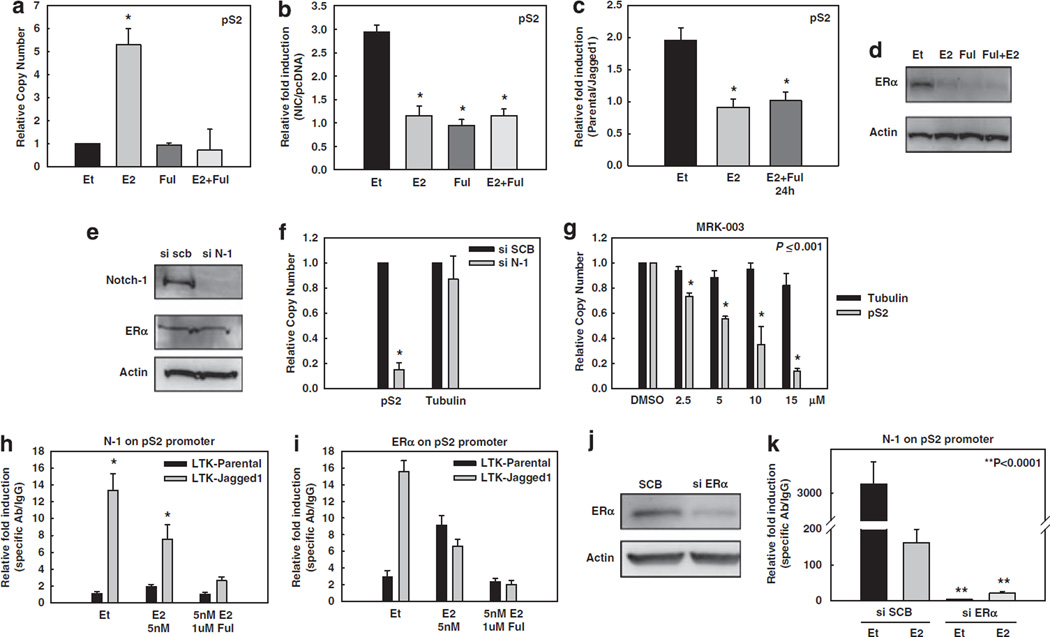
Figure 2.
Notch activation increases ER-dependent transcription. In all experiments, MCF-7 cells were grown in phenol red-free RPMI containing 10% DCC-fetal bovine serum for 3 days prior to harvest. (a–g) pS2 real-time RT–PCR experiments: (a) Untransfected MCF-7 cells were treated with 5 nm of E2 (4 h), 1 µm fulvestrant (24 h) or the combination before harvest. Data are expressed as relative copy number normalized to internal control (18S rRNA); *P ⩽ 0.001. (b) Twelve hours after serum starvation, MCF-7 cells were transfected with NIC or pcDNA vector control. Cells were treated with 5 nm of E2 (4 h), 1 µM fulvestrant (24 h) or a combination of both before harvest. Data are expressed as relative fold induction of NIC over pcDNA after normalization to the internal control 18S rRNA; *P ⩽ 0.001. (c) MCF-7 cells were treated with E2 alone or in combination with fulvestrant as described above, and co-cultured with LTK–JAG1 cells for 12 h. Data are expressed as relative fold induction by LTK–JAG1 over LTK–PAR cells after normalization to internal control RPL13a; *P ⩽ 0.001. (d) Western blot of ERα after the treatments described above. (e) Western blot of MCF-7 cells transfected with Notch-1 siRNA or scrambled control (SCB). (f) MCF-7 cells transfected with Notch-1 siRNA or SCB; *P ⩽ 0.001. (g) MCF-7 cells were treated with increasing concentrations of GSI for 24 h. Data are expressed as relative copy number normalized to internal control (18S rRNA). (h, i) ChIP assay on the pS2 promoter. MCF-7 cells were treated with 5 nm E2, ethanol control for 1 h or E2 in combination with 1 µm fulvestrant (24 h) after 3 days of charcoal stripping and co-cultured with LTK–JAG1 cells for 3 h. Data expressed as relative fold increase of specific antibody over IgG control, after normalization to internal control RPL13a. (j) MCF-7 cells were grown in charcoal-stripped media for 3 days and transfected with ERα siRNA or SCB. The western blot shows efficient downregulation of ERα. Actin was used as a loading control. (k) ChIP assay on the pS2 promoter with cells transfected with siRNA to ERα (as described above), co-cultured with LTK–JAG fibroblasts for 3 h and treated with 5 nm E2 or ethanol for 1 h. ChIP, chromatin immunoprecipitation; ERα, estrogen receptor-α; GSI, γ-secretase inhibitor; LTK–JAG1, mouse LTK fibroblasts expressing Notched ligand Jagged-1; LTK–PAR, control vector-transduced parental fibroblast; NIC, active form of Notch-1 (Notch-1IC); RPMI, Rosewell Park Memorial Institute; RT–PCR, reverse transcription–PCR; siRNA, short interfering RNA.