Abstract
Approximately 80% of breast cancers express the estrogen receptor-α (ERα) and are treated with anti-estrogens. Resistance to these agents is a major cause of mortality. We have shown that estrogen inhibits Notch, whereas anti-estrogens or estrogen withdrawal activate Notch signaling. Combined inhibition of Notch and estrogen signaling has synergistic effects in ERα-positive breast cancer models. However, the mechanisms whereby Notch-1 promotes the growth of ERα-positive breast cancer cells are unknown. Here, we demonstrate that Notch-1 increases the transcription of ERα-responsive genes in the presence or absence of estrogen via a novel chromatin crosstalk mechanism. Our data support a model in which Notch-1 can activate the transcription of ERα-target genes via IKKα-dependent cooperative chromatin recruitment of Notch–CSL–MAML1 transcriptional complexes (NTC) and ERα, which promotes the recruitment of p300. CSL binding elements frequently occur in close proximity to estrogen-responsive elements (EREs) in the human and mouse genomes. Our observations suggest that a hitherto unknown Notch-1/ERα chromatin crosstalk mediates Notch signaling effects in ERα-positive breast cancer cells and contributes to regulate the transcriptional functions of ERα itself.
Keywords: breast cancer, estrogen, ERα, IKKα, Notch-1
Introduction
The Notch pathway regulates cell fate specification, differentiation, proliferation and apoptosis (Artavanis-Tsakonas et al., 1999). Notch activates the expression of target genes via CSL factors (Artavanis-Tsakonas et al., 1999; Nickoloff et al., 2003; Miele, 2006). Notch targets include members of the HES (Artavanis-Tsakonas et al., 1999), HERP (Iso et al., 2001) and HEY (Maier and Gessler, 2000) families, p21Cip/Waf (Rangarajan et al., 2001), c-Myc (Klinakis et al., 2006; Weng et al., 2006), nuclear factor-κB subunits (Cheng et al., 2001), cyclin-D1 (Ronchini and Capobianco, 2001) and cyclin-A (Baonza and Freeman, 2005). Mammals have four Notch paralogs (Notch-1 through Notch-4) and five Notch ligands (delta-1, 3, 4 and Jagged-1 and 2). Notch also binds non-CSL transcription factors such as HIF-1α (hypoxia-inducible factor-1α; Gustafsson et al., 2005) and β-catenin (Hayward et al., 2005), as well as Nur77, a nuclear-receptor-superfamily protein (Jehn et al., 1999).
Recent data indicate that Notch signaling is critical in mammary development (Dontu et al., 2004) and in mammary stem cell function and luminal fate commitment (Bouras et al., 2008). Emerging evidence indicates that Notch signaling is frequently activated in breast cancer (Stylianou et al., 2006). Notch activity has been suggested to correlate with proliferation, antiapoptosis and tumor progression in breast cancer (Miele, 2008). High expression of Notch-1 and Jagged-1 is associated with poor prognosis (Reedijk et al., 2005, 2008; Dickson et al., 2007). Constitutively active Notch-1 or Notch-4 cause mammary tumors in mice (Gallahan et al., 1996; Gallahan and Callahan, 1997; Callahan and Raafat, 2001; Callahan and Egan, 2004; Kiaris et al., 2004).
17β-Estradiol (henceforth, E2) promotes the growth of estrogen receptor-α (ERα)-positive breast cancer cells. Canonical ERα-responsive genes contain estrogen-responsive elements (EREs; Green and Carroll, 2007), whereas other genes recruit ERα through transcription factors such as AP1 or SP1 (Porter et al., 1997; Jakacka et al., 2001). Numerous protein complexes participate in ERα-mediated gene regulation (Green and Carroll, 2007). The best known ER coactivators are p160 family members, including steroid receptor coactivator-1 (SRC-1), AIB1/SRC-3 and SRC-2/TIF2/GRIP1. p160 proteins can recruit other coactivators such as CBP, p300 and P/CAF, which possess histone acetyltransferase activity (Perissi and Rosenfeld, 2005; Green and Carroll, 2007). The phosphorylation status of ERα regulates its activity (Cenni and Picard, 1999). ERα can be phosphorylated at multiple serine residues by various kinases, including IKKα (Park et al., 2005), mitogen-activated protein kinases (Kato et al., 1995; Bunone et al., 1996) and Akt (Martin et al., 2000; Campbell et al., 2001). IKKα-kinase activity is required for E2-mediated ERα phosphorylation and activation of downstream gene expression (Park et al., 2005).
In addition to E2, multiple growth factors such as insulin, IGF-1 and EGF can activate ERα through mitogen-activated protein kinases (Kato et al., 1995; Bunone et al., 1996); cyclin-D1, which is frequently overexpressed or amplified in breast cancer, can bind ERα, recruit SRC-family coactivators and activate downstream gene expression in the absence of E2 (Neuman et al., 1997; Zwijsen et al., 1997).
We have recently shown that in ERα-positive breast cancer cells E2 inhibits Notch signaling by modulating Notch activation (Rizzo et al., 2008). Conversely, E2 deprivation or treatment with 4-hydroxytamoxifen re-activates Notch signaling and increases dependence on it for survival. Notch-1 knockdown causes growth arrest in T47D and MCF-7 cells, and potentiates the effects of 4-hydroxytamoxifen. Treatment of T47D xenografts with a combination of tamoxifen and a pharmacological Notch inhibitor (γ-secretase inhibitor (GSI)) caused tumor regression. These observations posed a fundamental question: Does Notch regulate a subset of ERα-target genes?
Results
Active Notch-1 facilitates transcription of ERα-target genes in the absence of E2
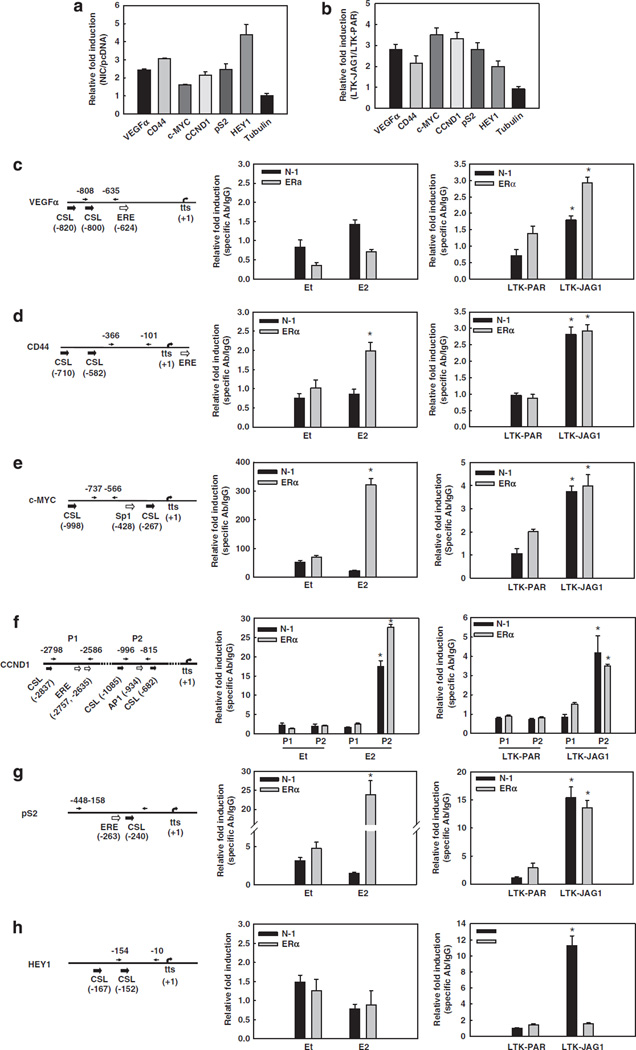
We hypothesized that Notch activation in the absence of E2 may rescue the expression of some critical E2-target genes. Two well-known E2-target genes, c-Myc and cyclin-D1, are also potential Notch targets in the mammary gland (Kiaris et al., 2004; Klinakis et al., 2006). Exploratory reverse transcription–PCR (RT– PCR) array studies indicated that expression of Notch-1IC (NIC) in MCF-7 cells in charcoal-stripped (E2-deficient) medium transactivated a number of E2-target genes (data not shown). Validation experiments by real-time RT–PCR confirmed that E2-target genes vascular endothelial growth factor-α (VEGFα), CD44, cyclin-D1, c-Myc and pS2, and Notch-target gene HEY1, but not β-tubulin, are upregulated by NIC in charcoal-stripped medium (Figure 1a). We confirmed that E2 induces the expression of VEGFα, CD44, cyclin-D1, c-Myc and pS2 in the cells used in this study (Supplementary Figure 1).
Figure 1.
