Abstract
Objective
To test the feasibility of implementing ask-advise-refer (AAR) in representative community chain pharmacies serving low socioeconomic areas, and to assess the effectiveness of a multimodal intervention on short-term implementation of AAR.
Design
Randomized controlled trial
Settings
Sixteen community chain pharmacies in South-central Wisconsin
Intervention
A multimodal intervention including: 1) training to implement AAR, 2) workflow integration recommendations, 3) a cessation poster to create awareness, and 4) a support visit.
Main outcome measures
Number of patrons asked about their tobacco use, number of tobacco users advised to quit, number of quitline cards given, and number of tobacco users enrolled in the quitline.
Results
As hypothesized, the multimodal intervention significantly predicted the number of patrons asked (estimate=4.84, incidence rate ratios[IRR]=127.2; p<0.001) tobacco users advised (estimate=2.12, IRR=8.33; p<0.01), quitline cards distributed (estimate=1.04, IRR=2.82; p<0.05), and tobacco users enrolled in the quitline (estimate=2.31, IRR=10.13; p<0.001).
Conclusion
This trial demonstrates the feasibility of implementing AAR in routine community pharmacy practice. This trial also indicates the short-term effectiveness of the intervention in facilitating AAR, implementation in partnership with other public health services and systems. More research is needed to evaluate the generalizability, effectiveness and sustainability of AAR, including factors influencing adoption and the impact on cessation.
Keywords: tobacco cessation, community pharmacy services, community pharmacists
INTRODUCTION
Cigarette smoking is the leading cause of preventable mortality in the United States, with current prevalence among adults at 20%.1,2 It is well established that tobacco dependence can cause and/or aggravate various health conditions including several types of cancers. 3 Additionally, it leads to undesirable effects on society in the form of billions of dollars in financial losses. 4
Brief tobacco cessation interventions by healthcare providers are effective and can be feasibly implemented.5 There has been steadily growing emphasis on promoting cessation interventions among non-physician providers,6,7 including pharmacists.8,9 The current evidence-based clinical practice guidelines5 recommend that all clinicians, including pharmacists, should routinely implement the 5As approach on a routine basis. In this 5-stage approach, clinicians should ask all patients whether they use tobacco, advise them to quit, assess their readiness to quit, provide assistance during quitting and arrange follow-up with those who quit. Recent evidence also suggests that interventions by more than one provider have the potential to substantially increase quitting rates.10
Given their easy accessibility, community pharmacists are in a unique position to promote and assist in tobacco cessation counseling. They have regular contact with patients, often on a monthly basis, and meeting with a pharmacist does not require an appointment. Research indicates that community pharmacist-led cessation interventions are effective;11 however, only 14% of pharmacies are involved in routine tobacco cessation counseling.12 Several personal and environmental barriers to cessation counseling have been reported by pharmacists, including lack of training and self-efficacy.13 Lack of time has been consistently identified as a key impediment. 13,14,15 Accepting this crucial barrier, pharmacy advocates are now promoting a recently introduced alternative approach derived from the 5As called Ask-Advise-Refer (AAR).9,16 The evidence-based guidelines specifically recommend AAR in situations where 5As might not be feasible (e.g. busy settings).5 The 3-stage AAR approach involves: asking patrons whether they use tobacco, advising tobacco users to quit, and referring tobacco users to an intensive program (e.g. telephone quitlines that effectively offer free behavioral counseling to help tobacco users quit 17). Users who are ready to quit in the next month skip the advise stage and go directly to the referral. Consistent with the 5As, advising involves ‘urging the tobacco user to stop using tobacco’.
Pharmacists might be more likely to implement AAR without having to compromise their immediate dispensing responsibilities. With over 175,000 pharmacists working in retail settings, each pharmacist enrolling one smoker to the quitline per month could result in over 2 million annual enrollments. Finally, this approach furthers the potential for pharmacy to collaborate with public health resources for the referral.
Several factors have been hypothesized as potential facilitators to implementing AAR in community pharmacies. These include pharmacists’ training, technician support, and having patients initiate cessation discussions or request services.18 Few studies have assessed the feasibility of AAR,6,7,19 particularly in community pharmacies,8,15 and the effectiveness of these facilitators in promoting AAR implementation. Purcell and colleagues8 demonstrated AAR feasibility in 2 independent pharmacies with low prescription volume. Baggarly and colleagues15 studied AAR implementation by 9 community pharmacists affiliated with a state university. Although informative, both studies employed weaker designs and contained a motivated sample. Thus there remains a need to evaluate the feasibility of AAR in real-world representative pharmacy settings, and to identify effective interventions to facilitate implementation. Additionally, there has been growing emphasis on trying to reach the high risk groups that need the most help, such as low socioeconomic groups.20 Tobacco use is more common in people living at or below the federal poverty level and people with lower levels of education.2 With the goal of addressing these gaps, this paper presents findings from the first pharmacy-based randomized controlled trial that evaluated feasibility of AAR in community chain pharmacies serving low socioeconomic areas.
