Abstract
Three types of beta adrenergic receptors (ARβ1–3) mediate the sympathetic activation of brown adipose tissue (BAT), the key thermogenic site for mice which is also present in adult humans. In this study, we evaluated adaptive thermogenesis and metabolic profile of a mouse with Arβ2 knockout (ARβ2KO). At room temperature, ARβ2KO mice have normal core temperature and, upon acute cold exposure (4 °C for 4 h), ARβ2KO mice accelerate energy expenditure normally and attempt to maintain body temperature. ARβ2KO mice also exhibited normal interscapular BAT thermal profiles during a 30-min infusion of norepinephrine or dobutamine, possibly due to marked elevation of interscapular BAT (iBAT) and of Arβ1, and Arβ3 mRNA levels. In addition, ARβ2KO mice exhibit similar body weight, adiposity, fasting plasma glucose, cholesterol, and triglycerides when compared with WT controls, but exhibit marked fasting hyperinsulinemia and elevation in hepatic Pepck (Pck1) mRNA levels. The animals were fed a high-fat diet (40% fat) for 6 weeks, ARβ2KO mice doubled their caloric intake, accelerated energy expenditure, and induced Ucp1 expression in a manner similar to WT controls, exhibiting a similar body weight gain and increase in the size of white adipocytes to the WT controls. However, ARβ2KO mice maintain fasting hyperglycemia as compared with WT controls despite very elevated insulin levels, but similar degrees of liver steatosis and hyperlipidemia. In conclusion, inactivation of the ARβ2KO pathway preserves cold- and diet-induced adaptive thermogenesis but disrupts glucose homeostasis possibly by accelerating hepatic glucose production and insulin secretion. Feeding on a high-fat diet worsens the metabolic imbalance, with significant fasting hyperglycemia but similar liver structure and lipid profile to the WT controls.
Keywords: β-adrenergic receptors, adaptive thermogenesis, brown adipose tissue (BAT), obesity
Introduction
Brown adipose tissue (BAT) is an important site of adaptive thermogenesis, preserving thermal homeostasis in response to cold exposure in both rodents and humans (Cannon & Nedergaard 2004, Nedergaard & Cannon 2010), as well as dissipating caloric excess in rodents in response to feeding with a high caloric diet (Rothwell & Stock 1979, Vosselman et al. 2013). Thus, understanding of the mechanisms regulating BAT activity may help in development of new strategies to accelerate energy expenditure and address the current high prevalence of obesity.
In mice, BAT develops during the 3-day window E16.5–18.5 (Hall et al. 2010) and after birth can be activated by the sympathetic nervous system (SNS) through stimulation of β-adrenergic receptors (ARβ) and cAMP production (Cannon & Nedergaard 2004). In BAT, cAMP signaling not only induces the expression of genes involved in the thermogenic capacity of the tissue but also promotes lipolysis and activates the mitochondrial uncoupling protein-1 (Ucp1) that leads to heat production. The importance of this pathway is illustrated by the fact that mice in which all three ARβ isoforms have been inactivated exhibit increased susceptibility to cold exposure as well as diet-induced obesity (Bachman et al. 2002, Jimenez et al. 2002).
The ARβs are members of a family of three G-protein-coupled receptors, i.e. β1, β2, and β3, that are distributed throughout the body and play their metabolic roles by controlling glucose homeostasis, lipolysis, and insulin secretion (Gardner & Shoback 2007). All three ARβ isoforms are expressed in BAT (Collins & Surwit 2001) and our recent studies have indicated that the ARβ1 is key for cold-and diet-induced BAT thermogenesis as the ARβ1KO mice develop hypothermia when exposed to cold and obesity due to being fed a high-fat diet (HFD) (Ueta et al. 2012). In contrast, the roles played by ARβ2 and ARβ3 are less clear. Inactivation of the Arβ3 gene seems to be very well tolerated in vivo given that mice with ARβ3KO exhibit only a modest increase in body fat, have normal basal metabolic rate, and respond normally to cold exposure (Susulic et al. 1995), which could be due to upregulation of ARβ1 (Atgie et al. 1997).
There is great interest in the study of the ARβ2 pathway in humans. For example, activation of the ARβ2 pathway with salbutamol accelerates energy expenditure, lipolysis, and fat oxidation without affecting glucose oxidation in humans (Hoeks et al. 2003). In fact, a specific genetic variation in the ARβ2 gene is associated with blunted in vivo ARβ-mediated lipolysis and fat oxidation during ARβ stimulation (Jocken et al. 2007). In addition, polymorphisms of the ARβ2 gene are frequent in obese humans (Large et al. 1997, Takenaka et al. 2012), but studies on associations between these polymorphisms and body weight and composition, glucose tolerance, and insulin sensitivity have produced mixed results (Echwald et al. 1998, Kortner et al. 1999, Oberkofler et al. 2000, Prior et al. 2011).