Active Notch-1 facilitates the transcription of ERα-target promoters in the absence of E2. In all experiments, MCF-7 cells were grown in phenol red-free RPMI containing 10% DCC-fetal bovine serum for 3 days prior to harvest. (a) MCF-7 cells were transiently transfected with the active form of Notch-1 (NIC) or pcDNA vector control. The mRNA levels of VEGFα, CD44, c-MYC, CCND1, pS2, HEY1 and β-tubulin were measured by real-time RT–PCR after 48 h after transfection. Values are expressed as relative fold induction by NIC over pcDNA, after internal normalization for 18S rRNA. (b) MCF-7 cells were co-cultured with mouse fibroblasts expressing Jagged-1 (LTK–JAG1) or vector-transfected controls (LTK–PAR) for 12 h prior to harvest. The mRNA levels of VEGFα, CD44, c-MYC, CCND1, pS2, HEY1 and β-tubulin were measured by real-time RT–PCR using validated human-specific primers. Values are expressed as relative fold induction by LTK–JAG1 over LTK–PAR, after internal normalization for RPL13a mRNA. (c–h) The schematics of the indicated promoters and ChIP assays. Charcoal-stripped MCF-7 cells were treated with 5 nm E2 or ethanol (vehicle) for 1 h (left), or co-cultured with LTK cells for 3 h (right) before formaldehyde fixation. ChIP assays were performed with antibodies to Notch-1 or ERα, followed by real-time PCR analysis of the indicated regions of each promoter (arrows). Values are expressed as relative fold increase of specific antibody pull-down over IgG control, after normalization for internal control RPL13a. tts, transcription start site; *P < 0.001. ChIP, chromatin immunoprecipitation; ERα, estrogen receptor-α; LTK–JAG1, mouse LTK fibroblasts expressing Notched ligand Jagged-1; LTK–PAR, control vector transduced parental fibroblast; NIC, active form of Notch-1 (Notch-1IC); RPMI, Rosewell Park Memorial Institute; RT–PCR, reverse transcription–PCR; VEGF, vascular endothelial growth factor.
We then activated endogenous Notch by co-culturing MCF-7 cells in charcoal-stripped medium with mouse LTK fibroblasts engineered to express Notch ligand Jagged-1 (LTK–JAG1) or control vector-transduced parental fibroblasts (LTK–PAR). After 12 h of co-culture, mRNA levels of all tested E2 targets were significantly upregulated by Jagged-1-expressing fibroblasts as compared with that by control fibroblasts (Figure 1b), whereas β-tubulin was not affected. Since Jagged-1 could potentially activate all four Notch receptors, we used a specific Notch-1 short interfering RNA (siRNA) and determined that Notch-1 knockdown prevented Jagged-1-expressing fibroblasts from activating pS2 transcription, whereas Notch-2 or Notch-4 siRNA did not affect the stimulation of pS2 expression by Jagged-1 (Supplementary Figure 2).
Next, we investigated the mechanism of Notch-mediated induction of these E2-target genes. Sequence analysis revealed putative CSL-binding sites in proximity of ERα-binding elements in these promoters. These elements included canonical EREs, and in the case of cyclin-D1, an AP1 consensus sequence known to mediate ERα recruitment (Shen et al., 2008). The c-Myc promoter contains an Sp1 site and an ERE half-site (Dubik and Shiu, 1992) within 2 kb of the transcriptional start (Figures 1c – g). We hypothesized that Notch-1 may be co-recruited with ERα. We performed a series of chromatin immunoprecipitation (ChIP) experiments to detect Notch-1 and ERα at each of those promoters under different conditions. First, we confirmed E2-dependent recruitment of ERα to those promoters after short-term E2 treatment (Figures 1c–g, left). Interestingly, E2 also caused significant recruitment of Notch-1 to the cyclin-D1 promoter, but not any other promoters that we analysed. This may suggest that Notch-1 participates in the transcriptional regulation of a subset of E2-target genes in the presence of E2. When we activated Notch in charcoal-stripped medium by co-culturing MCF-7 cells with Jagged-1-expressing mouse fibroblasts, Jagged-1 caused significant increases in promoter occupancy of both Notch-1 and ERα on all the promoters studied (Figures 1c–g, right). The induction was comparable to the increase of Notch-1 on the HEY1 promoter. Interestingly, ERα does not bind to the VEGFα promoter unless Notch is activated (Figure 1c). These observations supported the hypothesis that Notch can activate a subset of ERα-target genes under E2-free conditions, and that Notch-1 is co-recruited to these promoters with ERα.
Notch activation increases ER-dependent transcription
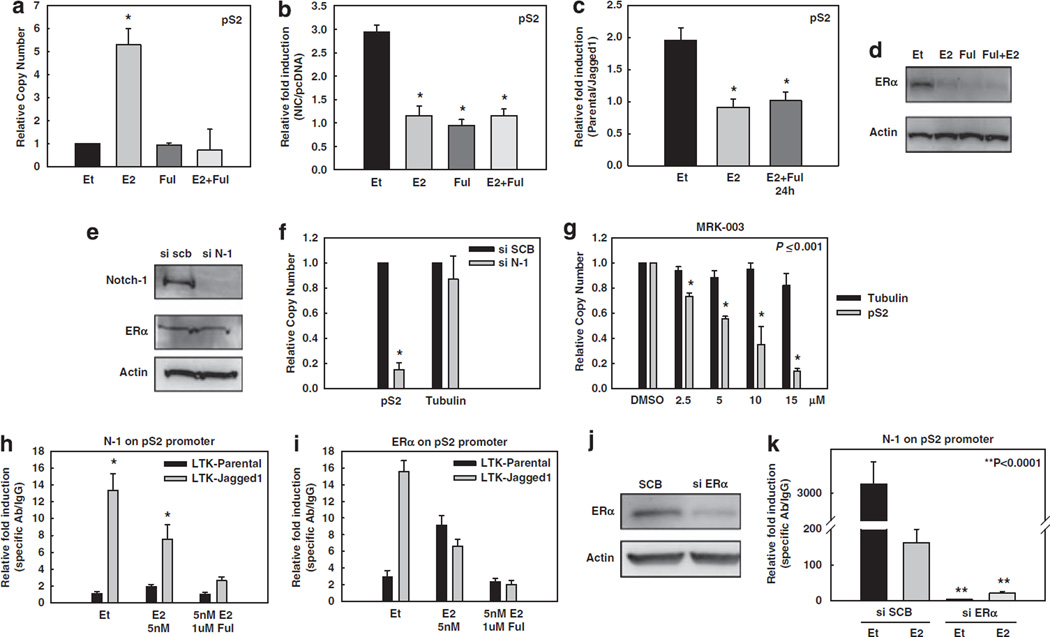
In order to further investigate the molecular mechanism(s) whereby Notch activates ER-dependent transcription, we chose a well-characterized experimental model. The gene for TFF1/pS2 is an extensively studied model of transcriptional regulation by E2. Its promoter contains a perfect half-ERE at position −263, which is used by ERα (Jeltsch et al., 1987) as well as other regulatory elements within a relatively short DNA sequence (Nunez et al., 1989; Gillesby et al., 1997). We detected a putative CSL-binding site at position −240. Real-time RT–PCR experiments showed that pS2 mRNA level was increased by E2 as expected. This effect was abrogated by fulvestrant, which causes ER degradation (Figure 2a). NIC expression in charcoal-stripped medium also significantly stimulated pS2 expression (Figure 2b). In the presence of E2, NIC did not cause any additional stimulation over that achieved by E2. Fulvestrant abolished NIC -mediated stimulation of pS2 expression, suggesting that ER is required for this Notch effect (Figure 2b). Conversely, neither E2 nor fulvestrant affected NIC -induced HEY1 expression (Supplementary Figure 3).
Figure 2.
Notch activation increases ER-dependent transcription. In all experiments, MCF-7 cells were grown in phenol red-free RPMI containing 10% DCC-fetal bovine serum for 3 days prior to harvest. (a–g) pS2 real-time RT–PCR experiments: (a) Untransfected MCF-7 cells were treated with 5 nm of E2 (4 h), 1 µm fulvestrant (24 h) or the combination before harvest. Data are expressed as relative copy number normalized to internal control (18S rRNA); *P ⩽ 0.001. (b) Twelve hours after serum starvation, MCF-7 cells were transfected with NIC or pcDNA vector control. Cells were treated with 5 nm of E2 (4 h), 1 µM fulvestrant (24 h) or a combination of both before harvest. Data are expressed as relative fold induction of NIC over pcDNA after normalization to the internal control 18S rRNA; *P ⩽ 0.001. (c) MCF-7 cells were treated with E2 alone or in combination with fulvestrant as described above, and co-cultured with LTK–JAG1 cells for 12 h. Data are expressed as relative fold induction by LTK–JAG1 over LTK–PAR cells after normalization to internal control RPL13a; *P ⩽ 0.001. (d) Western blot of ERα after the treatments described above. (e) Western blot of MCF-7 cells transfected with Notch-1 siRNA or scrambled control (SCB). (f) MCF-7 cells transfected with Notch-1 siRNA or SCB; *P ⩽ 0.001. (g) MCF-7 cells were treated with increasing concentrations of GSI for 24 h. Data are expressed as relative copy number normalized to internal control (18S rRNA). (h, i) ChIP assay on the pS2 promoter. MCF-7 cells were treated with 5 nm E2, ethanol control for 1 h or E2 in combination with 1 µm fulvestrant (24 h) after 3 days of charcoal stripping and co-cultured with LTK–JAG1 cells for 3 h. Data expressed as relative fold increase of specific antibody over IgG control, after normalization to internal control RPL13a. (j) MCF-7 cells were grown in charcoal-stripped media for 3 days and transfected with ERα siRNA or SCB. The western blot shows efficient downregulation of ERα. Actin was used as a loading control. (k) ChIP assay on the pS2 promoter with cells transfected with siRNA to ERα (as described above), co-cultured with LTK–JAG fibroblasts for 3 h and treated with 5 nm E2 or ethanol for 1 h. ChIP, chromatin immunoprecipitation; ERα, estrogen receptor-α; GSI, γ-secretase inhibitor; LTK–JAG1, mouse LTK fibroblasts expressing Notched ligand Jagged-1; LTK–PAR, control vector-transduced parental fibroblast; NIC, active form of Notch-1 (Notch-1IC); RPMI, Rosewell Park Memorial Institute; RT–PCR, reverse transcription–PCR; siRNA, short interfering RNA.
