Abstract
Purpose of review
The purpose of this review is to evaluate the most recent findings on indoor allergens and their impact on allergic diseases.
Recent findings
Indoor allergens are present inside buildings (home, work environment, school), and given the chronic nature of the exposures, indoor allergies tend to be associated with the development of asthma. The most common indoor allergens are derived from dust mites, cockroaches, mammals (including wild rodents and pets), and fungi. The advent of molecular biology and proteomics has led to the identification, cloning, and expression of new indoor allergens, which have facilitated research to elucidate their role in allergic diseases. This review is an update on new allergens and their molecular features, together with the most recent reports on their avoidance for allergy prevention and their use for diagnosis and treatment.
Summary
Research progress on indoor allergens will result in the development of new diagnostic tools and design of coherent strategies for immunotherapy.
Keywords: Indoor allergens, Mite, Cockroach, Cat, Dog, Fungi
Introduction
Sensitization and exposure to indoor allergens is a risk factor for allergic respiratory diseases, including rhinitis and asthma [1, 2]. In this report, an overview of the most important indoor allergens is presented, together with an update on the most current cutting edge research on their molecular structure and function (Table 1) [3•] and approaches to assess and treat indoor allergies.
Table 1.
Most relevant indoor allergens
| Source | Group | Allergen | Protein family |
|---|---|---|---|
| Mite | 1 | Der p 1, Der f 1 | Cysteine protease |
| 2 | Der p 2, Der f 2 | Immunoglobulin-like | |
| 5 | Der p 5 | Structural protein | |
| 7 | Der p 7, Der f 7 | LPS-binding protein | |
| 10 | Der p 10, Der f 10 | Tropomyosin | |
| 11 | Der p 11, Der f 11 | Paramyosin | |
| 23 | Der p 23 | Chitin-binding protein homologue | |
| Cockroach | 1 | Bla g 1, Per a 1 | Microvilli protein homologue |
| 2 | Bla g 2, Per a 2 | Inactive aspartic protease | |
| 4 | Bla g 4 | Lipocalin | |
| 5 | Bla g 5 | Glutathione S-transferase | |
| 9 | Per a 9 | Arginine kinase | |
| 11 | Bla g 11, Per a 11 | α-Amylase | |
| Cat | 1 | Fel d 1 | Uteroglobulin |
| 4 | Fel d 4 | Lipocalin | |
| Dog | 1 | Can f 1 | Lipocalin |
| 2 | Can f 2 | Lipocalin | |
| 5 | Can f 5 | Arginine esterase | |
| Rodents | 1 | Mus m 1, Rat n 1 | Lipocalin |
| Fungi | 1 | Asp f 1 | Mitogillin |
| 1 | Alt a 1 | Unknown | |
| 6 | Cla h 6 | Enolase | |
| 8 | Cla h 8 | Mannitol dehydrogenase | |
| 13 | Pen ch 13 | Serine protease | |
| 18 | Pen ch 18 | Serine protease |
Arthropods
Mites
House dust mites are an important cause of allergies worldwide, associated with diseases such as allergic rhinitis, atopic dermatitis, and asthma [1]. Mite allergens are classified into 33 groups, listed in the systematic Allergen Nomenclature Database maintained by the World Health Organization and International Union of Immunological Societies (WHO/IUIS) (www.allergen.org). More than 95 % of the allergen accumulating in mite cultures is associated with fecal particles, where Der p 1, the first allergen to be isolated, was estimated to be present at 10 mg/ml [4, 5]. These particles, from 10 to 40 μm of diameter, become airborne upon disturbance. Although inhalation is the most common mechanism of exposure, ingestion of foods made with mite-contaminated wheat flour has also been reported as a source of allergic reactions, including anaphylaxis (“the pancake syndrome”) [6]. The two most common species of dust mites are Dermatophagoides pteronyssinus and Dermatophagoides farinae. Additional mite species are Dermatophagoides microceras, Euroglyphus maynei, and Blomia tropicalis, as well as storage mites Glycyphagus domesticus, Lepidoglyphus destructor, Acarus siro, and Tyrophagus putrescentiae.