Despite great interest in ARβ2 in humans, available animal models indicate only a minor metabolic role for ARβ2 (Rohrer et al. 1999). For example, it is known that ARβ2KO mice have lower body weight and smaller epididymal fat pads (Chruscinski et al. 1999), but their metabolic rate remains unaffected when compared with WT control animals (Rohrer et al. 1999). However, ARβ2KO mice have not been studied under conditions in which thermogenesis is activated. Thus, herein we looked at different metabolic parameters in ARβ2KO animals that were acutely exposed to cold or fed with a HFD. We found that ARβ2KO mice expressed increased liver phosphoenolpyruvate carboxykinase (Pepck) mRNA levels and displayed marked hyperinsulinemia, which on a HFD also results in increased fasting blood glucose. However, these animals attempt to maintain their body temperature when exposed to cold and exhibit normal catecolamine-stimulated interscapular BAT (iBAT) thermal response. When fed a HFD, ARβ2KO mice exhibited similar susceptibility to diet-induced obesity when compared with WT controls. Notably, there was a dramatic increase in the expression of both Arβ1 and Arβ3 mRNAs in the BAT of ARβ2KO mice, possibly explaining the lack of a major thermogenic phenotype.
Materials and methods
Animals
Approximately 60-day-old Friend virus B (FVB) male ARβ2KO mice and WT FVB controls were studied, following an animal protocol approved by the Institutional Committee on Animal Research at the Center of Biological Sciences and Health-University Presbyterian Mackenzie. Each experiment was repeated two or three times on different sets of animals. As indicated, mice were kept on a chow diet (1.8 Cal/g) and water was available for drinking ad libitum in a room maintained at 25 °C. Food intake and body weight were measured daily. Acute cold exposure was performed in conscious mice housed individually in cages with no bedding in a cold room maintained at 4 °C (Eletrolab, São Paulo, SP, USA) for up to 4 h. Colonic temperature was measured hourly using a 1 mm wide rectal probe (Y4000, YSI, Yellow Springs, OH, USA) as previously described (de Jesus et al. 2001). Some animals were fed a HFD (5.44 Cal/g; 40% lipid; Rhoster, Sao Paulo, BR, USA) for 5 weeks.
Resting oxygen consumption (VO2)
VO2 was measured in an open-circuit respirometer system (O2–10, Sable System, Las Vegas, NV, USA) as described previously (Curcio et al. 1999). For cold exposure, mice were exposed to different temperatures (5, 15, 25, 30, 32, 34, and 36 °C) and VO2 was measured for 1 h at each temperature. The experiments were conducted between 1100 and 1900 h. Data were collected and analyzed using the Sable Systems’ software and results expressed as ml O2/min per g BW and ml O2/min as indicated. When at room temperature (25 °C), measurements were obtained over 30 min periods, in the afternoon (1400–1800 h) in animals that had access to food ad libitum.
Intraperitoneal glucose tolerance test and insulin tolerance test
Overnight fasting blood glucose was measured weekly in all animals by sampling the tail vein and using a glucose analyzer (LifeScan, Inc., Milipitas, CA, USA). For the glucose tolerance test (GTT), animals were fasted overnight and received glucose (2 g/kg) injected i.p. between 0900 and 1000 h. Blood samples were collected from the tail vein at the indicated times after the glucose load and assessed for glucose analysis. For the insulin tolerance test (ITT), food was removed 6 h before the experiment, which was carried out between 1400 and 1500 h. Blood samples were collected from the tail vein at the indicated times after injection of insulin (0.5 U/kg; i.p.) and glycemia was immediately determined using the glucose analyzer. At the end of the experimental period, blood samples were collected by cardiac puncture and assessed for insulin levels by ELISA (Marschner et al. 1974).
iBAT thermal response to norepinephrine or dobutamine infusion
This was determined under anesthesia as described previously (Ribeiro et al. 2001, Bianco et al. 2014). Briefly, mice were anesthetized with urethane (560 mg/kg, i.p.) and chloralose (38 mg/kg, i.p.) in the morning on the day of the experiment and a polyethylene (P-50) cannula was inserted into the left jugular vein. iBAT temperatures (°C) were measured using a precalibrated thermistor probe (YSI 427; Yellow Springs Instrument Co., Yellow Springs, OH, USA) surgically placed under the iBAT pad. Temperature was monitored until it reached a stable baseline (approximately 10 min), followed by infusion of norepinephrine (NE) (2 mg/ml) or the selective β1 agonist dobutamine (DB) (600 μg/ml) (Aikawa et al. 1996, Huang et al. 1998) through the left jugular vein using an infusion pump (model 2274, Harvard Apparatus, Holliston, MA, USA) at a rate of 0.643 μl/min for 30 min.
mRNA analysis
iBAT and liver were dissected and total RNA extracted using Trizol (Life Technologies, Inc.), according to the manufacturer’s instructions, and quantified by spectrophotometry. For the reverse transcriptase reaction, 1.0 μg total RNA was used in the ImProm-II Reverse Transcription System for RT-PCR (Promega), on a Robocycler thermocycler (Stratagene, La Jolla, CA, USA). Based on the reaction efficiency, about 120 ng of cDNA was used for amplification. Quantitative real-time PCR (RT-qPCR) was carried out using an IQ SYBR Green PCR kit (Bio-Rad) on an iCycler thermal cycler (Bio-Rad). The housekeeping gene cyclophilin A was used as an internal reference. Primer sequences were available upon request. The cycle conditions were as follows: 5 min at 94 °C, 30 s at 94 °C, 30 s at 58 °C, and 45 s at 72 °C for 50 cycles followed by the melting curve protocol to verify the specificity of amplicon generation. Gene expression was determined by the ΔΔCt method, as described previously (Christoffolete et al. 2004).