OBJECTIVES
The study objectives were to assess the feasibility of implementing AAR in community chain pharmacies and to assess the impact of a multimodal intervention on short-term implementation of AAR. It was hypothesized that compared to the control group (usual care pharmacies), significantly more tobacco users visiting the experimental group pharmacies would be: 1) asked whether they use tobacco, 2) advised to quit, 3) referred and enrolled in the quitline via the Fax-to-Quit program at each pharmacy, and 4) referred to the quitline by distributing quitline cards.
METHODS
Setting
The sampling frame for this study consisted of community pharmacies in South-central Wisconsin that met the following inclusion criteria: 1) they belonged to a specific large national chain pharmacy, 2) they were located within 75 miles radius from Madison, and 3) they were located in an area of lower socioeconomic status than the state average. Low socioeconomic areas were identified using US Census 2000 data (education and per capita income).
Study Design
A post-test only two-group randomized controlled trial (RCT) was conducted with pharmacies as the unit of analyses. This study was approved by the Institutional Review Board at the University of Wisconsin-Madison. The authors selected a one month study period given budget constraints and the primary goals of assessing: 1) feasibility of AAR, and 2) short-term impact of a multimodal intervention. The study was conducted between July 2008 (pharmacy recruitment) - March 2009 (data analysis), with data gathered in November 2008.
Eighteen pharmacies met the inclusion criteria and 16 were randomly selected to participate in the study. The 2 excluded pharmacies were similar to those selected, except for lower prescription volume per day. These 16 pharmacies were then randomly assigned to a control group or experimental group using blocked randomization which involves random assignment into groups after matching on a blocking covariate. Based on the review of literature, ‘pharmacists’ self-efficacy toward implementing AAR’ was used as the blocking covariate. Random assignment was carried out by research assistants blinded to the study goal. The authors were not involved in this assignment process. Following random selection and random assignment, 2 full-time pharmacists and 3 full-time technicians were invited from each pharmacy to participate in the study.
Following random assignment, both the groups were compared on various baseline characteristics including pharmacists’ self-efficacy toward implementing AAR, average number of prescriptions dispensed per day per dispensing staff employed (as a proxy for lack of time and pharmacy busyness), pharmacists’ prior tobacco cessation training, and pharmacist demographic variables.
To minimize any bias in their cessation counseling behavior and/or reporting of behavior, pharmacy staff were not informed about the existence of two groups in the study and were thus blinded to group assignment throughout. Although aware of the pharmacy group assignment, the primary author (who conducted staff training at all sites), was blinded to pharmacists’ self-efficacy scores throughout the study.
Intervention
The control group pharmacies received quitline cards, an informational presentation about the quitline and its services, and enrollment in a free service called Fax-to-Quit21 (FTQ). FTQ enabled pharmacies to proactively refer tobacco users to the quitline by faxing a signed consent form that allowed the quitline to directly call back users to initiate cessation treatment. The experimental group pharmacies received all that was provided to the control, as well as a multimodal intervention that involved training, recommendations for integrating AAR in pharmacy workflow, a cessation poster, and a support visit. (Table 1)
Table 1.
Comparison between interventions delivered to experimental group pharmacies and control group pharmacies
| Intervention Component | Experimental Pharmacies | Control Pharmacies |
|---|---|---|
| Quitline cards | ✓ | ✓ |
| Informational Presentation | ✓ | ✓ |
| Enrolling Pharmacy in Fax-to-Quit service | ✓ | ✓ |
| Pharmacist and Technician Training | ✓ | - |
| Recommendations for integrating AAR in pharmacy workflow | ✓ | - |
| Poster to create patient awareness | ✓ | - |
| Support Visit | ✓ | - |
The multimodal intervention, uniquely provided to the experimental group, directly drew from the authors’ formative qualitative study18, literature on smoking cessation, and constructs from the Social Cognitive Theory.22 The qualitative study concluded that multiple factors could influence successful AAR implementation including pharmacists’ comfort and confidence in conducting AAR, technicians’ support, setting up a system so a counseling pharmacist would know a tobacco users’ readiness to quit prior to cessation discussion with the user, encouraging tobacco users to proactively initiate cessation discussions, and integrating AAR in existing workflow. Following paragraphs discuss the various multimodal intervention elements.