Groups 1 and 2
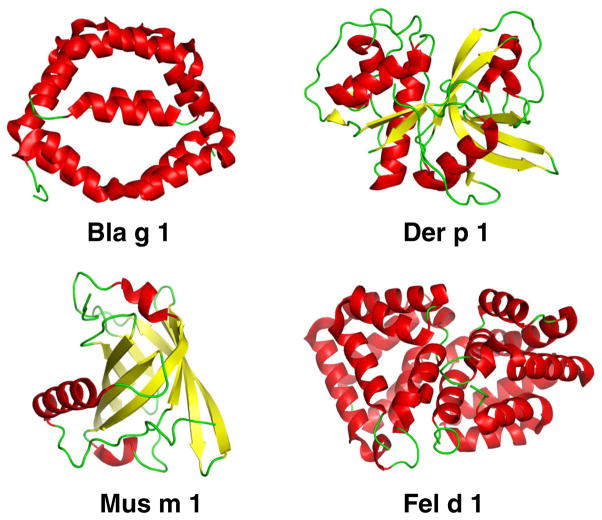
The group 1 and 2 allergens cause sensitization in >80 % of mite-allergic patients. These potent allergens account for 50–60 % of anti-house dust mite IgE antibodies in allergic subjects [7]. The group 1 allergens, Der p 1 and Der f 1, are cysteine proteases. The structures of natural Der p 1 and Der f 1 were determined, alone or in complex with fragments of monoclonal antibodies (mAb) that inhibit IgE antibody binding [8, 9, 10••] (Fig. 1). The proteolytic activity of group 1 has been reported to contribute to allergenicity by cleaving molecules involved in the immune response (i.e., CD23, CD25) and increasing membrane permeability [11]. Group 3, 6, and 9 allergens are serine proteases and may have similar effects.
Fig. 1.
X-ray crystal structures of Bla g 1 (Protein Data Bank ID code 4JRB), Der p 1 (3RVW), Mus m 1 (1MUP), and Fel d 1 (2EJN)
Group 2 mite allergens have immunoglobulin-like folds, which bind lipids in the internal cavity. Der p 2 activates the innate immune system through Toll-like receptors (TLR-4) by mimicking the action of human MD-2 (myeloid differentiation antigen-like lipid-binding protein), a structural homologue that loads lipopolysaccharide (LPS) onto these receptors [12]. A TLR4-associated phospholipase D1 activation has recently been reported to be crucial for Der f 2-induced IL-13 production [13].
Groups 4, 5, 7, and 21
Allergens belonging to groups 4, 5, 7, and 21 together account for 30 % of house dust mite IgE antibodies, and each of them binds IgE in approximately 50 % of mite-allergic subjects [7]. Allergens from group 4 are α-amylases. Groups 5 and 21 contain structurally related proteins with a three-helical bundle [14]. Allergens from group 7 have a similar structure to LPS-binding proteins that interact with Toll-like receptors upon binding of LPS and other bacterially derived lipid ligands [15].
Group 8 allergens are glutathione S-transferases (GST), and group 10 allergens are tropomyosins. The degree of homology of these mite allergens with allergens from other species is an important determinant of allergenic cross-reactivity or lack thereof [16•]. Recently, a tropomyosin was reported from Chortoglyphus arcuatus, a storage mite to whom some patients are mono-sensitized in the northwest of Spain [17]. A mixture of purified mite allergens from groups 1, 2, 5, 7, 8, and 10 bound, on average, 76 % of mite-specific IgE antibodies [18]. Group 11 allergens are paramyosins, and Der p 11 is a new marker allergen for house dust mite-allergic patients suffering from atopic dermatitis [19••].
Der p 23 has been described as a new major allergen with a high prevalence of IgE reactivity (74 %) [20, 21]. However, this allergen accounts for a small percentage of the IgE response to mite allergens, which is dominated by Der p 1 and Der p 2 [22]. Interestingly, RNA expression level of Der p 23 is the lowest of the major allergens. The allergen is a small, globular protein stabilized by two disulphide bonds [22]. Der p 23 is homologous to chitin-binding proteins, but recent studies have shown that the allergen does not bind chitin and must have a different function [22, 23].
In 2015, genomic-transcriptomic and/or proteomic approaches have been used to identify up to 33 mite allergen groups [24, 25••].
Cockroaches
The first report on positive skin test responses to cockroach allergen dates back to 1964 [26]. A strong association between cockroach allergy, allergic rhinitis, and asthma has been demonstrated [27–29]. Inner-city asthma studies in the USA have shown that exposure and sensitization to cockroach allergens are associated with increased asthma morbidity in children [28, 29]. The two most common species are German and American cockroach (Blattella germanica and Periplaneta americana, respectively). The WHO/IUIS Allergen Nomenclature database currently lists 12 groups of cockroach allergens.
Groups 1 and 2
The molecular structure of group 1 cockroach allergens (Bla g 1 and Per a 1) consists of tandem repeats of ~100 amino acids. The determination of the three-dimensional structure of these allergens, challenging due to protein fragmentation, has recently been achieved for Bla g 1 thanks to the expression of its basic structural unit. The Bla g 1 unit comprises two consecutive repeats of six helices each, which encapsulate a large hydrophobic cavity that contains lipids (Fig. 1). This structure allowed the definition of 1 unit of Bla g 1 as 104 ng of allergen, which facilitates allergen standardization [30].