Western blot
iBAT was also processed for mitochondrial isolation. Mitochondrial proteins were then size-fractionated by 12% SDS–PAGE and probed with α-UCP1 (Santa Cruz Biotechnology).
Blood chemistry
Total serum cholesterol and triglycerides were assessed via enzymatic methods using a commercial kit (Roche Molecular Biochemicals).
Histology
After dissection, tissues were immersed in buffered formaldehyde solution and fixed for 24 h. Paraffin-embedded tissues were sectioned and processed as described for staining with hematoxylin–eosin or Masson’s trichrome (Kerr et al. 1995). The cellular area of the white adipocytes was measured by analyzing pictures of at least 40 adipocytes per animal taken at 200× magnification.
Statistical analysis
The statistical analyses were done by ANOVA followed by the Student–Newman–Keuls post-test when P<0.05 and by analysis of covariance (ANCOVA) followed by the Bonferroni post-test as indicated.
Results
Thermogenic profile of ARβ2KO animals in response to cold or infusion with catecholamines
Mice acclimatized to room temperature were exposed to acute cold temperature at 4 °C and core body temperatures were measured hourly for up to 4 h. Both WT controls and ARβ2KO mice maintained their core temperature (Fig. 1A) by progressively accelerating resting energy expenditure (REE) (Fig. 1B). The iBAT thermal response to infusion with a defined dose of catecholamines provides information about the BAT thermogenic capacity (Ribeiro et al. 2001). In WT animals acclimatized to room temperature, infusion of NE over 30 min resulted in an increase of approximately 2.0 °C in iBAT temperature, similar to what was observed in the ARβ2KO animals (Fig. 1C).
Figure 1.
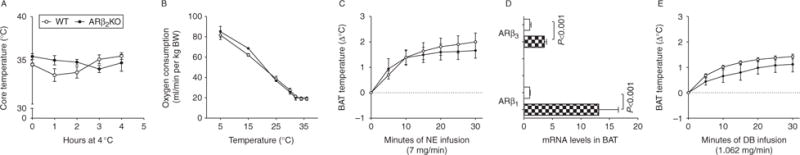
Effects of the lack of β2 adrenergic receptor on cold-induced thermogenesis. (A) Central body temperature during cold exposure (4 °C) for 3 h of WT and ARKOβ2; (B) oxygen consumption during exposure of WT and ARKOβ2 mice to decreasing temperatures; (C) brown adipose tissue thermogenic response to norepinephrine (NE) infusion of WTand ARKOβ2 mice; (D) gene expression of β1 and β3 adrenergic receptors in mice kept on a chow or HFD in BAT (cross hatched (black and white), WT; white, ARβ2KO); (E) brown adipose tissue thermogenic response during infusion of DB of WT and ARKOβ2 mice. Entries are mean±S.E.M. of five animals per group.
The possibility that ARβ2KO animals compensate for the disruption of the ARβ2 pathway by activating other β-adrenergic pathways was tested by measuring mRNA levels for Arβ1 and Arβ3 in the BAT of ARβ2KO animals. Indeed, mRNA levels for both ARs were greatly elevated in the ARβ2KO BAT (Fig. 1D). However, the iBAT thermal response to the selective β1 agonist DB was similar in WT and in β2KO animals (Fig. 1E), indicating that the ARβ2KO BAT maintains its thermogenic capacity, which can be maximally stimulated via the ARβ1 pathway.
Susceptibility of ARβ2KO mice to diet-induced obesity
Adult ARβ2KO mice exhibited similar caloric intake (Fig. 2A) and body weight (Fig. 2B). Mice were then fed a HFD and monitored for 8 weeks, these animals exhibited increased caloric intake with no differences between ARβ2KO and WT controls (Fig. 2A). At the same time, all animals gained weight and those on the HFD gained approximately twice as much, again with no differences between ARβ2KO and WT controls (Fig. 2B and C). REE as expressed in relation to BW (VO2 ml/min/g BW) was not different in ARβ2KO when compared with WT controls (Fig. 2D) even when data were plotted as a function of BW (Supplementary Figure 1A, see section on supplementary data given at the end of this article) as suggested previously (Tschop et al. 2012). At the same time, REE was accelerated by 8 weeks of HFD in both WT controls and ARβ2KO animals, albeit less so in the latter group (Fig. 2D). These differences between the two groups of animals remained even when data were plotted as a function of BW (Supplementary Figure 1B, C and D). When the same data are expressed in terms of absolute values (VO2 ml/min) and statistically controlled for the effects of body weight (covariant) then the HFD-induced acceleration in REE was only observed in the WT controls (Fig. 2E). The epididymal white adipocyte area (Fig. 2F) and Ucp1 levels also behaved similarly in both ARβ2KO and WT controls (Fig. 2G), except that ARβ2KO mice on a chow diet exhibited significantly higher Ucp1 levels when compared with WT controls.
Figure 2.