Training
Prior to the 1-month implementation, experimental group pharmacists and technicians received on-site training from the primary author (in groups of 2-3) to conduct AAR counseling. The training program lasted for about half an hour. Drawing from the constructs of behavioral capability and observational learning, the training element primarily consisted of: 1) a didactic presentation on the steps involved in AAR, 2) technician and pharmacist-specific model videos that demonstrated how to initiate and conduct the AAR-based brief tobacco cessation discussions with patrons who are at various stages of motivation to quit (precontemplation, contemplation, preparation), and 3) self-study scripts that they could use to initiate cessation discussions with tobacco users. Pharmacists were trained to ask tobacco users if they were ready to quit in the next month. Pharmacists were then told to advise tobacco users to quit who hadn’t already decided to quit in the next month. If users had already decided to quit, pharmacists did not advise to quit. Instead they referred them to the quitline by handing out a quitline card and encouraging them to enroll in the quitline by completing a fax-to-quit form. This form was then faxed to the quitline by the pharmacy staff. Tobacco users who were expecting to quit, but not in the next month, were also given the card.
Recommendations for integration of AAR in pharmacy workflow
The primary author made recommendations for implementing AAR, in a manner consistent with each experimental pharmacy’s workflow. This involved divided responsibilities between pharmacy staff, with the technicians trained to ask patrons whether they used tobacco and the pharmacists to subsequently advise tobacco users to quit and refer them to the quitline according to their stage of readiness to quit. (Figure 1)
Figure 1.
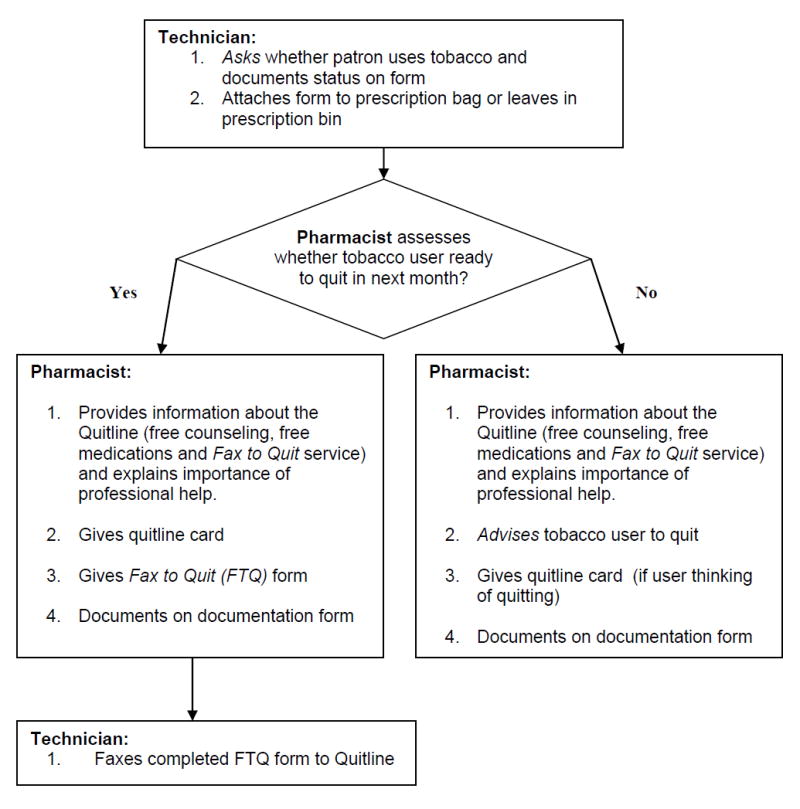
Guidance on Integrating Ask-Advise-Refer in Routine Pharmacy Workflow
RESUME proofread Cessation Poster. Copies of a cessation poster designed by the primary author were placed at directly visible spots at the drop-off, pick-up and drive-through windows of each experimental pharmacy (Figure 2). The goals of the poster were to create awareness and initiate tobacco user inquiry about the quitline. In addition to brief information about the quitline including availability of free smoking cessation medications, the poster also informed the patrons about AAR being conducted at the pharmacy staff and encouraged them to consult their pharmacist for cessation advice.
Support Visit
In the first week of implementation, a follow-up visit was made by the primary investigator to each experimental pharmacy. This visit identified and resolved any challenges faced in implementing AAR.