We conducted similar experiments, this time activating Notch by co-culture with Jagged-1-expressing mouse fibroblasts. The results were essentially identical. Jagged-1 stimulation increased pS2 expression in charcoal-stripped medium, but did not further enhance the effects of E2. Fulvestrant abolished the effects of Jagged-1 (Figure 2c). ERα levels were verified by western blotting (Figure 2d). Control experiments (Supplementary Figure 3) showed that expression of HEY1 mRNA was increased by Jagged-1-expressing cells. Consistent with our published observations (Rizzo et al., 2008), E2 inhibited HEY1 induction by Jagged-1 and fulvestrant restored it. We then explored the role of Notch-1 in basal pS2 expression. A specific siRNA to Notch-1 was used to knock down Notch-1 expression by approximately 70% (Figure 2e). Expression of pS2, but not β-tubulin, was dramatically decreased by Notch-1 siRNA in charcoal-stripped medium (Figure 2f). Similarly, a GSI inhibited pS2 expression in a dose-dependent manner (Figure 2g). The IC50 for this effect was virtually identical to that for HEY1 inhibition (not shown). These experiments support the hypothesis that Notch-1 regulates pS2 expression in the absence of E2, and basal pS2 expression under E2-free conditions is Notch-1-dependent.
We investigated the mechanism of this effect by ChIP. Co-culture of MCF-7 cells with Jagged-1-expressing fibroblasts in charcoal-stripped medium caused recruitment of both Notch-1 (Figure 2h) and ERα (Figure 2i) to the pS2 promoter. Short-term E2 treatment reduced the amount of Jagged-1-induced Notch-1 recruitment (Figure 2h), while increasing ERα as compared with that in the control. Fulvestrant nearly abolished the recruitment of Notch-1 and ERα to the pS2 promoter. We confirmed these results by knocking down ERα via siRNA (Figure 2j) before co-culture. In control cells transfected with scrambled siRNA, Jagged-1 co-culture caused dramatic upregulation of Notch-1 on the pS2 promoter in charcoal-stripped medium, which was significantly inhibited by E2. In cells transfected with ERα siRNA, Jagged-1 did not increase the binding of Notch-1 or ERα on the same promoter in the presence or absence of E2 (Figure 2k). These data support a model in which Jagged-1 causes co-recruitment of Notch-1 and ERα to the pS2 promoter, inducing gene expression. Notch-1 recruitment requires ERα even in charcoal-stripped medium, suggesting that unliganded ERα and Notch-1 cooperate in inducing pS2 expression. The inhibition of Notch recruitment by E2 is consistent with our previous data (Rizzo et al., 2008) and suggests a feedback mechanism.
IKKα and its kinase activity are required by Notch-1 for transcriptional activation of ER-dependent genes
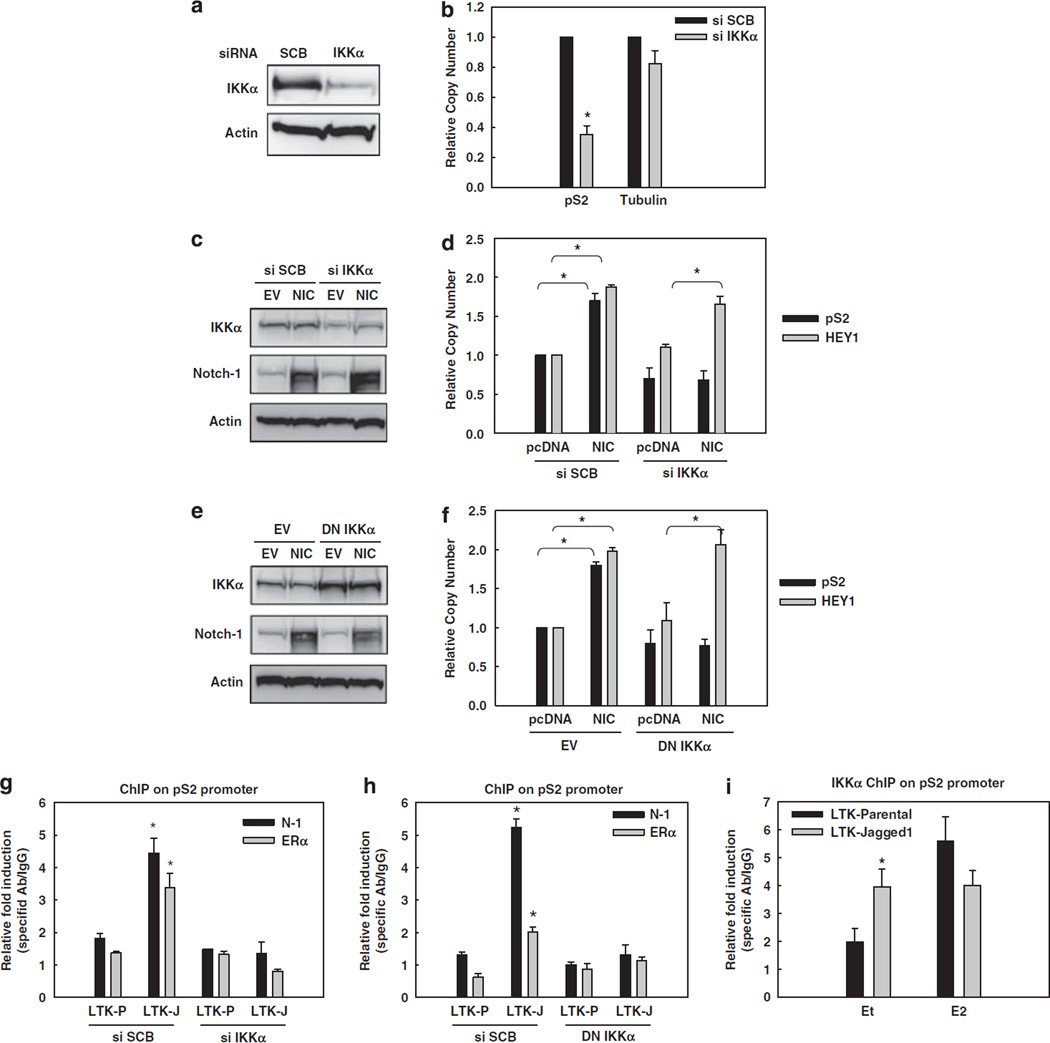
We have shown that active Notch-1 associates with IKKα, promoting its recruitment to nuclear factor-κB-dependent promoters (Song et al., 2008). IKKα has numerous nuclear functions (Perkins, 2007), and was described as a necessary component of ERα-dependent transcriptional complexes in the presence of E2 (Park et al., 2005). Thus, we investigated whether Notch-1 may stimulate ERα-dependent transcription by facilitating the association of IKKα with ERα in the absence of E2. First, we used a specific siRNA to IKKα. IKKα knockdown significantly decreased pS2 mRNA in charcoal-stripped medium (Figures 3a and b), indicating that MCF-7 cells require not only Notch-1 but also IKKα for basal pS2 expression in the absence of E2. To determine whether IKKα is required for Notch-mediated activation of ER-dependent transcription, we expressed NIC in the absence of IKKα (Figure 3c). IKKα knockdown abolished the induction of pS2, but not HEY1, by NIC (Figure 3d), suggesting that IKKα is important for Notch-mediated activation of pS2 expression, but not for canonical Notch signaling in these cells. To rule out possible siRNA artifacts, we also used a dominant-negative (DN) form of IKKα (AA) in which two crucial catalytic residues are replaced by alanine (Arsura et al., 2003). We coexpressed DN-IKKα with NIC in MCF-7 cells in charcoal-stripped medium (Figure 3e), and obtained similar results as with IKKα siRNA: DN-IKKα prevented NIC from activating pS2 but not HEY1 transcription (Figure 3f). Real-time RT– PCR data were complemented by ChIP experiments in which we activated Notch in MCF-7 cells by co-culture with Jagged-1-expressing fibroblasts in charcoalstripped medium, and measured Notch-1 or ERα occupancy of the pS2 promoter. While Jagged-1 stimulated the binding of Notch-1 and ERα to the promoter, either IKKα siRNA (Figure 3g) or DN-IKKα (Figure 3h) completely abolished this effect. Additionally, Jagged-1 co-culture increased the binding of IKKα to the pS2 promoter in the absence of E2 (Figure 3i). Taken together, these data support a model in which Jagged-1 stimulates the recruitment of Notch-1, IKKα and ERα to the pS2 promoter. IKKα and its kinase activity are required for Jagged-1-induced promoter recruitment and pS2 transcriptional activation.