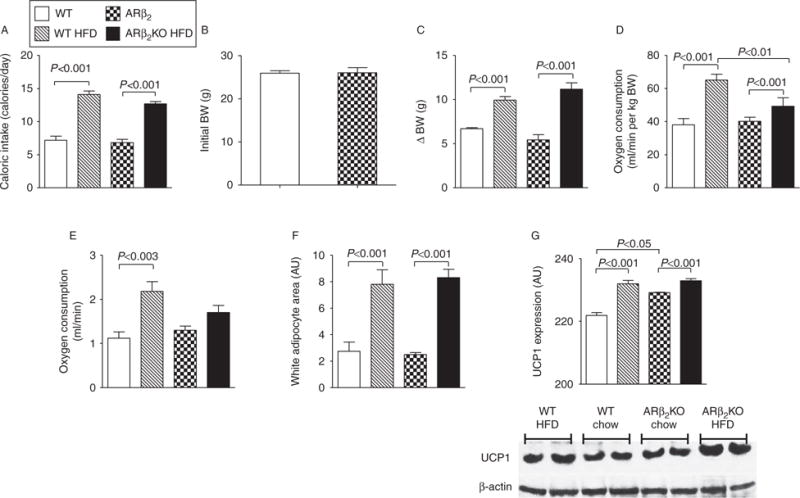
Effects of the lack of β2 adrenergic receptor on diet-induced thermogenesis. (A) Caloric intake calculated based on daily food consumption; (B) initial BW; (C) variances of BW (Δ BW) are shown; (D) VO2 assessed during 30 min at the end of the experiment expressed relative to BW (VO2 ml/min/g per BW); (E) VO2 assessed during a period of 30 min at the end of the experiment expressed as absolute values (VO2 ml/min) and statistically controlled for the effects of body weight (covariant); (F) estimated individual epididymal adipocytes area; 40 cells for each group were analyzed; (G) UCP1 expression determined by western blot. Values are mean±S.E.M. of five animals per group.
Glucose and insulin tolerance in ARβ2KO mice
Fasting blood glucose levels were monitored weekly during the 7-week experimental period (Fig. 3A) and no differences were observed until the end of the first week of HFD. From that point on, all animals on the HFD developed fasting hyperglycemia that was transiently more intense in the WT controls but that during weeks 5–7 became more pronounced in the ARβ2KO animals (Fig. 3A). Upon further investigation during week 8 of the experiment, all animals fed a HFD exhibited an apparent greater intolerance to glucose as assessed by the area under the curve of the GTT (Fig. 3B and C). However, when blood glucose was expressed at each time-point as a percentage of basal values, only the WT controls fed with the HFD exhibited mild glucose intolerance, whereas no differences were found in the ARβ2KO animals (Fig. 3D and E). Sensitivity to insulin was similar in ARβ2KO and WT controls, with both groups exhibiting a slight but significant decrease in the AUC when kept on a HFD (Fig. 3F and G). As expected, during week 7, insulin levels after overnight fasting were elevated in WT controls placed on the HFD (Fig. 3H). At the same time, insulin levels were dramatically elevated in the ARβ2KO animals regardless of the type of diet (Fig. 3H). The elevated fasting glucose – not associated with decreased insulin sensitivity or glucose intolerance – indicates that hepatic glucose production is accelerated in the ARβ2KO animals. This is in fact supported by the observation that liver Pepck (Pck1) mRNA levels are doubled in the ARβ2KO animals kept on chow diet and further elevated in both strains by HFD (Fig. 3I).
Figure 3.
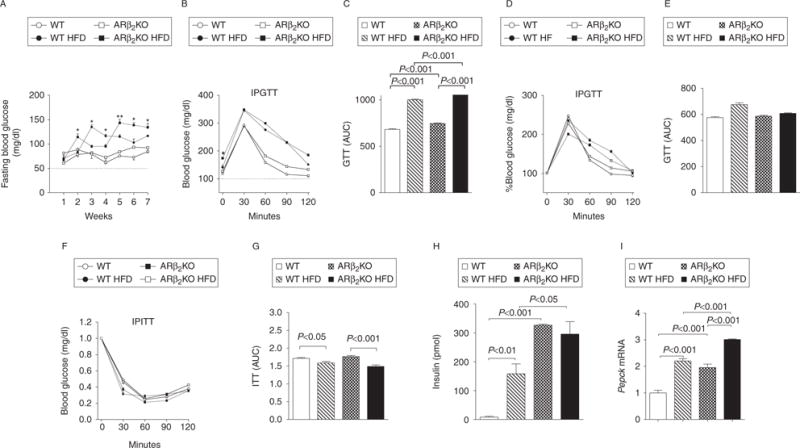
Effects of the lack of β2 adrenergic receptor on glucose and insulin tolerance of mice kept on a chow diet or on a HFD. (A) Fasting blood glucose levels during the 7-week experimental period; (B and C) blood glucose levels before and after i.p. administration of 2 g/kg glucose (GTT) expressed as absolute values and (D and E) expressed at each time-point as a percentage of basal values; (F and G) blood glucose levels after i.p. injection of 0.5 U/kg insulin (ITT); (H) insulin serum levels after overnight fasting and (I) Pepck mRNA levels in liver of WT and ARβ2KO animals kept on chow or high-fat diets. (A) *P<0.05 and **P<0.01 vs WT HFD. Entries are mean±S.E.M. of five animals per group.