Measures and Data Collection
The outcome measures were number of patrons asked about tobacco use, number of tobacco users advised to quit, number of users enrolled in the quitline via FTQ (primary referral measure) at each pharmacy, and number of quitline cards given. Asking was operationalized as ‘making a direct verbal inquiry regarding a patron’s current tobacco use’, and advising was operationalized as ‘urging the tobacco user to stop using tobacco’. Advising was only done with tobacco users who were not willing to quit in the next month.
Referral was measured in two ways. The primary measure consisted of Fax-To-Quit data from the quitline. Data on number of users enrolled in the quitline via FTQ was directly obtained from the quitline’s FTQ reports. These reports provided the number of tobacco users proactively enrolled by each pharmacy on a monthly basis. The second measure of referral was collected by self-report as described below.
Data on number of patrons asked, number of users advised and number of cards distributed were gathered using simple check-off documentation forms designed with input and feedback from practicing pharmacists. Staff was instructed to check-off asking only if they made a direct verbal inquiry, and not when they were able to identify a patron’s tobacco use status indirectly (e.g. patron picking up a cessation medication). The same 3 items were included on the documentation forms for the control and the experimental groups. In addition, to prevent these items from triggering AAR in the control group, documentation forms for control pharmacies also included 5 other items relating to cessation medications (e.g. helped patron choose smoking cessation medication). In all pharmacies, check-off forms were readily (and conspicuously) available at various locations where interaction between staff and patrons could occur. Staff were instructed to use one form per patron/tobacco user, and to check-off relevant items for the activity performed (asked, advised, gave card).
A 21-item baseline survey was self-administered by all pharmacists. This survey assessed pharmacists’ self-efficacy toward AAR, average number of prescriptions dispensed per day per dispensing staff employed (AvgRx/Day/Staff)23, pharmacists’ prior tobacco cessation training, and pharmacist demographics (age range, gender, years since graduation). A self-efficacy index score was created for each pharmacy by adding the individual pharmacist’s self-efficacy scores at baseline. Self-efficacy for each pharmacist was measured using an 11-item 7-point rating scale developed by the author (1=not at all and 7=extremely), using standard scale development criteria 23 and adopting item structures from Hudmon’s 5As self-efficacy scale.13 experience AvgRx/Day/Staff was calculated by dividing store-specific data on average number of prescriptions dispensed per day (obtained directly from the chain’s database) by the pharmacy manager’s self-report of number of dispensing staff employed per day.24 Prior tobacco cessation training experience was assessed by using a single item with a dichotomous response option (Yes/No).14
All tools were reviewed by 4 pharmacists for ease of understanding followed by cognitive interviews, 35 and pilot tested on 33 pharmacists who did not participate in the trial. Cognitive interviewing involved interviewing survey participants for their comprehension and interpretation of the survey questions in order to reduce error. Also, the primary author assessed the ease of reporting and completeness of the documentation forms via on-site observations. Lastly, to minimize the chance of staff improving their counseling activities because they were being researched,36 the authors emphasized in written and oral communication with all staff that the goal of this project was to assess the feasibility of AAR, and there were no expectations regarding counseling (e.g. ‘how many patrons to ask).
Data Analysis
Given that the outcome variables were counts, and due to evidence of overdispersion (sample variance exceeded sample mean), data were analyzed using negative binomial regression for each outcome variable (α = 0.05).37 Given limited sample size, only bivariate regression models were run using the multimodal intervention as the dichotomous predictor. Data were analyzed using STATA Version 10. The two groups were compared at baseline using two-tailed significant tests at α = 0.05.
RESULTS
Study Participation
The participation rates for this trial (pharmacy and pharmacy staff) were very high. All randomly selected pharmacies (n=16) agreed to participate, and completed the study. Additionally, all but 1 of the 32 pharmacists and 1 of the 48 technicians agreed to participate in the study (who were then substituted with another pharmacist and technician from those pharmacies), with no dropouts.
Baseline Assessment
There were no significant between group differences on any pharmacy or pharmacist characteristic (Table 2). The AAR self-efficacy scale showed high reliability (Cronbach’s α =0.95) and all items loaded on one factor (65% variance explained). None of the pharmacists had systematically implemented AAR in the past. Only a few (control=3; experimental=4) reported some AAR-related experience, mainly as discrete counseling events that involved tobacco users with worsening health problems.
Table 2.