Bla g 2 is a globular protein that belongs to the family of aspartic proteases, but amino acid substitutions in the catalytic site render it inactive [31]. The antigenic surface of Bla g 2 has been analyzed by determining the structure of the allergen in complex with fragments (Fab or Fab′) of mAb that interfere with IgE antibody binding and by site-directed mutagenesis of residues involved in the epitopes [32–34, 35•]. These studies revealed IgE antibody binding sites and mechanisms of allergen-antibody interaction.
Group 3 allergens are homologous to arylphorins and insect hemocyanins. Different isoallergens and variants have been reported for Per a 3 with a wide range of skin test reactivities (26–95 %) [36]. Therefore, the relevance of this allergen remains controversial.
Group 4 allergens are lipocalins, with a similar molecular structure to mammalian lipocalin allergens, e.g., from cow, dog, cat, horse, rat, and mouse (Fig. 1). The molecular structure of Bla g 4 consists of an eight-stranded β-barrel and a C-terminal α-helix [37]. Most lipocalins share a low degree of amino acid identity (~20 %), and no significant cross-reactivity among them is expected.
Group 5 allergens are GST, thought to be involved in detoxification of toxic compounds. Bla g 5, together with Bla g 2, is one of the most important cockroach allergens in USA patients [38]. Recently, the X-ray crystal structures of Bla g 5 and the homologous allergens Der p 8 and Blo t 8 from mites and Asc s 13 from the nematode Ascaris suum were determined. These GST allergens showed a significant lack of cross-reactivity in a US population, suggesting that each individual allergen would be required for molecular diagnostic purposes [16•].
Groups 6, 7, and 8 are structural molecules including troponin C (group 6), tropomyosin (group 7), and myosin light chain. Tropomyosins are ubiquitous inhalant and food panallergens. In Brazil, cockroach tropomyosin is an important allergen, showing potential cross-reactivity with mite and shrimp tropomyosins [27]. Allergens from groups 6 and 8 are minor allergens and regulatory proteins involved in muscle contraction, which undergo structural changes upon calcium binding to EF-hand motifs [39].
Groups 9, 10, 11, and 12 are enzymes: arginine kinases, serine proteases, α-amylases, and chitinases, respectively, and mostly reported for P. americana, except for Bla g 11 [40–43]. A Per a 9-homolog from German cockroach and Bla g 11 have recently been reported to be immunodominant, together with Bla g 5, for T cell responses in asthmatic subjects [44]. The prevalence of IgE to these allergens was relatively high in the Asian countries where they were identified and needs to be evaluated in other parts of the world.
Mammalian Allergens
Animal allergens are primarily produced in the liver or secretory glands and are present on animal skin and in body fluids, such as urine, saliva, and blood. With the exception of the main cat allergen Fel d 1, most major animal allergens belong to the lipocalin family of proteins [45]. The proteins adhere to fur and can thus be efficiently distributed in the environment where they accumulate on fabrics, carpets, upholstery, and mattresses. Numerous studies have confirmed that the distribution of animal allergens in the environment is ubiquitous. Thus, allergy to animal proteins is considered a significant public health problem [46].
Cat (Felis domesticus)
Fel d 1 is a 38 kD tetrameric glycoprotein with a structure similar to that of uteroglobulin [47] (Fig. 1). IgE from over 90 % of cat-sensitized individuals reacts with this major cat allergen [48]. Fel d 1 is produced in sebaceous, anal, and salivary glands and transferred to the fur by grooming [49, 50]. While airborne Fel d 1 is mostly associated with larger particles (>9 μm), about 23 % of airborne Fel d 1 is carried on small particles (<4.7 μm diameter) that stay suspended in the air for several days, favoring distribution of the allergen in the environment [51]. In fact, the quantity of cat allergen in schools is directly correlated to the number of children in the class who live with cats in their homes [52]. Other relevant cat allergens include the cross-reactive serum albumin Fel d 2 and the lipocalins Fel d 4 and Fel d 7, which react with 15–40, 63, and 38 % of IgE from cat-allergic patients, respectively [53–55].
Dog (Canis familiaris)
Four of six currently identified dog allergens, Can f 1, Can f 2, Can f 4, and Can f 6, are lipocalins [56]. About ~70 % of dog-allergic individuals have IgE antibodies specific to the major dog allergen Can f 1 [57, 58]. Can f 1 is detectable not only in all homes with a dog, but also in one third of homes without a dog [59, 60••]. The size distribution of particles associated with Can f 1 is similar to that of Fel d 1 [60••]. A wide variability in Can f 1 levels can be found between dog breeds, but there is no evidence for a hypoallergenic breed [61, 62]. Can f 5 is also considered a major allergen, with up to 70 % of dog-allergic patients having Can f 5-specific IgE. Interestingly, 38 % of dog-allergic patients were monosensitized to Can f 5 [63]. Other relevant dog allergens are the lipocalins Can f 2 and Can f 4 and the cross-reactive dog serum albumin Can f 3, which react with 20–30, 15–50, and 81 % of IgE from dog-allergic patients, respectively [64–67].