Figure 3.
Notch-1 requires IKKα and its kinase activity for the transcriptional activation of ER-dependent genes. In all experiments, MCF-7 cells were grown in charcoal-stripped medium for a total of 3 days. (a) MCF-7 cells were transfected with IKKα siRNA. The expression level of IKKα was measured by western blotting. (b) pS2 real-time RT–PCR was performed with the same cells as in panel a. Data are expressed as relative copy number after normalization to internal control 18 S rRNA. Tubulin was used as negative control; *P < 0.0001. (c) MCF-7 cells were co-transfected with IKKα siRNA or scrambled control and the construct expressing NIC or pcDNA control. Overexpression of NIC and downregulation of IKKα were validated by western blotting, using actin as loading control. (d) HEY1 and pS2 real-time RT–PCR were performed with the same cells as in panel c; * P ⩽ 0.001. (e) MCF-7 cells were co-transfected with DN-IKKα (AA) or the empty vector and NIC or pcDNA control. Overexpression of DN-IKKα (AA) and Notch-1 was validated by western blotting, using actin as loading control. (f) HEY1 and pS2 real-time RT–PCR were performed with the same cells as in panel e; *P ⩽ 0.001. (g, h) MCF-7 cells were transfected with IKKα siRNA (g) or DN-IKKα (h) and co-cultured with LTK–JAG1 or LTK–PAR fibroblasts for 3 h. ChIP assays were performed with antibodies to Notch-1 or ERα, followed by real-time PCR analysis of the pS2 promoter; *P ⩽ 0.001. (i) MCF-7 cells were co-cultured with LTK–JAG1 or LTK–PAR fibroblasts for 3 h and treated with 5 nm E2 or ethanol control for 1 h prior to harvest. ChIP assay was performed with IKKα antibody followed by real-time PCR for the pS2 promoter; *P ⩽ 0.001. ChIP, chromatin immunoprecipitation; DN-IKKα, the dominant-negative form of IKKα; ERα, estrogen receptor-α; GSI, γ-secretase inhibitor; LTK–JAG1, mouse LTK fibroblasts expressing Notched ligand Jagged-1; LTK–PAR, control vector-transduced parental fibroblast; NIC, active form of Notch-1 (Notch-1IC); RT–PCR, reverse transcription–PCR.
SRC-1 and SRC-3 are not required, but CSL and MAML1 are indispensable for the effect of Notch-1 on the pS2 promoter
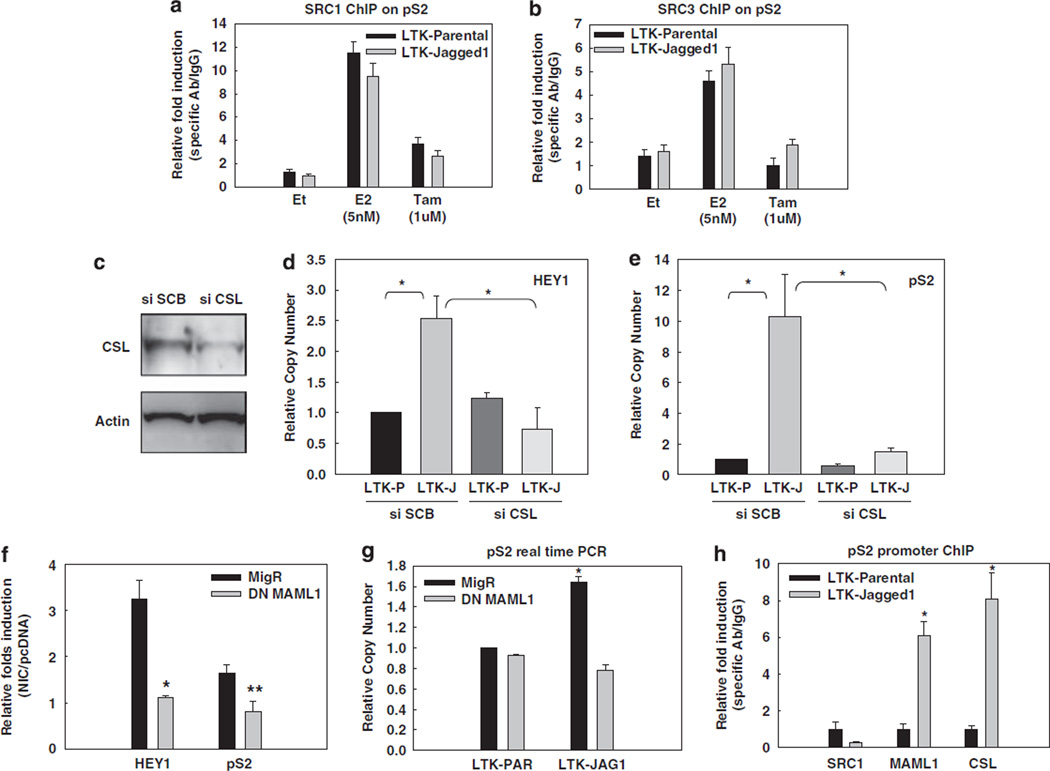
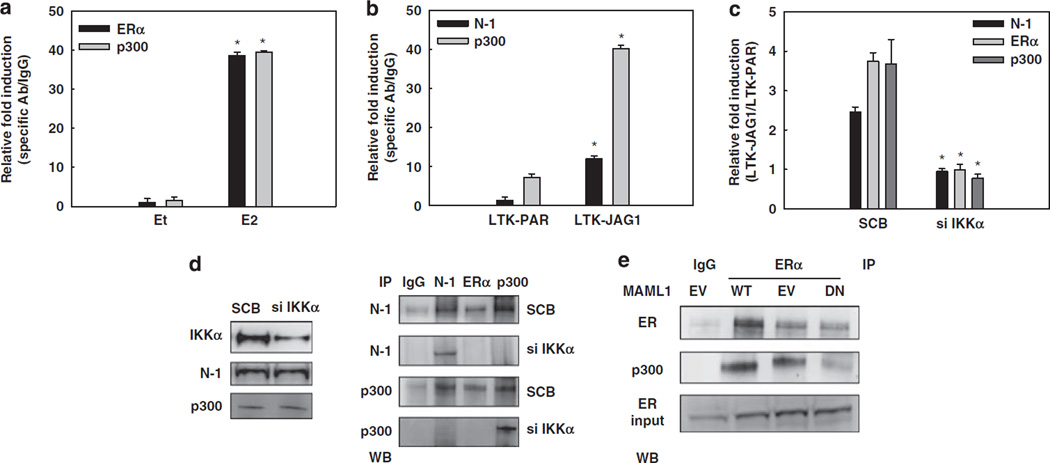
Coactivators SRC-1 and/or SRC-3/AIB1 play an important role in ligand-dependent or independent ERα activation. These coactivators possess histone acetyltransferse activity and recruit additional histone acetyltransferses, such as p300 (Green and Carroll, 2007). ERα phosphorylation at S118, S167 and other residues increases its affinity for SRC-3 (Likhite et al., 2006). IKKα is one of the kinases that can phosphorylate ERα S118. Thus, we asked whether Notch-1 can induce the recruitment of SRC-1 or SRC-3 to the pS2 promoter in the absence of E2 via IKKα. Tamoxifen and its active metabolite 4-hydroxytamoxifen can prevent coactivator recruitment by ERα. ChIP assays showed that E2, but not Jagged-1-mediated Notch activation, stimulated SRC-1 and SRC-3 binding to the pS2 promoter (Figures 4a and b). Thus, these canonical ERα coactivators are dispensable for Notch-1-mediated ERα activation. Consistent with this model, 4-hydro-xytamoxifen did not affect pS2 or HEY1 induction by NIC, whereas fulvestrant did (Supplementary Figure 4).
Figure 4.
SRC-1 or SRC-3 are not required, but CSL and MAML1 are indispensable for the effect of Notch-1 on the pS2 promoter. In all experiments, MCF-7 cells were grown in charcoal-stripped medium for a total of 3 days. SRC-1 (a) and SRC-3 (b) were detected by ChIP on the pS2 promoter. Charcoal-stripped MCF-7 cells were co-cultured with LTK–JAG1 or LTK–PAR fibroblasts for 3 h, and treated with 5 nm E2 (1 h) or 1 µm 4-hydroxytamoxifen (24 h); *P ⩽ 0.001. (c) MCF-7 cells were transfected with CSL siRNA or scrambled control. CSL knockdown was verified by western blotting. (d, e) HEY1 and pS2 real-time RT–PCR was performed with cells transfected with CSL siRNA and co-cultured with LTK fibroblasts. (f) MCF-7 cells were co-transfected with NIC or pcDNA control and DN-MAML1 or the empty vector MigR. HEY1 and pS2 real-time RT–PCR was performed 48 h after transfection. Data are expressed as relative fold induction by NIC over pcDNA (*P ⩽ 0.001, **P ⩽ 0.005). (g) MCF-7 cells were transfected with DN-MAML1 or the empty vector MigR under charcoal-stripped conditions and co-cultured with LTK cells for 12 h. pS2 mRNA level was measured by real-time RT–PCR; *P ⩽ 0.001. (h) ChIP–PCR of SRC1, MAML1, CSL on pS2 promoter with MCF-7 cells co-cultured with LTK–JAG1 or LTK–PAR fibroblasts; *P ⩽ 0.001. ChIP, chromatin immunoprecipitation; DN-MAML1, the dominant-negative form of MAML1; GSI, g-secretase inhibitor; LTK–JAG1, mouse LTK fibroblasts expressing Notched ligand Jagged-1; LTK–PAR, control vector-transduced parental fibroblast; NIC, active form of Notch-1 (Notch-1IC); RT–PCR, reverse transcription–PCR; siRNA, short interfering RNA; SRC, steroid receptor coactivator.