Between-group comparison on baseline characteristics
| Variable | Experimental Group | Control Group | p-valuea |
|---|---|---|---|
| Pharmacy Characteristics (n=16) | |||
| Self-efficacy Index Score, mean(SD) | 7.39 (2.15) | 7.44 (2.07) | 0.96 |
| Prescriptions dispensed per day , mean (SD) | 287.41 (130.72) | 293.89 (155.92) | 0.93 |
| Prescriptions dispsensed per day per dispensing staff employed, mean (SD) | 67.39 (17.92) | 65.98 (13.75) | 0.86 |
| Pharmacist Characteristics (n=30) | |||
| Years since graduation, mean (SD) | 10.80 (12.3) | 14.87 (12.80) | 0.38 |
| Sex, n (%) | |||
| Female | 8 (57) | 6 (43) | |
| Male | 7 (44) | 9 (57) | 0.71 |
| Age range, n (%) | |||
| 25-34 years | 9 (60) | 6 (40) | |
| 35-44 years | 3 (50) | 3 (50) | |
| 45-54 years | 1 (25) | 3 (75) | |
| 55-64 years | 2 (40) | 3 (60) | 0.61 |
| Self-efficacy toward AAR, n (%) | |||
| Low (1- 3.5) | 5 (50) | 5 (50) | |
| High (3.6 – 7) | 10 (50) | 10 (50) | 1.0 |
| Past training in smoking cessation, n (%) | |||
| Yes | 9 (50) | 9 (50) | |
| No | 12 (50) | 12 (50) | 1.0 |
| Past discrete AAR-like experiences, n (%) | |||
| Yes | 3 (42.9) | 4 (57.1) | |
| No | 12 (52.2) | 11 (47.8) | 1.0 |
two-tailed significance tests
Study Hypotheses
All study hypotheses were confirmed. Descriptive statistics (Table 3) and regression analyses (Table 4) for each hypothesis are presented below. The (pharmacy level) self-report based subjective measure for ask (r=0.78; p<0.05) and advise (r=0.77; p<0.05) correlated significantly with the objective measure for number of tobacco users enrolled obtained directly from the quitline’s FTQ enrollment reports, thus indicating strong concurrent validity for the subjective self-report items, plus valid and complete data entry.
Table 3.
Descriptive analysis of AAR outcomes at experimental and control group pharmacies
| Outcome Variable | Experimental | Control | ||
|---|---|---|---|---|
| Total (no. of pharmacies = 8) | Mean (Std. Dev.) | Total (no. of pharmacies =8) | Mean (Std. Dev.) | |
| No. of patrons asked whether they use tobacco | 636 | 79.5 (91.94) | 5 | 0.63 (0.92) |
| No. of tobacco users advised to quit (who were not interested in the next month)a | 25 | 3.13 (3) | 3 | 0.38 (0.74) |
| No. of quitline cards distributed to tobacco users thinking of quitting (passive referral)b | 240 | 30 (26.17) | 85 | 10.63 (9.83) |
| No. of quitline enrollments via FTQ (active referral) | 81 | 10.13 (9.28) | 8 | 1.0 (1.69) |
Control pharmacy staff was not given any instructions on whether to advising only those who were ready to quit in the next month.
Control pharmacy staff was not given any instructions on who should receive quitline cards.
Table 4.
Effect of multimodal intervention on AAR implementation
| Outcome variable (n = 16, number of pharmacies) | Regression Coefficienta | IRRb | Robust Standard Error | P value | 95% Confidence Interval |
|---|---|---|---|---|---|
| No. of patrons asked | 4.84 | 127.2 | 0.64 | .000c | 3.56 – 6.10 |
| No. of tobacco users advised | 2.12 | 8.33 | 0.75 | .005d | 0.64 – 3.60 |
| No. of cards distributed | 1.04 | 2.82 | 0.43 | .017e | 0.19 – 1.89 |
| No. of quitline enrollments | 2.31 | 10.13 | 0.97 | .000c | 1.03 – 3.60 |
Independent variable: Multimodal training intervention (dichotomous; 0=No, 1=Yes)
IRR is the ‘incidence rate ratio’ obtained by exponentiating the regression coefficient. IRR compares experimental pharmacies to control pharmacies on each of the four dependent variables (asked, advised, cards distributed, quitline enrollments).
p < 0.001; two-tailed test
p < 0.01; two-tailed test
p < 0.05; two-tailed test
A total of 636 patrons (Mean=79.5, Std.Dev.=91.94) were asked whether they used tobacco in all experimental group pharmacies, compared to 5 in all control pharmacies (Mean=0.63, Std. Dev.=0.92). This difference was statistically significant (estimate=4.84, IRR[incidence rate ratio]=127.2, p<0.001).