Rodents (Mus musculus, Rattus norvegicus)
Allergy to mice and rats is an important occupational health problem. The prevalence of rodent allergy among technicians, animal care takers, physicians, and scientists working in pharmaceutical industry, university laboratories, and animal facilities ranges from 11 to 44 % [68]. Besides exposure in occupational settings, rodent exposure also occurs in domestic environments as was shown in inner-city children with asthma in the USA, where mouse and rat sensitization rates were 11–47 and 21 %, respectively [69, 70•, 71]. In contrast, a recent study from Europe has reported very low sensitization prevalence for mouse and rat (1.5 and 0.5 %, respectively) in urban atopic populations without occupational exposures [72]. Mouse sensitization has also been associated with allergic rhinitis in urban children in the USA with comorbid asthma [73]. Urine is the main source of allergenic proteins in both mice and rats, and the major allergens Mus m 1 from mouse and Rat n 1 from rat are lipocalins. Mus m 1 is carried on small particles that stay airborne for a long time, favoring the distribution of the allergen within the facility and even outside the facility into the homes of laboratory animal workers [74, 75]. Indeed, children of parents who are occupationally exposed to rodents have a higher prevalence of sensitization to mouse, rat, and hamster compared to children of non-exposed parents [76].
Fungi
From more than 15 genera of fungi measurable in inner-city homes, Cladosporium, Penicillium, Aspergillus, and Alternaria species were the most commonly detected [77]. Alternaria and Cladosporium species also produce important outdoor allergens, and sensitization and exposure to species of these genera is associated with the development of asthma and rhinitis, as well as epidemics of asthma exacerbations, some of which are life threatening [78].
Alternaria alternata
The prevalence of sensitization to Alternaria is approximately 5 % and is strongly associated with asthma and allergic rhinitis [79]. Alt a 1 is the most important Alternaria allergen with a seroprevalence of over 90 % among Alternaria-sensitized individuals [80]. The structure of this dimeric allergen has been recently determined [81]. Other relevant Alternaria alternata allergens include Alt a 2, a 25 kD aldehyde dehydrogenase and major allergen, as well as Alt a 5, an enolase, which is recognized by approximately 20 to 50 % of Alternaria-sensitized individuals [82, 83].
Cladosporium herbarum
Similar to Alternaria, C. herbarum is frequently found in indoor and outdoor air and is a major source of fungal inhalant allergens [84]. While Alternaria alternata is a major allergen in humid climates, Cladosporium is the leading allergenic mold in cooler climates [85]. No dominant Cladosporium allergen had been found until the identification of Cla h 8, a NADP-dependent mannitol dehydrogenase. Cla h 8 is recognized by IgE antibodies of 57 % of all Cladosporium-allergic patients [86]. In addition, Cla h 6 (enolase) is recognized by ~50 % of sera from Cladosporium-sensitized patients [87].
Aspergillus fumigatus
Aspergillus fumigatus is a thermo-tolerant fungus with worldwide distribution. A. fumigatus is the principal etiologic agent of allergic bronchopulmonary aspergillosis (ABPA) and is also associated with asthma [88]. Both ABPA and allergic asthma are characterized by hypersensitivity and presence of A. fumigatus-specific IgE, but the sensitization patterns to individual allergens differ [89]. The major allergen, Asp f 1, is an 18 kD ribotoxin that is recognized by 85 % of Aspergillus-sensitized patients [90]. Besides Asp f 1, several other Aspergillus allergens (Asp f 2, Asp f 3, Asp f 4, Asp f 5, Asp f 9, Asp f 11, Asp f 15, and Asp f 18) are associated with a high prevalence of reactivity among Aspergillus-sensitized patients [78].
Penicillium
Penicillium species are prevalent indoor fungi that are associated with allergic disease in sensitized individuals. Penicillium citrinum and Penicillium chrysogenum are the most studied and the two most abundant species in the USA. The major allergens of P. chrysogenum include the serine proteases Pen ch 13 and Pen ch 18 with specific IgE reactivities of 88 and 82 %, respectively [91, 92]. Generally, IgE reactivity to allergens from P. citrinum was lower, and the highest reactivity of 46 % among Penicillium-sensitized asthmatic patients was reported for Pen c 3, an 18 kD membrane protein [93].
Cross-Reactive Indoor Allergens
The most relevant protein families involved in cross-reactivity with indoor allergens are tropomyosins and serum albumins. Tropomyosin is a highly conserved protein found in both muscle and non-muscle cells of all species of vertebrates and invertebrates. Allergenic tropomyosins are found in invertebrates such as crustaceans, arachnids (house dust mites), insects (cockroaches), and mollusks (squid). Immunological cross-reactivity has been demonstrated between crustaceans, cockroaches, and house dust mites, suggesting that tropomyosin is an important cross-sensitizing panallergen. More than 50 % of European house dust mite-allergic patients with IgE sensitization to tropomyosin (Der p 10) have clinically relevant cross-reactivity to eating seafood [94]. However, there is a lack of allergenic cross-reactivity between these tropomyosins and those from vertebrates such as bony fish, beef, pork, or chicken, which are considered nonallergenic [95].
