPV17 genes using genomic DNA from the peripheral leukocytes, and all patients had mutations in the MPV17 gene (Table 1). Four different MPV17 mutations were found. Three mutations, p.Gly94Arg, p.Pro98Leu, and p.Leu151Profs*39, were previously reported [5], [7]. The other mutation, p.Val66Glu, was a novel mutation and was not found in the 1000 Genomes database (www.1000genomes.org). Being located in the 2nd transmembrane domain, this mutation was predicted as pathogenic by two in silico analyses, MutationTaster (http://www.mutationtaster.org) and SIFT (http://blocks.fhcrc.org/sift/SIFT.html). Despite conservative management, all the patients died of liver failure between 6 and 28 mos of age.
Fig. 1.
Histological findings in the hepatic tissues of a patient with MPV17-related hepatocerebral MDS (Patient1). A) Hepatocytes with macrovesicular steatosis (hematoxylin and eosin stain, × 400). B) Portal inflammation with bile ductular reaction and septal fibrosis (Masson's trichrome stain, × 400). C) Hepatocytes with densely packed mitochondria showing a granular appearance and loss of cristae (electron micrographs, × 120,000).
2. Discussion
The prevalence of MPV17-related hepatocerebral MDS is unknown but is expected to be very rare. In addition, as more nuclear genetic defects are identified in various kinds of MDS patients, the identification of each genetic defect is becoming more complicated in these cases. Because MDS involves multiple systems, muscular, hepatic, gastrointestinal, or neuronal, the clinical classification of MDS into 1 of the 4 subgroups can be difficult in some patients with overlapping phenotypes. In this respect, multigene testing using next-generation sequencing techniques is becoming more popular for the genetic confirmation of MDS [4]. Nevertheless, our own experience indicates that these four genes can be screened in infantile patients who exhibit cholestatic hepatopathy, lactic acidosis (increased lactate/pyruvate ratio), developmental delay and motor hypotonia, as well as showing histological evidence of MDS such as an increase in dysmorphic mitochondria. Although we did not test for C10orf2, we identified MPV17 mutations in all four patients.
In the liver of a patient with hepatocerebral MDS, the mitochondrial DNA copy number is profoundly reduced and mitochondrial respiratory chain activities are decreased, except complex II that is encoded by the nuclear DNA. However, these measurements were not available for our patients. Instead, we directly screened for genomic mutations in the genes responsible for this condition. We cannot conclude that MPV17 is the most common gene responsible for hepatocerebral MDS in Korean or other East Asian populations but we suggest that MPV17 can be the first gene to be screened in MDS patients in these populations.
To date, MPV17 mutations have been detected as widely distributed along the coding region of its 8 exons [6], and p.Arg50Gln is the most common mutation that is only found in a homozygous state [5]. Excluding p.Arg50Gln, most mutations have been reported as private. Among the families studied here, 1 frameshift mutation—p.Leu151Profs*39—was homozygous in the siblings, and this has been first reported in Japanese siblings with MDS [7]. In addition, p.Pro98Leu was recurrently found in two unrelated families of our patients, which was first reported in Caucasian children [5]. It remains to be seen whether these mutations are common mutations in MDS patients from Eastern Asian countries, but there are no other reports on MPV17 mutations in these populations.
Genotype-phenotype correlations are poorly understood because fewer than 50 patients have been identified to date, and most cases have private mutations, experience liver failure, and do not survive their first decade of life. A milder phenotype has been described only for the most common mutation—p.Arg50Gln—in some homozygotes [5]. In our present patients, the siblings with the homozygous frameshift mutation—p.Leu151Profs*39—experienced the most rapid progression of liver failure and died within 6 mos of age.
Liver transplantation is controversial in MDS patients due to its multisystemic involvement but only conservative management is otherwise possible for this devastating condition. Due to the autosomal recessive inheritance of MDS, genetic diagnosis is very important to provide genetic counseling to affected patients and their families regarding future reproduction.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Acknowledgements
We thank the patient and her family for participating in this study, which was supported by a grant from the National Research Foundation of Korea, funded by the Ministry of Education, Science, and Technology (NRF-2015R1D1A1A01058192).
References
- 1.El-Hattab A.W., Scaglia F., Craigen W.J. MPV17-Related Hepatocerebral Mitochondrial DNA Depletion Syndrome. In: Pagon R.A., Adam M.P., Ardinger H.H., Wallace S.E., Amemiya A., Bean L.J.H., Bird T.D., Fong C.T., Mefford H.C., Smith R.J.H., Stephens K., editors. GeneReviews(R) University of Washington, Seattle; Seattle WA: 1993. [Google Scholar]
- 2.Spinazzola A., Viscomi C., Fernandez-Vizarra E. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat. Genet. 2006;38:570–575. doi: 10.1038/ng1765. [DOI] [PubMed] [Google Scholar]
- 3.Korean Children and Adolescents Growth Standard, Korea Center for Disease Control and Prevention, The Korean Pediatric Society, The Committee for the Developmentof Growth Standard for Korean Children and Adolescents. 2007.
- 4.Vilarinho S., Choi M., Jain D. Individual exome analysis in diagnosis and management of paediatric liver failure of indeterminate aetiology. J. Hepatol. 2014;61:1056–1063. doi: 10.1016/j.jhep.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Hattab A.W., Li F.Y., Schmitt E., Zhang S. MPV17-associated hepatocerebral mitochondrial DNA depletion syndrome: new patients and novel mutations. Mol. Genet. Metab. 2010;99:300–308. doi: 10.1016/j.ymgme.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Uusimaa J., Evans J., Smith C. Clinical, biochemical, cellular and molecular characterization of mitochondrial DNA depletion syndrome due to novel mutations in the MPV17 gene. Eur. J. Hum. Genet. 2014;22:184–191. doi: 10.1038/ejhg.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaji S., Murayama K., Nagata I. Fluctuating liver functions in siblings with MPV17 mutations and possible improvement associated with dietary and pharmaceutical treatments targeting respiratory chain complex II. Mol. Genet. Metab. 2009;97:292–296. doi: 10.1016/j.ymgme.2009.04.014. [DOI] [PubMed] [Google Scholar]