A total of 25 tobacco users who were not ready to quit in the next 30 days were advised to quit in the experimental group pharmacies (Mean=3.13, Std. Dev.=3) compared to 3 in the control (Mean=0.38, Std. Dev.=0.74). Advise was defined as ‘urging the tobacco users to stop using tobacco’. The number advised to quit was significantly higher in the experimental than the control group (estimate=2.12, IRR=8.33; p<0.01).
Eighty-one tobacco users were enrolled in the quitline via FTQ (active referral) from the experimental group (Mean=10.13, Std. Dev.=9.28) as compared to 8 from the control (Mean=1, Std. Dev.=1.69) As hypothesized, the number of enrollments from the experimental group were significantly higher than from the control (estimate=2.31, IRR=10.13; p<0.001).
A total of 240 quitline cards (passive referral) were reportedly distributed (Mean=30, Std. Dev.=26.17) in the experimental pharmacies; whereas, the control pharmacies distributed 85 cards (Mean=10.63, Std. Dev.=9.8). The between-group difference was found to be statistically significant (estimate=1.04, IRR=2.82; p<0.05).
DISCUSSION
This trial demonstrates the feasibility of implementing AAR in routine practice in community chain pharmacies. It also suggests the potential for a successful partnership between community pharmacy and other public health entitities. A significant number of tobacco users were counseled and subsequently enrolled in the quitline at the experimental pharmacies. Further, far more quitline cards were distributed and quitline enrollments triggered, than number of tobacco users advised to quit in the experimental group of pharmacies. This suggests that community-pharmacy based quitline referrals might be a particularly good match for busy pharmacists interested in delivering brief cessation interventions to tobacco users who want to quit in the next month. The results also indicate the short-term effectiveness of the multimodal intervention in facilitating AAR. These results are similar to those from other community pharmacy-based studies of AAR,26, 27 and those from other healthcare fields.22-25
Consistent with the existing literature, very few patrons visiting the control pharmacies were asked whether they used tobacco.14,16,30,31 In addition to the lack of training, this could be attributed to pharmacists’ misperceptions of offending patrons , lack of confidence and their attitudes toward cessation counseling.21,29,30,32
Although experimental group pharmacies were significantly more likely to distribute quitline cards than control pharmacies were, the mean number of cards distributed at the control group pharmacies was high (see Table 3). This was particularly interesting since proactive ask and advise outcomes at control pharmacies were minimal.
As hypothesized, there were also statistically significant group differences on the number of tobacco users who enrolled in the quitline via FTQ. Given that the data on the number of quitline enrollments came directly from FTQ program’s quitline reports, these objective data are especially important for evluating the intervention’s impact. The FTQ data was highly correlated with the self-report data obtained from the documentation forms. The significant group differences in these FTQ data further validate the self-report measures used for ask and advise behaviors performed by pharmacy staff.
Although the experimental group pharmacies were successful in asking, advising and referring tobacco users to the quitline compared to the control group pharmacies (Table 3), the absolute number of patrons asked, advised, and referred was relatively low with significant within group variability. (Table 3) In addition to some of the reasons discussed earlier, it is also possible that lack of time and attention to smoking cessation counseling (given other immediate responsibilities such as dispensing medications) continued to present significant barriers to a larger scale implementation of AAR. Further, differential availability of time and staff attitudes toward tobacco cessation counseling between the various pharmacies could potentially explain the within group variability. Future larger scale studies should adjust for such confounders, and should also assess the challenges faced by pharmacy staff in implementating AAR.
A key strength of this study lies in its randomized controlled trial design with 16 busy chain pharmacies. Another strength was the use of an objective measure of impact, which was the fax-to-quit enrollment data directly obtained from the quitline. Finally, this randomized trial adds to the nascent literature on trials that systematically assess facilitators of expanded services in community pharmacies. It documents that pharmacists’ adoption of programs such as AAR requires more than providing pharmacists with referral cards and mechanisms of documentation.
LIMITATIONS
This study was based on a 16-pharmacy sample from a specific region. Thus findings need to be confirmed in other geographic locations with larger samples and follow-ups over longer periods of time to assess AAR sustainability. Future studies could also assess quit rates which this feasibility study was not designed to do. Percentages instead of number of patrons/tobacco users would have been ideal. However, total number of patrons visiting pharmacies was not available in their database.
Leaving out the five additional items from the experimental pharmacies’ self-documentation forms could have led them to endorse the only items they were given (ask, advise, gave card). However, as evidence of concurrent validation, self-documented behaviors were found to correlate highly with the objective data obtained from the quitline. Finally, it is possible that regression estimates could be biased given the sample size. However, it is important to note that the authors conducted Mann-Whitney U tests which confirmed the findings regarding the intervention effectiveness.