Allergic sensitization to serum albumin can occur by inhalation as well as ingestion [96]. Serum albumins are found in dander and saliva and are important inhalant allergens of cat (Fel d 2) and dog (Can f 3). Chicken serum albumin (Gal d 5) is a major hen egg allergen that is associated with the bird-egg syndrome, a cross-reactivity between ingested egg allergens and inhaled feather and dander allergens [97]. Similarly, the cat-pork syndrome is based on cross-reactivity between Fel d 2 and pork serum albumin (Sus s 6). In this rare syndrome, patients develop an IgE antibody response specific for cat serum albumin Fel d 2 that cross-reacts with porcine albumin Sus s 6 and can lead to severe or even fatal allergic reactions on occasions when pork is consumed [98].
Indoor Allergens for Diagnosis
Commercial allergen extracts are standardized based on in-house assays and standards and are not comparable between different manufacturers [99]. Efforts to improve standardization and enable cross-product comparisons using individual recombinant allergens were initiated in 2001 with the CREATE project of the European Union [100]. Purified allergens from birch, timothy grass, olive pollen, and dust mites were compared with the natural counterparts, and allergen-specific candidate ELISA systems were investigated. Two allergens, rBet v 1 and rPhl p 5, were further characterized through the BSP090 Biological Standardization Programme (BSP) of the European Directorate for the Quality of Medicines and HealthCare (EDQM) [101]. These allergens have since been included in the European Pharmacopeia.
The selection of an optimal set of allergens for diagnosis needs to be evaluated for each source. Sensitization to Fel d 1 in childhood is a good predictor of cat allergy symptoms during adolescence [102]. However, other allergen sources such as cockroach do not have a dominant allergen. Early studies revealed that Bla g 1, Bla g 2, Bla g 4, and Bla g 5 identify ~95 % of cockroach-allergic patients in the USA [103]. As more allergens have been identified around the world, different patterns of IgE sensitization to cockroach have been found in other populations. IgE reactivity to cockroach tropomyosin was found to be dominant in Brazil [27]. Overall, a cocktail of five cockroach allergens from groups 1, 2, 4, 5, and 7 would allow to diagnose 50–65 % of patients worldwide [103].
For mites, Der p 1 and Der p 2 will diagnose most mite-allergic patients. Der p 23 has also been defined as a major allergen [20]. However, the contribution of Der p 23 to mite-specific IgE was small (4 %) compared to Der p 1 and Der p 2 combined (85 %) in a North American population [22]. Der p 1 and Der p 2 diagnosed 96 % of mite-allergic patients, and addition of Der p 23 did not show further improvement. Similarly, a European study reported an IgE prevalence of 89 % for Der p 1 and Der p 2 combined, and the addition of Der p 23 increased the percentage only by 3 % [104].
Allergen Exposure Assessment
Measurements of major allergens in dust and air samples have proved to be an effective approach to assess allergen exposure and to relate exposure to allergic sensitization. While in the past, these measurements were made by ELISA, they are increasingly being replaced by multiplex technology (MARIA) which enables 6–10 allergens to be measured in a single assay [105]. In keeping with the CREATE project, ELISA and MARIA use purified allergens as standards. For example, cockroach allergens are used to be measured in arbitrary units but are now measured in absolute units using purified allergen standards [30, 106]. The advantages of immunoassays are that they provide high throughput and are ideally suited to large cohorts involving hundreds or thousands of environmental samples. Alternatively, much progress has recently been made using mass spectrometry for allergen measurements, and this highly sensitive technology is increasingly being used in the pharmaceutical industry (reviewed in [107]). The joint task force of the AAAAI and ACAAI recently published several practice parameters on environmental exposure assessments as part of allergy practice [108–111]. The parameters provided systematic reviews of the categories of evidence linking allergen exposure and allergic symptoms. The practice parameters did not make specific recommendations regarding allergen exposure thresholds for health effects. Further work in this area is needed to improve indoor air quality in the homes of allergic patients.
Indoor Allergens and Therapy
Avoidance of indoor allergen exposure is an important factor that may ameliorate symptoms but is not always sufficient. Immunotherapy via either subcutaneous or sublingual routes has shown benefits in patients with allergic rhinitis and allergic asthma induced by house dust mites. However, there is a lack of consensus on basic treatment parameters (i.e., dose and duration) and a need for rigorous, long-term, double-blind, placebo-controlled randomized clinical trials for house dust mite allergies [112]. Four pilot studies of cockroach immunotherapy suggest that immunotherapy with cockroach allergen is more likely to be effective with SCIT than SLIT [113].