Liver steatosis in ARβ2KO mice
Feeding on a HFD caused liver steatosis indistinctly in both groups of animals, i.e. ARβ2KO and WT animals (Fig. 4).
Figure 4.
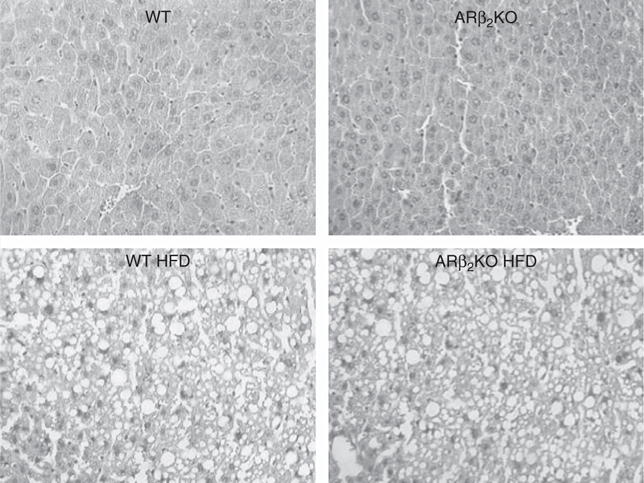
Liver steatosis in ARβ2KO mice fed HFD. Liver sections stained by H&E; magnification is 180×.
Cholesterol and triglyceride plasma levels in ARβ2KO mice
No differences in serum cholesterol and triglycerides were observed between ARβ2KO and WT animals. Both parameters increased similarly in both groups of animals after they were fed a HFD.
Discussion
It is well recognized that ARβs play a significant role in metabolic control and energy homeostasis, including adaptive thermogenesis (Bachman et al. 2002). The results of the present studies indicate that inactivation of the ARβ2 pathway interferes with glucose homeostasis, most probably by accelerating hepatic neoglucogenesis and glucose production, which is largely neutralized by a marked elevation in insulin levels. However, feeding the animals with a HFD stresses the systems further, beyond what can be compensated for by an elevation of insulin secretion, resulting in fasting hyperglycemia and relative glucose intolerance.
Although data obtained in the ARβ1KO mouse indicated that the ARβ1 pathway is important for BAT thermogenesis (Ueta et al. 2012), the present studies indicate that the role played by ARβ2 in BAT thermogenesis is much less significant and possibly redundant. Animals with inactivation of the ARβ2 pathway exhibit a normal baseline metabolic profile and respond indistinguishably from WT controls when exposed to cold, or treated with infusions of adrenergic agonists, but exhibit lesser acceleration of REE when fed a HFD. Alternatively, it is conceivable that the ARβ2 pathway plays an important role in BAT thermogenesis and the apparent lack of a thermogenic phenotype is explained by the overexpression of ARβ1 and ARβ3 in BAT. Thus, ARβ2KO mice provide a suitable model for studying the interrelationships between the ARβ2 pathway and glucose homeostasis, which is also observed in humans. However, the minor role played by the ARβ2 pathway in BAT thermogenesis is in contrast to the wealth of literature supporting a role for ARβ2 in energy homeostasis and adaptive thermogenesis in humans (Hoeks et al. 2003). If confirmed, this could indicate important differences in the mechanisms involved in energy homeostasis between the two species.
ARβ2 activation promotes dilation of smooth muscle in different organs and structures but also plays an important role in energy homeostasis by slowing peristaltic movements and gastrointestinal secretion, as well as promoting lipolysis, insulin secretion, gluconeogenesis, and glycogenolysis, thus leading to overall lower levels of blood glucose (Gardner & Shoback 2007). Therefore, it is notable that ARβ2KO animals exhibit a marked elevation in serum insulin when on a chow diet and, when on a HFD they exhibit greater disruption in glucose homeostasis, with fasting hyperglycemia and relative glucose intolerance. Such findings are reminiscent of those observed in humans exposed to long-term overfeeding. Subjects with the ARβ2 Gln27Gln polymorphism or the 3.7/3.4 kb BanI variant experienced a greater increase in insulin resistance than Glu27Glu/Gln27Glu subjects (Ukkola & Bouchard 2001). Furthermore, in obese postmenopausal women, different ARβ2 haplotypes were associated with glucose intolerance, and thus may mediate insulin action, glucose tolerance, and potentially risk for type 2 diabetes mellitus (Prior et al. 2011).
Previous studies with β agonists-induced activation of glucose uptake in astrocytes and skeletal muscle cells indicated a key role of β2 in SNS-mediated glucose uptake, allowing for synergy between SNS and insulin signaling (Nevzorova et al. 2006, Catus et al. 2011). This is in contrast to the present observation that overall sensitivity to insulin is not modified in the ARβ2KO mice (Fig. 3F and G). At the same time, ARβ1 and ARβ3 have been recognized as key mediators in glucose uptake (Nikami et al. 1996, Chernogubova et al. 2004) and our data supports these findings. In addition, while metoprolol, a β1 antagonist, worsens insulin resistance, carvedilol, a β2 and α1 antagonist, decreases insulin resistance in type 2 diabetes patients (Phillips et al. 2008).