Next, we asked whether canonical Notch partners, CSL and MAML1, are involved in this novel transactivation complex. We downregulated CSL by specific siRNA in charcoal-stripped medium (Figure 4c) and found that induction of HEY1 (Figure 4d) or pS2 (Figure 4e) by Jagged-1 was abolished. Similar results were obtained when we overexpressed a DN form of MAML1 (DN-MAML1), which binds to Notch-1 and CSL but lacks the domain responsible for coactivator recruitment (Weng et al., 2003). In the presence of DN-MAML1, neither transient transfection of NIC (Figure 4f), nor Jagged-1 co-culture (Figure 4g), could activate HEY1 or pS2 expression. ChIP data indicated that CSL and MAML1, but not SRC1, are recruited to the pS2 promoter when Notch is activated in the absence of E2 (Figure 4h).
The Notch-1 and ERα transcriptional complexes interact in chromatin-enriched nuclear extracts, leading to MAML-1-dependent p300 recruitment
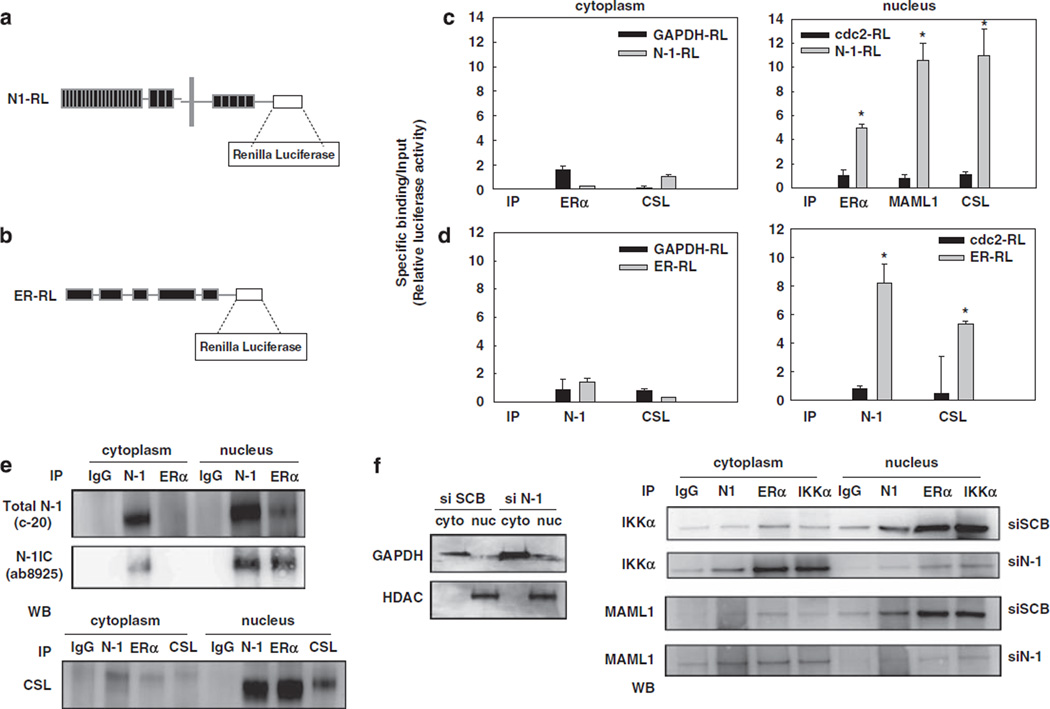
Based on our ChIP results showing that Notch-1 binds to the pS2 promoter in an ER-dependent manner, we hypothesized that the Notch-1 and ERα transcriptional complexes may physically interact on the chromatin. We took two different IP strategies to test this hypothesis. First, we used a quantitative co-IP assay using a chimeric construct comprised of full-length Notch-1 fused at the C-terminus with Renilla luciferase (N1-RL) (Vooijs et al., 2004; Figure 5a). We immunoprecipitated cytoplasmic extracts and chromatinenriched nuclear extracts from cells transfected with N1-RL, with an ERα-specific antibody or nonspecific IgG. We determined the fraction of luciferase activity immunoprecipitated by anti-ERα as compared with that by nonspecific IgG. We could specifically co-precipitate N1-1RL and ERα in chromatin-enriched nuclear lysates but not in cytoplasmic lysates. N1-RL could also be co-immunoprecipitated with known Notch-binding partners CSL and MAML1, which were used as positive controls (Figure 5c). Negative controls, glyceraldehyde-3-phosphate dehydrogenase-RL (GAPDH-RL) and Cdc2-RL, could not be co-immunoprecipitated with ERα. Similar results were observed in reverse IP experiments where we transfected cells with an ERα-RL construct and immunoprecipitated luciferase activity with either Notch-1 or CSL antibodies (Figure 5d).
Figure 5.
The Notch-1 and ERα transcriptional complexes interact in chromatin-enriched nuclear extracts. (a, b) Schematics for the constructs of N1-RL (a) and ERα-RL (b). (c) MCF-7 cells were transfected with N1-RL or the negative controls GAPDH-RL (for cytoplasmic proteins) and Cdc2-RL (for nuclear proteins). Cells were harvested after 3 days E2 starvation and nuclear extraction was performed. Cytoplasmic or nuclear extracts were immunoprecipitated with ERα, CSL and MAML1 antibodies. (d) MCF-7 cells were transfected with ERα-RL or negative controls. Cells were harvested after 3 days E2 starvation and nuclear extraction was performed. Cytoplasm lysates or nuclear extracts were immunoprecipitated with Notch-1 and CSL antibodies; *P ⩽ 0.001. (e) Standard IP-western blot was performed on cytoplasmic or nuclear extracts from MCF-7 cells grown in charcoal-stripped medium for 3 days with antibodies to Notch-1, ERα, and CSL. (f) MCF-7 cells were grown in charcoal-stripped medium for a total of 3 days. Cells were transfected with Notch-1 siRNA or scrambled control, and harvested 48 h after transfection. The efficiency of nuclear extraction was verified by western blotting (left). IP–western blot was performed with antibodies to Notch-1, ERα and IKKα. A 10-µl volume of eluted material from each IP was analysed by western blotting for IKKα or MAML1. ChIP, chromatin immunoprecipitation; ERα, estrogen receptor-α; ERα-RL, Renilla luciferase-tagged ERα; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; N1-RL, Renilla luciferase-tagged Notch-1; siRNA, short interfering RNA.
We confirmed the interaction between ERα and the Notch-1/CSL transcriptional complex by traditional IP–western blot from chromatin-enriched nuclear extracts. Figure 5e shows that Notch-1 and ERα can be co-immunoprecipitated only in nuclear extracts, and CSL is part of the complex. To verify the identity of the Notch-1 band, we used two Notch-1 antibodies to detect the interaction between Notch-1 and ERα: we immunoprecipitated with a polyclonal C-terminal antibody, C-20, or ERα, and detected western blots with either C-20 or a specific antibody to NIC, which recognizes the N-terminal epitope generated by γ-secretase cleavage. We confirmed that the Notch-1 band we co-immunoprecipitate with ERα is NIC. This interaction appeared to be DNA-dependent, since it was abolished by pretreatment of nuclear lysates with the DNA intercalator ethidium bromide before IP (Supplementary Figure 5). These data indicate that the Notch-1 and ERα transcriptional complexes are in close proximity to each other and may form a large DNA-bound supramolecular complex. Additionally, IKKα and MAML1 could be detected in association with ERα only when Notch-1 was present (Figure 5f). Notch-1 knockdown abolished complex formation, and completely prevented the nuclear translocation of IKKα. Interestingly, after Notch-1 knockdown IKKα was found in association with ERα in the cytoplasm but not in the nucleus, indicating that Notch may affect the nuclear localization of ERα in the absence of E2. To test this hypothesis, we transfected the MCF-7 cells with the ER–RL construct, and measured luciferase activity in cytoplasmic or nuclear fractions after co-culturing MCF-7 cells with Jagged-1-expressing fibroblasts. We observed significant shift of luciferase activity from the cytoplasm to the nucleus in MCF-7 cells co-cultured with Jagged-1-expressing fibroblasts, which do not express ERα. Western blots on the same lysates confirmed this observation (Supplementary Figure 6). These data support a model in which Notch-1 is required for chromatin recruitment of IKKα, as we showed in a different model (Song et al., 2008), and can trigger nuclear migration of ERα in the absence of estrogen.