Modified Indoor Allergens for Immunotherapy
The availability of natural and recombinant purified allergens has led to the design of new immunotherapeutic molecules [114]. The rationale for using modified allergens for immunotherapy is to reduce side effects due to IgE cross-linking during the administration of increasing doses of allergen, while maintaining immunogenicity. Hypoallergenic chemically modified extracts (allergoids) are successfully used in Europe for rhinitis, asthma, and atopic dermatitis. These include carbamylated allergoids and depigmented-polymerized extracts [115–117]. However, the Federal Drug Administration has not approved their use in the USA, because these preparations lack structurally well-defined molecules and are difficult to standardize.
Alternative approaches to immunotherapy are under study, based on current knowledge of the molecular structure of allergens. One of them uses short T cell epitope synthetic peptides from the allergen sequence and has been extensively studied for cat allergy and less for mite [118, 119].
Additional approaches to immunotherapy have become possible with the advent of molecular biology to generate modified allergens expressed as recombinant proteins [114]. The design of protein modifications is based on structural features of the allergens and aims to reduce IgE antibody reactivity while preserving immunogenicity. For example, hybrid molecules were obtained by either combining two allergens, such as Der p 1 and Der p 2 [120, 121], or by combining two fragments in inverse order [122]. Allergens fused to viral domains or viral-like particles have shown immunomodulatory capacity [21]. Another strategy is to perform site-directed mutagenesis of known IgE epitopes, based on the X-ray crystal structures of the allergens alone or in complex with monoclonal antibodies that interfere with IgE antibody binding [9, 10••, 32, 34, 35•, 123, 124].
Conclusions
In the past 20 years, a broader knowledge of indoor allergens from mite, cockroach, cat, dog, rodents, and fungi has led to the development of new strategies for the diagnosis and treatment of allergic disease. These include the use of modified allergens with reduced IgE reactivity, allergen peptides, or fusion proteins with molecules that modulate immune responses.
Acknowledgments
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI077653. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Drs. Pomés, Chapman, and Wünschmann declare a grant from NIAID. Dr. Chapman is founder and a co-owner of Indoor Biotechnologies Inc.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100:S2–24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- 2.Kanchongkittiphon W, Mendell MJ, Gaffin JM, Wang G, Phipatanakul W. Indoor environmental exposures and exacerbation of asthma: an update to the 2000 review by the Institute of Medicine. Environ Health Perspect. 2015;123:6–20. doi: 10.1289/ehp.1307922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Pomés A, et al. 100 years later: celebrating the contributions of x-ray crystallography to allergy and clinical immunology. J Allergy Clin Immunol. 2015;136:29–37. doi: 10.1016/j.jaci.2015.05.016. This is a commemorative review of the 100th anniversary of X-ray crystallography, showing the contributions of this discipline to allergy and clinical immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman MD, Platts-Mills TA. Purification and characterization of the major allergen from Dermatophagoides pteronyssinus-antigen P1. J Immunol. 1980;125:587–92. [PubMed] [Google Scholar]
- 5.Tovey ER, Chapman MD, Platts-Mills TA. Mite faeces are a major source of house dust allergens. Nature. 1981;289:592–3. doi: 10.1038/289592a0. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Borges M, Suarez CR, Capriles-Hulett A, Caballero-Fonseca F, Fernandez-Caldas E. Anaphylaxis from ingestion of mites: pancake anaphylaxis. J Allergy Clin Immunol. 2013;131:31–5. doi: 10.1016/j.jaci.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Thomas WR. House dust allergy and immunotherapy. Hum Vaccin Immunother. 2012;8:1469–78. doi: 10.4161/hv.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chruszcz M, et al. Crystal structures of mite allergens Der f 1 and Der p 1 reveal differences in surface-exposed residues that may influence antibody binding. J Mol Biol. 2009;386:520–30. doi: 10.1016/j.jmb.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chruszcz M, et al. Molecular determinants for antibody binding on group 1 house dust mite allergens. J Biol Chem. 2012;287:7388–98. doi: 10.1074/jbc.M111.311159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Osinski T, et al. Structural analysis of Der p 1-antibody complexes and comparison with complexes of proteins or peptides with monoclonal antibodies. J Immunol. 