The role of the ARβ2 pathway in adaptive thermogenesis was tested during cold exposure or feeding on a HFD, two processes that involve multiple organs and tissues, e.g. hypothalamus, skeletal muscle, liver, and white adipose tissue and BAT. The latter is where most heat is generated in response to SNS stimulation (Enerback et al. 1997). Thus, the present data do not support a major role for ARβ2 in cold- or diet-induced thermogenesis. First, the ARβ2KO animals maintained their core temperature and increased VO2 during acute cold exposure (4 h), indicating normal BAT function. That this is a process primarily dependent on BAT is illustrated by the dramatic hypothermia developed during a similar time frame of cold exposure in UCP-1KO mice (Enerback et al. 1997). That the BAT thermogenesis is largely preserved in the ARβ2KO animals is further verified by the observation that their iBAT responds normally to NE infusion. Notably, the only biochemical difference observed in the ARβ2KO BAT was a slightly elevated baseline mitochondrial UCP1 content, a finding of undetermined significance. Second, when fed a HFD the overall thermogenic response seems to be preserved in the ARβ2KO animals given that caloric intake, weight gained, and the size of white fat depots behaved similarly to those of WT controls. This occurred despite lesser acceleration of the REE, at least as assessed through a limited 1 h-diurnal VO2 study.
ARβ2 is present (Sell et al. 2004) and fully functional (Atgie et al. 1997) in the murine BAT. Although its inactivation would be expected to result in a detectable phenotype, previous studies have indicated that over-expression of other Arβ isoforms could provide a compensatory mechanism. For example, increased signaling via the ARβ1 or ARβ2 pathways was thought to explain the resistance to diet-induced obesity of ARβ3KO mice (Atgie et al. 1997). In fact, there is marked overexpression of Arβ1 and Arβ3 mRNA in the BAT of ARβ2KO animals. However, when treated with infusions of the ARβ1-selective agonist, DB, ARβ2KO animals exhibited a similar iBAT thermal response, indicating that the ARβ1 is not hyper-responsive to stimulation. Although this could reflect limitations in other steps of the BAT thermogenic pathway, at face value these data strengthen the argument that ARβ2 plays only a minor role if any in BAT thermogenesis.
The present data indicate that ARβ2 does not play a significant role in lipid metabolism, given that ARβ2KO mice exhibit similar hyperlipidemia when fed a HFD. In addition, ARβ2KO animals fed a HFD developed liver steatosis indistinguishable from WT controls. These data are relevant considering that ARβ blockers have been implicated in the increase in triglycerides and cholesterol plasma levels of hypertensive patients (Sarafidis & Bakris 2006, Bell et al. 2009). Also, patients with the ARβ2-Glu27 variant have hypertriglyceridemia with elevated cholesterol levels (Iaccarino et al. 2005). However, long-term treatment with oral broxaterol, a selective ARβ2 agonist, has minimal metabolic effects on lipid metabolism (Petraglia et al. 1990). Thus, it is not clear that ARβ2KO mice would be useful for modelling the role played by the ARβ2 pathway in lipid metabolism in humans.
In conclusion, the ARβ2KO pathway does play a significant role in glucose homeostasis, probably by accelerating hepatic glucose production that is largely neutralized by increased insulin secretion. Fasting hyperglycemia only occurs if the system is further stressed, for example by feeding a HFD. The study of the ARβ2KO mouse also indicated that the ARβ2 pathway plays a less significant role in the acute BAT thermogenic response and overall diet-induced thermogenesis.
Supplementary Material
Acknowledgments
Funding
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and MackPesquisa.
Footnotes
Supplementary data
This is linked to the online version of the paper at http://dx.doi.org/10.1530/JOE-13-0526.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Aikawa J, Fukazawa M, Ishikawa M, Moroi M, Namiki A, Yamaguchi T. Vascular smooth muscle relaxation by α-adrenoceptor blocking action of dobutamine in isolated rabbit aorta. Journal of Cardiovascular Pharmacology. 1996;27:33–36. doi: 10.1097/00005344-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Atgie C, D’Allaire F, Bukowiecki LJ. Role of β1- and β3-adrenoceptors in the regulation of lipolysis and thermogenesis in rat brown adipocytes. American Journal of Physiology. 1997;273:C1136–C1142. doi: 10.1152/ajpcell.1997.273.4.C1136. [DOI] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. βAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- Bell DS, Bakris GL, McGill JB. Comparison of carvedilol and metoprolol on serum lipid concentration in diabetic hypertensive patients. Diabetes, Obesity & Metabolism. 2009;11:234–238. doi: 10.1111/j.1463-1326.2008.00927.x. [DOI] [PubMed] [Google Scholar]