Notch activation induces recruitment of p300 to the pS2 promoter
p300/CBP functions as a coactivator for ERα (Green and Carroll, 2007) and Notch/CSL (Miele, 2006). SRC–p300 complexes are essential for transcriptional initiation on ER-dependent promoters (Green and Carroll, 2007). Thus, we explored p300 recruitment to the pS2 promoter upon Notch activation. ChIP experiments showed similar levels of p300 on the pS2 promoter when cells were treated with E2 or co-cultured with Jagged-1-expressing fibroblasts after 3 days of hormone deprivation (Figures 6a and b). p300 recruitment was abolished by IKKα siRNA (Figure 6c). Notch-1 and ERα could be co-immunoprecipitated with p300. This interaction was inhibited by IKKα knockdown (Figure 6d). MAML1 recruits p300 to the CSL–Notch transcriptional complex (Saint Just et al., 2007). When we transfected MCF-7 cells with DN-MAML1, the interaction between ERα and p300 was inhibited (Figure 6e). These data support a model in which in the absence of E2 MAML1 rather than p160-family coactivators is responsible for Notch-1-induced p300 recruitment to ERα-dependent promoters.
Figure 6.
Notch activation recruits p300 to the pS2 promoter. (a) ChIP on the pS2 promoter from MCF-7 cells treated with 5 nm E2 for 1 h after 3 days charcoal stripping. (b) ChIP on the pS2 promoter from MCF-7 cells co-cultured with LTK–JAG1 or LTK–PAR fibroblasts for 3 h after 3 days charcoal stripping. Data in panels a and b are expressed as relative fold increase of specific antibody over IgG control, after normalization to human-specific internal control RPL13a. (c) ChIP on the pS2 promoter from MCF-7 cells grown in charcoal-stripped medium for a total of 3 days, transfected with IKKα siRNA or scrambled control, and co-cultured with LTK–JAG1 or LTK–PAR fibroblasts for 3 h. Data are expressed as relative fold induction by LTK–JAG1 over LTK–PAR after normalization to IgG control and internal control RPL13a. (d) Right: Co-IP experiments on MCF-7 cells, charcoal-stripped for 3 days and transfected with IKKα siRNA or scrambled control. Left: IKKα knockdown was confirmed by western blotting. (e) Co-IP of ERα and p300 on MCF-7 cells, charcoal-stripped for 3 days and transfected with either wild-type or DN-MAML1, or the empty vector control; *P ⩽ 0.05. ChIP, chromatin immunoprecipitation; DN-MAML1, the dominant-negative form of MAML1; LTK–JAG1, mouse LTK fibro-blasts expressing Notched ligand Jagged-1; LTK–PAR, control vector-transduced parental fibroblast; siRNA, short interfering RNA.
Discussion
We describe a novel crosstalk between Notch-1 and ERα, whereby Notch-1 transactivates a subset of ERα-responsive genes via a mechanism that requires IKKα and MAML1, but not SRC1 or SRC3. The working model supported by our data is shown in Figure 7. The transcriptional effects of Notch activation are notoriously context-dependent. Part of this context dependence may result from a complex interplay between the Notch transcriptional complex (NTC) and other cell type-specific transcription factors. Evidence (Nam et al., 2007) supports a model in which two DNA-bound NTCs bind cooperatively to some Notchresponsive genes. Our data suggest that the NTC is also capable of forming complexes with ERα. Thus, in breast cancer cells, the effects of Notch activation would depend on ERα expression and E2 concentration.
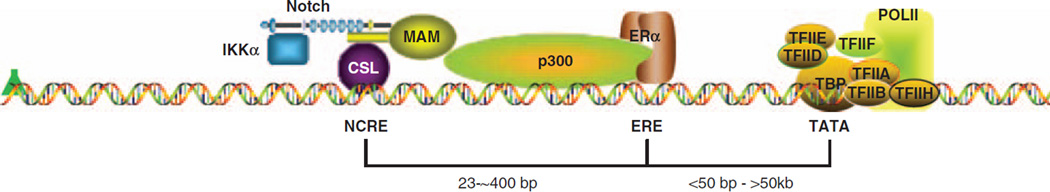
Figure 7.
Working model of the Notch–ERα crosstalk. The Notch transcriptional complex (NTC) including Notch-1, CSL, MAML1 (MAM) and other coactivators (not shown for clarity) binds to Notch-CSL-responsive elements (NCRE). IKKα is recruited to the NTC in a Notch-dependent manner, although it is still unclear whether the interaction with Notch is direct and is necessary for formation of the supramolecular complex. ERα is recruited to the nucleus in a Notch-1-dependent manner, binds to its responsive element (ERE) or possibly through other transcription factors or pioneer factors, and is necessary for NTC recruitment to ERα-responsive genes. In the absence of E2, MAML1 recruits p300 to the complex, taking over the functions normally carried out by p160 ERα coactivators. The formation of a supramolecular complex between the NTC and the ERα transcriptional complex contributes to activate transcription of a subset of ERα-responsive genes in the absence of E2, and for some genes also in its presence. The pol-II complex is depicted at the TATA box with its most important components. The angled green arrow indicates transcriptional start. ER, estrogen receptor; ERα, estrogen receptor-α.
Estrogen receptor-a can regulate transcription positively and negatively, and it can bind DNA directly via EREs or other transcription factors such as AP1 or SP1 (Carroll et al., 2006). Pioneer factors such as FoxA1 play a key role in recruiting ERα to specific DNA sites upon E2 treatment (Laganiere et al., 2005). ERα-binding DNA sites can be found as far as 50–100 kb from regulated genes (Lin et al., 2007). Several sites can cooperate in the regulation of individual genes (Eeckhoute et al., 2006). A widely accepted model of E2 regulation of cyclin-D1 suggests that recruitment of FoxA1 to a downstream enhancer facilitates E2-activated binding of ERα to this site and additional upstream sites (Laganiere et al., 2005). Among these, an AP1 site at −934 recruits ERα and p300. This site is contained in the amplicon that we analysed (P2 site in Figure 1f), as is a putative ERE. A bioinformatic scan of the human and mouse genomes revealed that canonical EREs are found at specific spacing distances from CSL-responsive elements with highly statistically significant frequencies (Supplementary Figure 7). Many of these sequence pairs are found within 2 kb of genes conserved between humans and mice (not shown). Genome-wide studies will be necessary to determine which of these genes are co-regulated by Notch-1 and ERα, which subset of the known ERα binding sites is close to CSL responsive elements, whether Notch-1 influences DNA site usage by ERα, and whether AP1-tethered or SP1-tethered ERα also cooperate with Notch-1. This is possible, based on our data on the cyclin-D1 and c-Myc promoters. Notch-1 is recruited both by E2 and by Jagged-1 to a region in the cyclin-D1 promoter known to bind ERα via AP1 (Shen et al., 2008). The SP1 site in the c-Myc promoter thought to be important in mediating E2 transcriptional effects is very close to our amplicon (Dubik and Shiu, 1992). We focused on a relatively small and well-characterized promoter, pS2, whose active regulatory elements are found in the vicinity of the transcriptional start.
Our data demonstrate a key role for nuclear IKKα in Notch-1-ERα cooperation. IKKα stimulates ERα activity in the presence of E2 (Park et al., 2005), by phosphorylating ERα and/or histone H3, and is necessary for transcriptional induction of cyclin-D1 by mitogenic signals (Albanese et al., 2003). We have previously shown that in CaSki cervical cancer cells, Notch-1 associates with IKKα and is required for its recruitment to the c-IAP2 promoter (Song et al., 2008). This study indicates that IKKα is found in association with ERα and MAML1 in the nucleus only in the presence of Notch-1. Taken together, these findings suggest that promoting the chromatin recruitment of IKKα is a novel function of Notch-1, through which Notch-1 can mediate crosstalk with other transcription factors. IKKα has been suggested to regulate Notch signaling via phosphorylation of nuclear IkBa in 3T3 cells (Aguilera et al., 2004). Abnormal IKKα activity in colon cancer cells was reported to increase Notch transcriptional activity (Fernandez-Majada et al., 2007). Our data from CaSki cells (Song et al., 2008) are consistent with that model. However, in MCF-7 cells, IKKα was not required for Notch-mediated induction of HEY1, whereas being absolutely required for crosstalk with ERα. It is likely that the accessory role of IKKα in Notch signaling depends on cell type and target genes.
A physical association between Notch-1 and a nuclear receptor, Nur77, was shown in 1999 in T-cells (Jehn et al., 1999). In our model, the interaction between the Notch-1 and ERα transcriptional complexes appears to require DNA, and is likely to be indirect. However, direct contact between Notch-1 and DNA-bound ERα may contribute to complex formation.
The possible physiological role of Notch in ERα signaling deserves further investigation. Under our experimental conditions, Notch-1 knockdown inhibits c-Myc basal expression in the presence, but not absence, of E2, whereas for pS2 and cyclin-D1, Notch-1 is required for expression in the presence or absence of E2 (Supplementary Figure 8). Knockdown of either Notch-1 or IKKα prevents transactivation of cyclin-D1 and pS2 by E2 (Supplementary Figure 9). This suggests that Notch-1 may be a physiological cofactor of E2 for some ERα-target genes, whereas for other genes Notch-1 may be sufficient to activate ERα-mediated transcription even in the absence of E2. The latter phenomenon may be pathogenetically important in endocrine-resistant breast cancers. Physiologically, this effect may contribute to luminal differentiation during mammary gland development, as Notch-1 has been shown to play a role in ERα expression during luminal cell fate determination (Dontu et al., 2004; Bouras et al., 2008).