CONCLUSION
This trial demonstrated the feasibility of implementing AAR in community chain pharmacies, and indicated the high potential for successfully integrating quitline referrals in real-world community pharmacy settings. This study also modeled how public health organizations can effectively collaborate with community pharmacies to provide evidence-based tobacco cessation treatments. As the next step, larger scale effectiveness trials involving quit rate assessment and longer periods of implementation are needed. Future research can also disentangle elements of the multimodal intervention to identify most critical facilitators of AAR.
Acknowledgments
To David H. Kreling, PhD, RPh; David A. Mott, PhD, RPh; Nora C. Schaeffer, PhD; Beth A. Martin, PhD, RPh; and Henry N. Young, PhD, University of Wisconsin Madison; Brian McIlhone, RPh, Walgreens Co; all participating pharmacists and technicians; David B. Abrams, PhD; Amanda L. Graham, PhD; and Raymond Niaura, PhD, American Legacy Foundation.
Funding Sources: Wisconsin Department of Health Services & Sonderegger Research Center, School of Pharmacy, University of Wisconsin–Madison. Also supported by grant 1UL1RR025011 from the Clinical and Translational Science Award program of the National Center for Research Resources, National Institutes of Health.
Biography
Dr. Patwardhan was a graduate student at the University of Wisconsin-Madison when this research was conducted.
Footnotes
Disclosure: The authors declare no conflicts of interest or financial interests in any product or service mentioned in this article, including grants, employment, gifts, stock holdings, or honoraria.
Conference Presentation: Podium presentation at the American Pharmacists Association Annual Meeting, Washington DC, March 15, 2010.
Contributor Information
Pallavi D. Patwardhan, The Schroeder Institute for Tobacco Research & Policy Studies, American Legacy Foundation, Washington DC.
Betty A. Chewning, Social and Administrative Sciences in Pharmacy, School of Pharmacy, University of Wisconsin-Madison, Madison, WI.
References
- 1.Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6(4):e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults and trends in smoking cessation - United States, 2008. Morb Mortal Wkly Rep. 2009;58(44):1227–1232. [PubMed] [Google Scholar]
- 3.The health consequences of smoking: a report of the Surgeon General. Atlanta, Ga: Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. Washington, D.C.: For sale by the Supt. of Docs., U.S. G.P.O. [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000-2004. Morb Mortal Wkly Rep. 2008;57(45):1226–1228. [PubMed] [Google Scholar]
- 5.Fiore MC, Jaen CR, Baker TB, et al. Clinical practice guideline. Rockville, MD: U.S Department of Health and Human Services Public Health Service; May, 2008. Treating tobacco use and dependence: 2008 update. [Google Scholar]
- 6.Mahabee-Gittens EM, Gordon JS, Krugh ME, et al. A smoking cessation intervention plus proactive quitline referral in the pediatric emergency department: a pilot study. Nicotine Tob Res. 2008;10(12):1745–1751. doi: 10.1080/14622200802443494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon JS, Andrews JA, Crews KM, Payne TJ, Severson HH, Lichtenstein E. Do faxed quitline referrals add value to dental office-based tobacco-use cessation interventions? J Am Dent Assoc. 2010;141(8):1000–1007. doi: 10.14219/jada.archive.2010.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purcell JL, Farris KB, Aquilino ML. Feasibility of brief smoking cessation intervention in community pharmacies. J Am Pharm Assoc. 2006;46(5):616–618. doi: 10.1331/1544-3191.46.5.616.purcell. [DOI] [PubMed] [Google Scholar]
- 9.Creating a road map for pharmacy’s role in the cessation of tobacco use. Am J Health Syst Pharm. 2006;63(6):564–566. doi: 10.2146/ajhp050350. [DOI] [PubMed] [Google Scholar]
- 10.An LC, Foldes SS, Alesci NL, et al. The impact of smoking-cessation intervention by multiple health professionals. Am J Prev Med. 2008;34(1):54–60. doi: 10.1016/j.amepre.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007;27(7):1040–1051. doi: 10.1592/phco.27.7.1040. [DOI] [PubMed] [Google Scholar]