- Bianco AC, Anderson G, Forrest D, Galton VA, Gereben B, Kim BW, Kopp PA, Liao XH, Obregon MJ, Peeters RP, et al. American thyroid association guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid. 2014;24:88–168. doi: 10.1089/thy.2013.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Catus SL, Gibbs ME, Sato M, Summers RJ, Hutchinson DS. Role of β-adrenoceptors in glucose uptake in astrocytes using β-adrenoceptor knockout mice. British Journal of Pharmacology. 2011;162:1700–1715. doi: 10.1111/j.1476-5381.2010.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernogubova E, Cannon B, Bengtsson T. Norepinephrine increases glucose transport in brown adipocytes via β3-adrenoceptors through a cAMP, PKA, and PI3-kinase-dependent pathway stimulating conventional and novel PKCs. Endocrinology. 2004;145:269–280. doi: 10.1210/en.2003-0857. [DOI] [PubMed] [Google Scholar]
- Christoffolete MA, Linardi CC, de Jesus L, Ebina KN, Carvalho SD, Ribeiro MO, Rabelo R, Curcio C, Martins L, Kimura ET, et al. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes. 2004;53:577–584. doi: 10.2337/diabetes.53.3.577. [DOI] [PubMed] [Google Scholar]
- Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the β2 adrenergic receptor gene. Journal of Biological Chemistry. 1999;274:16694–16700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- Collins S, Surwit RS. The β-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Progress in Hormone Research. 2001;56:309–328. doi: 10.1210/rp.56.1.309. [DOI] [PubMed] [Google Scholar]
- Curcio C, Lopes AM, Ribeiro MO, Francoso OA, Jr, Carvalho SD, Lima FB, Bicudo JE, Bianco AC. Development of compensatory thermogenesis in response to overfeeding in hypothyroid rats. Endocrinology. 1999;140:3438–3443. doi: 10.1210/endo.140.8.6906. [DOI] [PubMed] [Google Scholar]
- Echwald SM, Sorensen TI, Tybjaerg-Hansen A, Andersen T, Pedersen O. Gln27Glu variant of the human β2-adrenoreceptor gene is not associated with early-onset obesity in Danish men. Diabetes. 1998;47:1657–1658. doi: 10.2337/diabetes.47.10.1657. [DOI] [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- Gardner D, Shoback D. Greenspan’s Basic and Clinical Endocrinology. 8th. New York: McGraw-Hill Companies, Incorporated; 2007. [Google Scholar]
- Hall JA, Ribich S, Christoffolete MA, Simovic G, Correa-Medina M, Patti ME, Bianco AC. Absence of thyroid hormone activation during development underlies a permanent defect in adaptive thermogenesis. Endocrinology. 2010;151:4573–4582. doi: 10.1210/en.2010-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeks J, van Baak MA, Hesselink MK, Hul GB, Vidal H, Saris WH, Schrauwen P. Effect of β1- and β2-adrenergic stimulation on energy expenditure, substrate oxidation, and UCP3 expression in humans. American Journal of Physiology Endocrinology and Metabolism. 2003;285:E775–E782. doi: 10.1152/ajpendo.00175.2003. [DOI] [PubMed] [Google Scholar]
- Huang Y, Kwok KH, Chan NW, Lau CW, Chen ZY. Role of endothelium and K+ channels in dobutamine-induced relaxation in rat mesenteric artery. Clinical and Experimental Pharmacology & Physiology. 1998;25:405–411. doi: 10.1111/j.1440-1681.1998.tb02223.x. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Trimarco V, Lanni F, Cipolletta E, Izzo R, Arcucci O, De Luca N, Di Renzo G. β-blockade and increased dyslipidemia in patients bearing Glu27 variant of β2 adrenergic receptor gene. Pharmacogenomics Journal. 2005;5:292–297. doi: 10.1038/sj.tpj.6500324. [DOI] [PubMed] [Google Scholar]
- de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. Journal of Clinical Investigation. 2001;108:1379–1385. doi: 10.1172/JCI200113803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez M, Leger B, Canola K, Lehr L, Arboit P, Seydoux J, Russell AP, Giacobino JP, Muzzin P, Preitner F. β1/β2/β3-adrenoceptor knockout mice are obese and cold-sensitive but have normal lipolytic responses to fasting. FEBS Letters. 2002;530:37–40. doi: 10.1016/S0014-5793(02)03387-2. [DOI] [PubMed] [Google Scholar]
- Jocken JW, Blaak EE, Schiffelers S, Arner P, van Baak MA, Saris WH. Association of a beta-2 adrenoceptor (ADRB2) gene variant with a blunted in vivo lipolysis and fat oxidation. International Journal of Obesity. 2007;31:813–819. doi: 10.1038/sj.ijo.0803499. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Gobe GC, Winterford CM, Harmon BV. Anatomical methods in cell death. Methods in Cell Biology. 1995;46:1–27. doi: 10.1016/S0091-679X(08)61921-4. [DOI] [PubMed] [Google Scholar]
- Kortner B, Wolf A, Wendt D, Beisiegel U, Evans D. Lack of association between a human β-2 adrenoceptor gene polymorphism (gln27glu) and morbid obesity. International Journal of Obesity and Related Metabolic Disorders. 1999;23:1099–1100. doi: 10.1038/sj.ijo.0801063. [DOI] [PubMed] [Google Scholar]