2015;195:307–16. doi: 10.4049/jimmunol.1402199. Detailed antigenic analysis of Der p 1 based on the X-ray crystal structures of three complexes of Der p 1 with monoclonal antibodies that inhibit IgE antibody binding. A comparative analysis of these complexes with proteins/peptides-antibody complexes that are available in the Protein Data Bank is presented. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shakib F, Ghaemmaghami AM, Sewell HF. The molecular basis of allergenicity. Trends Immunol. 2008;29:633–42. doi: 10.1016/j.it.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Trompette A, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–8. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi HJ, et al. The TLR4-associated phospholipase D1 activation is crucial for Der f 2-induced IL-13 production. Allergy. 2015;70:1569–79. doi: 10.1111/all.12764. [DOI] [PubMed] [Google Scholar]
- 14.Mueller GA, et al. Der p 5 crystal structure provides insight into the group 5 dust mite allergens. J Biol Chem. 2010;285:25394–401. doi: 10.1074/jbc.M110.128306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller GA, et al. The structure of the dust mite allergen Der p 7 reveals similarities to innate immune proteins. J Allergy Clin Immunol. 2010;125:909–17. doi: 10.1016/j.jaci.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Mueller GA, et al. Analysis of glutathione S-transferase allergen cross-reactivity in a North American population: relevance for molecular diagnosis. J Allergy Clin Immunol. 2015;136:1369–77. doi: 10.1016/j.jaci.2015.03.015. This article provides the molecular structural basis for the observed lack of significant IgE cross-reactivity observed among four homologous glutathione S-transferase allergens from cockroach (Bla g 5), mite (Der p 8, Blo t 8) and the helminth Ascaris (Asc s 13), in a North American population. This study has implications for the use of each GST for accurate molecular diagnosis in different geographic areas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Matas MA, et al. Cloning and characterization of tropomyosin from the mite Chortoglyphus arcuatus. Mol Immunol. 2015;68:634–40. doi: 10.1016/j.molimm.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Weghofer M, et al. Comparison of purified Dermatophagoides pteronyssinus allergens and extract by two-dimensional immunoblotting and quantitative immunoglobulin E inhibitions. Clin Exp Allergy. 2005;35:1384–91. doi: 10.1111/j.1365-2222.2005.02345.x. [DOI] [PubMed] [Google Scholar]
- 19••.Banerjee S, et al. Der p 11 is a major allergen for house dust mite-allergic patients suffering from atopic dermatitis. J Invest Dermatol. 2015;135:102–9. doi: 10.1038/jid.2014.271. This study reports paramyosin Der p 11 as a major marker for house dust mite allergic patients suffering from atopic dermatitis. Der p 11 is, together with Der p 10, Der p 14 and Der p 18, one of the few dust mite allergens present primarily in mite bodies, but not in feces. Patients might become sensitized to these mite body-associated allergens by skin contact. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weghofer M, et al. Identification of Der p 23, a peritrophin-like protein, as a new major Dermatophagoides pteronyssinus allergen associated with the peritrophic matrix of mite fecal pellets. J Immunol. 2013;190:3059–67. doi: 10.4049/jimmunol.1202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerjee S, et al. Conversion of Der p 23, a new major house dust mite allergen, into a hypoallergenic vaccine. J Immunol. 2014;192:4867–75. doi: 10.4049/jimmunol.1400064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller GA, et al. Serological, genomic and structural analyses of the major mite allergen Der p 23. Clin Exp Allergy. 2016;46:365–76. doi: 10.1111/cea.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soh WT, et al. The house dust mite major allergen Der p 23 displays O-glycan-independent IgE reactivities but no chitin-binding activity. Int Arch Allergy Immunol. 2015;168:150–60. doi: 10.1159/000442176. [DOI] [PubMed] [Google Scholar]
- 24.An S, et al. Dermatophagoides farinae allergens diversity identification by proteomics. Mol Cell Proteomics. 2013;12:1818–28. doi: 10.1074/mcp.M112.027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Chan TF, et al. The draft genome, transcriptome, and microbiome of Dermatophagoides farinae reveal a broad spectrum of dust mite allergens. J Allergy Clin Immunol. 2015;135:539–48. doi: 10.1016/j.jaci.2014.09.031. Genomic, transcriptomic, and proteomic experiments were used to produce a house dust mite genome draft from Dermatophagoides farinae that revealed allergen genes and a diverse endosymbiotic microbiome. This study will allow identification and characterization of new mite allergens. [DOI] [PubMed] [Google Scholar]
- 26.Bernton HS, Brown H. Insect allergy—preliminary studies of the cockroach. J Allergy Clin Immunol. 1964;35:506–13. doi: 10.1016/0021-8707(64)90082-6. [DOI] [PubMed] [Google Scholar]