- 12.Doucette WR, Kreling DH, Schommer JC, et al. Evaluation of community pharmacy service mix: evidence from the 2004 National Pharmacist Workforce Study. J Am Pharm Assoc. 2006;46(3):348–355. doi: 10.1331/154434506777069471. [DOI] [PubMed] [Google Scholar]
- 13.Hudmon KS, Prokhorov AV, Corelli RL. Tobacco cessation counseling: pharmacists’ opinions and practices. Patient Educ Couns. 2006;61(1):152–160. doi: 10.1016/j.pec.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Aquilino ML, Farris KB, Zillich AJ, et al. Smoking-cessation services in Iowa community pharmacies. Pharmacotherapy. 2003;23(5):666–673. doi: 10.1592/phco.23.5.666.32192. [DOI] [PubMed] [Google Scholar]
- 15.Baggarly SA, Jenkins TL, Biglane GC, Smith GW, Smith CM, Blaylock BL. Implementing a referral to telephone tobacco cessation services in louisiana community pharmacies: a pilot study. Ann Pharmacother. 2010;44(9):1395–1402. doi: 10.1345/aph.1P226. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder SA. What to do with a patient who smokes. JAMA. 2005;294(4):482–487. doi: 10.1001/jama.294.4.482. [DOI] [PubMed] [Google Scholar]
- 17.Zhu SH, Anderson CM, Tedeschi GJ, et al. Evidence of real-world effectiveness of a telephone quitline for smokers. N Engl J Med. 2002;347(14):1087–1093. doi: 10.1056/NEJMsa020660. [DOI] [PubMed] [Google Scholar]
- 18.Patwardhan PD, Chewning B. Ask, advise and refer: hypothesis generation to promote a brief tobacco-cessation intervention in community pharmacies. IJPP. 2009;17:221–229. doi: 10.1211/ijpp/17.04.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothemich SF, Woolf SH, Johnson RE, et al. Promoting primary care smoking-cessation support with quitlines: the QuitLink Randomized Controlled Trial. Am J Prev Med. 2010;38(4):367–374. doi: 10.1016/j.amepre.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Orleans CT. Increasing the demand for and use of effective smoking-cessation treatments reaping the full health benefits of tobacco-control science and policy gains--in our lifetime. Am J Prev Med. 2007;33(6 Suppl):S340–8. doi: 10.1016/j.amepre.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Kobinsky KH, Redmond LA, Smith SS, et al. The Wisconsin Tobacco Quit Line’s Fax to Quit program: participant satisfaction and effectiveness. WMJ. 2010;109(2):79–84. [PubMed] [Google Scholar]
- 22.Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, N.J: Prentice-Hall; 1986. [Google Scholar]
- 23.DeVellis RF. Scale Development: Theory and Applications. Second ed. United States of America: Sage Publications; 2003. [Google Scholar]
- 24.Gadkari AS, Mott DA, Kreling DH, et al. Pharmacy characteristics associated with the provision of drug therapy services in nonmetropolitan community pharmacies. J Rural Health. 2009;25(3):290–295. doi: 10.1111/j.1748-0361.2009.00232.x. [DOI] [PubMed] [Google Scholar]
- 25.Willis G. Cognitive interviewing: A tool for implementing questionnaire design. United Stated of America, Thousand Oaks, California: Sage Publications; 2004. [Google Scholar]
- 26.Campbell D, Stanley J. Experimental and quasi-experimental designs for research. U.S.A: Houghton Mifflin Company; 1963. p. 20. [Google Scholar]
- 27.Hilbe JM. Negative Binomial Regression. United Kingdom: Cambridge University Press; 2008. [Google Scholar]
- 28.Patwardhan PD, Chewning BA. Tobacco users’ perceptions of a brief tobacco cessation intervention in community pharmacies. J Am Pharm Assoc (2003) 2010;50(5):568–574. doi: 10.1331/JAPhA.2010.09207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashley MJ, Brewster JM, Victor JC. Pharmacists’ smoking cessation practices: relationship to their knowledge and skills, attitudes, and perceptions of roles. J Am Pharm Assoc. 2006;46:729–37. doi: 10.1331/1544-3191.46.6.729.ashley. [DOI] [PubMed] [Google Scholar]
- 30.Anderson C, Blenkinsopp A, Armstrong M. The Contribution of Community Pharmacy to Improving the Public’s Health. Report 1: Evidence from the Peer-reviewed Literature 1990–2001. 2003:39. doi: 10.1111/j.1369-7625.2004.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An LC, Foldes SS, Alesci NL, et al. The impact of smoking-cessation intervention by multiple health professionals. Am J Prev Med. 2008;34(1):54–60. doi: 10.1016/j.amepre.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 32.Monson AL. Barriers to tobacco cessation counseling and effectiveness of training. J Dent Hyg. 2004;78(3):5. [PubMed] [Google Scholar]