We (Rizzo et al., 2008) have recently shown that in breast cancer cells E2 inhibits Notch activation and E2 withdrawal reactivates Notch. This study shows that Notch can activate ERα-dependent transcription, suggesting the existence of a feedback mechanism controlling the Notch–ERα crosstalk.
These findings have significant therapeutic implications. We have shown that anti-estrogens or estrogen withdrawal increases the dependence of breast cancer cells on Notch. Notch-1 knockdown inhibited the growth of MCF-7 cells in charcoal-stripped serum as potently as fulvestrant (Supplementary Figure 10). We have previously shown that NIC induces proliferation in these cells (Rizzo et al., 2008). However, we did not observe accelerated CFSE dilution (a measure of doubling time) in co-culture assays with Jagged-1-expressing fibroblasts (not shown). Thus, it is possible that the main effect of Notch-1 in this setting is to maintain survival, thus allowing proliferation, rather than directly accelerating proliferation.
We confirmed that regulation of E2-target genes by Notch is not limited to MCF-7 cells, and can be observed in T47D:A18 cells as well (Supplementary Figure 11). Similarly, in T47:A18 xenografts treated with tamoxifen and an oral GSI, pS2 expression was downregulated more effectively than canonical Notch-target genes (Supplementary Figure 12), indicating that inhibition of E2-target genes may mediate the effects of GSIs in ERα-positive breast cancers and may be an efficacy biomarker. Finally, our study suggests that targeting IKKα, alone or with Notch, may affect the expression of ERα-target genes without further decreasing the expression of canonical Notch-target genes. This may allow us to circumvent or reduce, thus, the systemic toxicity of Notch inhibitors while selectively affecting breast cancer cells.
Materials and methods
Cell culture
MCF-7 cells from ATCC were grown in RPMI with 10% fetal bovine serum and 6 ng/ml insulin. LTK–PAR and LTK-Jagged-1 cells were generously provided by Dr G Weinmaster (University of California at Los Angeles, Los Angeles, CA, USA) were expanded in Dulbecco’s Modified Eagle’s Medium with 10% fetal bovine serum.
Plasmids and reagents
Notch-1IC (Rizzo et al., 2008) and DN-IKKα (Arsura et al., 2003) constructs have been described. See Supplementary Information for details on additional constructs and reagents.
Western blotting and antibodies
Cell lysis and western blotting were performed as described (Rizzo et al., 2008). Antibodies were as follows: Notch-1 (C-20; Santa Cruz Biotechnology, Santa Cruz, CA, USA), ERα (G-20; Santa Cruz Biotechnology), RBP-Jk (CBF1, D-20; Santa Cruz Biotechnology), MAML1 (AB5975; Chemicon International, Temecula, CA, USA), Notch-1IC (ab8925; Abcam, Cambridge, MA, USA), IKKα (Imgenex, San Diego, CA, USA), p300 (N-15; Santa Cruz Biotechnology), GAPDH (MAB374; Chemicon International), histone deacetylase (H-51; Santa Cruz Biotechnology) and β-actin (Sigma-Aldrich, St Louis, MO, USA).
Real-time RT–PCR
Total RNA was extracted using the RNeasy Mini kit (Qiagen, Valencia, CA, USA); cDNA was produced using the First Strand cDNA synthesis kit (Fermentas, Glen Burnie, MD, USA). Real-time PCR reactions were conducted with an ABI 7300 system using the iTaq SYBR Green Supermix with ROX (Bio-Rad, Hercules, CA, USA). See Supplementary Figure 13 for primers. The RT–PCR internal controls were 18S rRNA for MCF-7 cells grown alone and RPL13a for co-culture experiments where validated human-specific primers were needed to avoid mouse RNA contamination.
Quantitative ChIP
Quantitative ChIP was performed as described (Wu et al., 2005). See Supplementary Information for detailed experimental procedure, and Supplementary Figure 13 for the primers used in real-time PCR. The following antibodies were used: Notch-1 (C-20), RBP-Jk (CBF1, D-20), SRC-1 (M-341) and p300 (N-15) (Santa Cruz Biotechnology); ERα (AB10) (Lab Vision-Neomarkers, Fremont, CA, USA); MAML1 (AB5975) (Chemicon International); IKKα (IMG-136) (Imgenex) and SRC-3 (MA1-845) (Affinity BioReagents, Rockford, IL, USA).
Quantitative Renilla IP
Quantiative IP was performed as previously described (Vooijs et al., 2004), with the following antibodies: Notch-1 (C-20), RBP-Jk (CBF1, D-20) (Santa Cruz Biotechnology); ERα (AB10) (Lab Vision-Neomarkers) and MAML1 (AB5975) (Chemicon).
Nuclear extraction and co-IP
Nuclear extraction was performed as described (ElShamy and Livingston, 2004). The following antibodies were used: Notch-1 (C-20), RBP-Jκ (CBF1, D-20) and p300 (N-15) (Santa Cruz Biotechnology); MAML1 (AB5975) (Chemicon); IKKα (IMG-136) (Imgenex) and ERα (AB10) (Lab Vision-Neomarkers).
Statistical analysis
For pairwise comparisons, two-tailed unpaired Student’s t-tests were used with α = 0.05. SigmaStat software (Jandel Scientific, San Jose, CA, USA) was used for statistical analysis.
Supplementary Material
Acknowledgments
This work was supported by Grant P01AG025531 (LM, BAO) and the Schmitt Fellowship Foundation (LH). We are grateful to Geraldine Weinmaster, Peter Strack and Rafi Kopan for the gift of reagents and cell lines, and to Sarah Bray for helpful discussions.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Aguilera C, Hoya-Arias R, Haegeman G, Espinosa L, Bigas A. Recruitment of IkappaBalpha to the hes1 promoter is associated with transcriptional repression. Proc Natl Acad Sci USA. 2004;101:16537–16542. doi: 10.1073/pnas.0404429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese C, Wu K, D’Amico M, Jarrett C, Joyce D, Hughes J, et al. IKKalpha regulates mitogenic signaling through transcriptional induction of cyclin D1 via Tcf. Mol Biol Cell. 2003;14:585–599. doi: 10.1091/mbc.02-06-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsura M, Panta GR, Bilyeu JD, Cavin LG, Sovak MA, Oliver AA, et al. Transient activation of NF-kappaB through a TAK1/ IKK kinase pathway by TGF-beta1 inhibits AP-1/SMAD signaling and apoptosis: implications in liver tumor formation. Oncogene. 2003;22:412–425. doi: 10.1038/sj.onc.1206132. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Baonza A, Freeman M. Control of cell proliferation in the Drosophila eye by Notch signaling. Dev Cell. 2005;8:529–539. doi: 10.1016/j.devcel.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, et al. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3:429–441. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- Callahan R, Egan SE. Notch signaling in mammary development and oncogenesis. J Mammary Gland Biol Neoplasia. 2004;9:145–163. doi: 10.1023/B:JOMG.0000037159.63644.81. [DOI] [PubMed] [Google Scholar]
- Callahan R, Raafat A. Notch signaling in mammary gland tumorigenesis. J Mammary Gland Biol Neoplasia. 2001;6:23–36. doi: 10.1023/a:1009512414430. [DOI] [PubMed] [Google Scholar]
- Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- Cenni B, Picard D. Ligand-independent activation of steroid receptors: new roles for old players. Trends Endocrinol Metab. 1999;10:41–46. doi: 10.1016/s1043-2760(98)00121-0. [DOI] [PubMed] [Google Scholar]
- Cheng P, Zlobin A, Volgina V, Gottipati S, Osborne B, Simel EJ, et al. Notch-1 regulates NF-kappaB activity in hemopoietic progenitor cells. J Immunol. 2001;167:4458–4467. doi: 10.4049/jimmunol.167.8.4458. [DOI] [PubMed] [Google Scholar]
- Dickson BC, Mulligan AM, Zhang H, Lockwood G, O’Malley FP, Egan SE, et al. High-level JAG1 mRNA and protein predict poor outcome in breast cancer. Mod Pathol. 2007;20:685–693. doi: 10.1038/modpathol.3800785. [DOI] [PubMed] [Google Scholar]
- Dontu G, Jackson KW, McNicholas E, Kawamura M, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:605–615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubik D, Shiu RP. Mechanism of estrogen activation of c-myc oncogene expression. Oncogene. 1992;7:1587–1594. [PubMed] [Google Scholar]
- Eeckhoute J, Carroll JS, Geistlinger TR, Torres-Arzayus MI, Brown M. A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev. 2006;20:2513–2526. doi: 10.1101/gad.1446006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElShamy WM, Livingston DM. Identification of BRCA1-IRIS, a BRCA1 locus product. Nat Cell Biol. 2004;6:954–967. doi: 10.1038/ncb1171. [DOI] [PubMed] [Google Scholar]
- Fernandez-Majada V, Aguilera C, Villanueva A, Vilardell F, Robert-Moreno A, Aytes A, et al. Nuclear IKK activity leads to dysregulated Notch-dependent gene expression in colorectal cancer. Proc Natl Acad Sci USA. 2007;104:276–281. doi: 10.1073/pnas.0606476104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallahan D, Callahan R. The mouse mammary tumor associated gene INT3 is a unique member of the NOTCH gene family (NOTCH4) Oncogene. 1997;14:1883–1890. doi: 10.1038/sj.onc.1201035. [DOI] [PubMed] [Google Scholar]