- Large V, Hellstrom L, Reynisdottir S, Lonnqvist F, Eriksson P, Lannfelt L, Arner P. Human Beta-2 adrenoceptor gene polymorphisms are highly frequent in obesity and associate with altered adipocyte Beta-2 adrenoceptor function. Journal of Clinical Investigation. 1997;100:3005–3013. doi: 10.1172/JCI119854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner I, Bottermann P, Erhardt F, Linke R, Loffler G, Maier V, Schwandt P, Vogt W, Scriba PC. Group experiments on the radioimmunological insulin determination. Hormone and Metabolic Research. 1974;6:293–296. doi: 10.1055/s-0028-1093851. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metabolism. 2010;11:268–272. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Nevzorova J, Evans BA, Bengtsson T, Summers RJ. Multiple signalling pathways involved in β2-adrenoceptor-mediated glucose uptake in rat skeletal muscle cells. British Journal of Pharmacology. 2006;147:446–454. doi: 10.1038/sj.bjp.0706626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikami H, Shimizu Y, Sumida M, Minokoshi Y, Yoshida T, Saito M, Shimazu T. Expression of β3-adrenoceptor and stimulation of glucose transport by β3-agonists in brown adipocyte primary culture. Journal of Biochemistry. 1996;119:120–125. doi: 10.1093/oxfordjournals.jbchem.a021196. [DOI] [PubMed] [Google Scholar]
- Oberkofler H, Esterbauer H, Hell E, Krempler F, Patsch W. The Gln27Glu polymorphism in the β2-adrenergic receptor gene is not associated with morbid obesity in Austrian women. International Journal of Obesity and Related Metabolic Disorders. 2000;24:388–390. doi: 10.1038/sj.ijo.0801180. [DOI] [PubMed] [Google Scholar]
- Petraglia A, Scarpitta M, Ansalone D, Gurrieri G, Galzerano V, Calvanese RC, Federico S, Zinno A. Negligible metabolic effects of long-term oral treatment with a new β2-agonist: broxaterol. International Journal of Clinical Pharmacology Research. 1990;10:299–304. [PubMed] [Google Scholar]
- Phillips RA, Fonseca V, Katholi RE, McGill JB, Messerli FH, Bell DS, Raskin P, Wright JT, Jr, Iyengar M, Anderson KM, et al. Demographic analyses of the effects of carvedilol vs metoprolol on glycemic control and insulin sensitivity in patients with type 2 diabetes and hypertension in the Glycemic Effects in Diabetes Mellitus: Carvedilol-Metoprolol Comparison in Hypertensives (GEMINI) study. Journal of the Cardiometabolic Syndrome. 2008;3:211–217. doi: 10.1111/j.1559-4572.2008.00017.x. [DOI] [PubMed] [Google Scholar]
- Prior SJ, Goldberg AP, Ryan AS. ADRB2 haplotype is associated with glucose tolerance and insulin sensitivity in obese postmenopausal women. Obesity. 2011;19:396–401. doi: 10.1038/oby.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro MO, Carvalho SD, Schultz JJ, Chiellini G, Scanlan TS, Bianco AC, Brent GA. Thyroid hormone–sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform-specific. Journal of Clinical Investigation. 2001;108:97–105. doi: 10.1172/JCI200112584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK. Cardiovascular and metabolic alterations in mice lacking both β1- and β2-adrenergic receptors. Journal of Biological Chemistry. 1999;274:16701–16708. doi: 10.1074/jbc.274.24.16701. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- Sarafidis PA, Bakris GL. Do the metabolic effects of β blockers make them leading or supporting antihypertensive agents in the treatment of hypertension. Journal of Clinical Hypertension. 2006;8:351–356. doi: 10.1111/j.1524-6175.2005.04679.x. quiz 357–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell H, Deshaies Y, Richard D. The brown adipocyte: update on its metabolic role. International Journal of Biochemistry & Cell Biology. 2004;36:2098–2104. doi: 10.1016/j.biocel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, Himms-Hagen J, Flier JS, Lowell BB. Targeted disruption of the β3-adrenergic receptor gene. Journal of Biological Chemistry. 1995;270:29483–29492. doi: 10.1074/jbc.270.49.29483. [DOI] [PubMed] [Google Scholar]
- Takenaka A, Nakamura S, Mitsunaga F, Inoue-Murayama M, Udono T, Suryobroto B. Human-specific SNP in obesity genes, adrenergic receptor beta 2 (ADRB2), beta 3 (ADRB3), and PPARγ2 (PPARG), during primate evolution. PLoS ONE. 2012;7:e43461. doi: 10.1371/journal.pone.0043461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, Eckel RH, Farese RV, Jr, Galgani JE, Hambly C, et al. A guide to analysis of mouse energy metabolism. Nature Methods. 2012;9:57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta CB, Fernandes GW, Capelo L, Fonseca TL, Maculan FD, Gouveia C, Brum PC, Christoffolete MA, Aoki MS, Lancelloti CL, et al. β1 adrenergic receptor is key to cold- and diet-induced thermogenesis in mice. Journal of Endocrinology. 2012;214:359–365. doi: 10.1530/JOE-12-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukkola O, Bouchard C. Clustering of metabolic abnormalities in obese individuals: the role of genetic factors. Annals of Medicine. 2001;33:79–90. doi: 10.3109/07853890109002062. [DOI] [PubMed] [Google Scholar]
- Vosselman MJ, Brans B, van der Lans AA, Wierts R, van Baak MA, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue activity after a high-calorie meal in humans. American Journal of Clinical Nutrition. 2013;98:57–64. doi: 10.3945/ajcn.113.059022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