- Gallahan D, Jhappan C, Robinson G, Hennighausen L, Sharp R, Kordon E, et al. Expression of a truncated Int3 gene in developing secretory mammary epithelium specifically retards lobular differentiation resulting in tumorigenesis. Cancer Res. 1996;56:1775–1785. [PubMed] [Google Scholar]
- Gillesby BE, Stanostefano M, Porter W, Safe S, Wu ZF, Zacharewski TR. Identification of a motif within the 5′ regulatory region of pS2 which is responsible for AP-1 binding and TCDD-mediated suppression. Biochemistry. 1997;36:6080–6089. doi: 10.1021/bi962131b. [DOI] [PubMed] [Google Scholar]
- Green KA, Carroll JS. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nat Rev Cancer. 2007;7:713–722. doi: 10.1038/nrc2211. [DOI] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Hayward P, Brennan K, Sanders P, Balayo T, DasGupta R, Perrimon N, et al. Notch modulates Wnt signalling by associating with Armadillo/beta-catenin and regulating its transcriptional activity. Development. 2005;132:1819–1830. doi: 10.1242/dev.01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso T, Sartorelli V, Poizat C, Iezzi S, Wu HY, Chung G, et al. HERP, a novel heterodimer partner of HES/E(spl) in Notch signaling. Mol Cell Biol. 2001;21:6080–6089. doi: 10.1128/MCB.21.17.6080-6089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem. 2001;276:13615–13621. doi: 10.1074/jbc.M008384200. [DOI] [PubMed] [Google Scholar]
- Jehn BM, Bielke W, Pear WS, Osborne BA. Cutting edge: protective effects of notch-1 on TCR-induced apoptosis. J Immunol. 1999;162:635–638. [PubMed] [Google Scholar]
- Jeltsch JM, Roberts M, Schatz C, Garnier JM, Brown AM, Chambon P. Structure of the human oestrogen-responsive gene pS2. Nucleic Acids Res. 1987;15:1401–1414. doi: 10.1093/nar/15.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Kiaris H, Politi K, Grimm LM, Szabolcs M, Fisher P, Efstratiadis A, et al. Modulation of notch signaling elicits signature tumors and inhibits hras1-induced oncogenesis in the mouse mammary epithelium. Am J Pathol. 2004;165:695–705. doi: 10.1016/S0002-9440(10)63333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinakis A, Szabolcs M, Politi K, Kiaris H, Artavanis-Tsakonas S, Efstratiadis A. Myc is a Notch1 transcriptional target and a requisite for Notch1-induced mammary tumorigenesis in mice. Proc Natl Acad Sci USA. 2006;103:9262–9267. doi: 10.1073/pnas.0603371103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganiere J, Deblois G, Lefebvre C, Bataille AR, Robert F, Giguere V. From the cover: location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc Natl Acad Sci USA. 2005;102:11651–11656. doi: 10.1073/pnas.0505575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhite VS, Stossi F, Kim K, Katzenellenbogen BS, Katzenellenbogen JA. Kinase-specific phosphorylation of the estrogen receptor changes receptor interactions with ligand, deoxyribonucleic acid, and coregulators associated with alterations in estrogen and tamoxifen activity. Mol Endocrinol. 2006;20:3120–3132. doi: 10.1210/me.2006-0068. [DOI] [PubMed] [Google Scholar]
- Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, et al. Whole-genome cartography of estrogen receptor alpha binding sites. PLoS Genet. 2007;3:e87. doi: 10.1371/journal.pgen.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier MM, Gessler M. Comparative analysis of the human and mouse hey1 promoter: hey genes are new notch target genes [In Process Citation] Biochem Biophys Res Commun. 2000;275:652–660. doi: 10.1006/bbrc.2000.3354. [DOI] [PubMed] [Google Scholar]
- Martin MB, Franke TF, Stoica GE, Chambon P, Katzenellenbogen BS, Stoica BA, et al. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology. 2000;141:4503–4511. doi: 10.1210/endo.141.12.7836. [DOI] [PubMed] [Google Scholar]
- Miele L. Notch signaling. Clin Cancer Res. 2006;12:1074–1079. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- Miele L. Rational targeting of Notch signaling in breast cancer. Expert Rev Anticancer Ther. 2008;8:1197–1202. doi: 10.1586/14737140.8.8.1197. [DOI] [PubMed] [Google Scholar]
- Nam Y, Sliz P, Pear WS, Aster JC, Blacklow SC. Cooperative assembly of higher-order Notch complexes functions as a switch to induce transcription. Proc Natl Acad Sci USA. 2007;104:2103–2108. doi: 10.1073/pnas.0611092104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman E, Ladha MH, Lin N, Upton TM, Miller SJ, DiRenzo J, et al. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol. 1997;17:5338–5347. doi: 10.1128/mcb.17.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff BJ, Osborne BA, Miele L. Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene. 2003;22:6598–6608. doi: 10.1038/sj.onc.1206758. [DOI] [PubMed] [Google Scholar]
- Nunez AM, Berry M, Imler JL, Chambon P. The 5′ flanking region of the pS2 gene contains a complex enhancer region responsive to oestrogens, epidermal growth factor, a tumour promoter (TPA), the c-Ha-ras oncoprotein and the c-jun protein. EMBO J. 1989;8:823–829. doi: 10.1002/j.1460-2075.1989.tb03443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KJ, Krishnan V, O’Malley BW, Yamamoto Y, Gaynor RB. Formation of an IKKalpha-dependent transcription complex is required for estrogen receptor-mediated gene activation. Mol Cell. 2005;18:71–82. doi: 10.1016/j.molcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Perissi V, Rosenfeld MG. Controlling nuclear receptors: the circular logic of cofactor cycles. Nat Rev Mol Cell Biol. 2005;6:542–554. doi: 10.1038/nrm1680. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Porter W, Saville B, Hoivik D, Safe S. Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol Endocrinol. 1997;11:1569–1580. doi: 10.1210/mend.11.11.9916. [DOI] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65:8530–8537. doi: 10.1158/0008-5472.CAN-05-1069. [DOI] [PubMed] [Google Scholar]
- Reedijk M, Pinnaduwage D, Dickson BC, Mulligan AM, Zhang H, Bull SB, et al. JAG1 expression is associated with a basal phenotype and recurrence in lymph node-negative breast cancer. Breast Cancer Res Treat. 2008;111:439–448. doi: 10.1007/s10549-007-9805-3. [DOI] [PubMed] [Google Scholar]
- Rizzo P, Miao H, D’Souza G, Osipo C, Yun J, Zhao H, et al. Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res. 2008;68:5226–5235. doi: 10.1158/0008-5472.CAN-07-5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchini C, Capobianco AJ. Induction of cyclin D1 transcription and CDK2 activity by Notch(ic): implication for cell cycle disruption in transformation by Notch(ic) Mol Cell Biol. 2001;21:5925–5934. doi: 10.1128/MCB.21.17.5925-5934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint Just RM, Hansson ML, Wallberg AE. A proline repeat domain in the Notch co-activator MAML1 is important for the p300-mediated acetylation of MAML1. Biochem J. 2007;404:289–298. doi: 10.1042/BJ20061900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Uray IP, Li Y, Krisko TI, Strecker TE, Kim HT, et al. The AP-1 transcription factor regulates breast cancer cell growth via cyclins and E2F factors. Oncogene. 2008;27:366–377. doi: 10.1038/sj.onc.1210643. [DOI] [PubMed] [Google Scholar]
- Song LL, Peng Y, Yun J, Rizzo P, Chaturvedi V, Weijzen S, et al. Notch-1 associates with IKKalpha and regulates IKK activity in cervical cancer cells. Oncogene. 2008;27:5833–5844. doi: 10.1038/onc.2008.190. [DOI] [PubMed] [Google Scholar]
- Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006;66:1517–1525. doi: 10.1158/0008-5472.CAN-05-3054. [DOI] [PubMed] [Google Scholar]
- Vooijs M, Schroeter EH, Pan Y, Blandford M, Kopan R. Ectodomain shedding and intramembrane cleavage of mammalian Notch proteins is not regulated through oligomerization. J Biol Chem. 2004;279:50864–50873. doi: 10.1074/jbc.M409430200. [DOI] [PubMed] [Google Scholar]
- Weng AP, Millholland JM, Yashiro-Ohtani Y, Arcangeli ML, Lau A, Wai C, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng AP, Nam Y, Wolfe MS, Pear WS, Griffin JD, Blacklow SC, et al. Growth suppression of pre-T acute lymphoblastic leukemia cells by inhibition of notch signaling. Mol Cell Biol. 2003;23:655–664. doi: 10.1128/MCB.23.2.655-664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Iwata F, Grass JA, Osborne CS, Elnitski L, Fraser P, et al. Molecular determinants of NOTCH4 transcription in vascular endothelium. Mol Cell Biol. 2005;25:1458–1474. doi: 10.1128/MCB.25.4.1458-1474.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwijsen RM, Wientjens E, Klompmaker R, van der SJ, Bernards R, Michalides RJ. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88:405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.