- 27.Barbosa MC, et al. Efficacy of recombinant allergens for diagnosis of cockroach allergy in patients with asthma and/or rhinitis. Int Arch Allergy Immunol. 2013;161:213–9. doi: 10.1159/000346318. [DOI] [PubMed] [Google Scholar]
- 28.Rosenstreich DL, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 29.Gruchalla RS, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–85. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Mueller GA, et al. The novel structure of the cockroach allergen Bla g 1 has implications for allergenicity and exposure assessment. J Allergy Clin Immunol. 2013;132:1420–6. doi: 10.1016/j.jaci.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wünschmann S, Gustchina A, Chapman MD, Pomés A. Cockroach allergen Bla g 2: an unusual aspartic proteinase. J Allergy Clin Immunol. 2005;116:140–5. doi: 10.1016/j.jaci.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 32.Li M, et al. Crystal structure of a dimerized cockroach allergen Bla g 2 complexed with a monoclonal antibody. J Biol Chem. 2008;283:22806–14. doi: 10.1074/jbc.M800937200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li M, et al. Carbohydrates contribute to the interactions between cockroach allergen Bla g 2 and a monoclonal antibody. J Immunol. 2011;186:333–40. doi: 10.4049/jimmunol.1002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glesner J, et al. Mechanisms of allergen-antibody interaction of cockroach allergen Bla g 2 with monoclonal antibodies that inhibit IgE antibody binding. PLoS ONE. 2011;6:e22223. doi: 10.1371/journal.pone.0022223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Woodfolk JA, et al. Antigenic determinants of the bilobal cockroach allergen Bla g 2. J Biol Chem. 2016;291:2288–301. doi: 10.1074/jbc.M115.702324. A rational design of site-directed mutagenesis was effective in producing a Bla g 2 mutant, which maintained the same fold as wild type Bla g 2 and showed T-cell modulatory capacity. This antigenic analysis of Bla g 2 will be useful for the subsequent development of recombinant allergen vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu CH, Lee MF, Wang NM, Luo SF. Sequencing and immunochemical characterization of the American cockroach per a 3 (Cr-PI) isoallergenic variants. Mol Immunol. 1997;34:1–8. doi: 10.1016/s0161-5890(97)00009-6. [DOI] [PubMed] [Google Scholar]
- 37.Tan YW, et al. Structures of two major allergens, Bla g 4 and Per a 4, from cockroaches and their IgE binding epitopes. J Biol Chem. 2009;284:3148–57. doi: 10.1074/jbc.M807209200. [DOI] [PubMed] [Google Scholar]
- 38.Satinover SM, et al. Specific IgE and IgG antibody-binding patterns to recombinant cockroach allergens. J Allergy Clin Immunol. 2005;115:803–9. doi: 10.1016/j.jaci.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Hindley J, et al. Bla g 6: a troponin C allergen from Blattella germanica with IgE binding calcium dependence. J Allergy Clin Immunol. 2006;117:1389–95. doi: 10.1016/j.jaci.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 40.Sudha VT, Arora N, Gaur SN, Pasha S, Singh BP. Identification of a serine protease as a major allergen (Per a 10) of Periplaneta americana. Allergy. 2008;63:768–76. doi: 10.1111/j.1398-9995.2007.01602.x. [DOI] [PubMed] [Google Scholar]
- 41.Chuang JG, Su SN, Chiang BL, Lee HJ, Chow LP. Proteome mining for novel IgE-binding proteins from the German cockroach (Blattella germanica) and allergen profiling of patients. Proteomics. 2010;10:3854–67. doi: 10.1002/pmic.201000348. [DOI] [PubMed] [Google Scholar]
- 42.Jeong KY, et al. Identification of novel allergenic components from German cockroach fecal extract by a proteomic approach. Int Arch Allergy Immunol. 2013;161:315–24. doi: 10.1159/000347034. [DOI] [PubMed] [Google Scholar]
- 43.Fang Y, et al. Two new types of allergens from the cockroach, Periplaneta americana. Allergy. 2015;70:1674–8. doi: 10.1111/all.12766. [DOI] [PubMed] [Google Scholar]
- 44.Dillon MB, et al. Different Bla-g T cell antigens dominate responses in asthma versus rhinitis subjects. Clin Exp Allergy. 2015;45:1856–67. doi: 10.1111/cea.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Virtanen T, Kinnunen T, Rytkonen-Nissinen M. Mammalian lipocalin allergens—insights into their enigmatic allergenicity. Clin Exp Allergy. 2012;42:494–504. doi: 10.1111/j.1365-2222.2011.03903.x. [DOI] [PubMed] [Google Scholar]
- 46.Kuehn A, Hilger C. Animal allergens: common protein characteristics featuring their allergenicity. Front Immunol. 2015;6:40. doi: 10.3389/fimmu.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaiser L, et al. Structural characterization of the tetrameric form of the major cat allergen Fel d 1. J Mol Biol. 2007;370:714–27. doi: 10.1016/j.jmb.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 48.van Ree R, van Leeuwen WA, Bulder I, Bond J, Aalberse RC. Purified natural and recombinant Fel d 1 and cat albumin in in vitro diagnostics for cat allergy. J Allergy Clin Immunol. 1999;104:1223–30. doi: 10.1016/s0091-6749(99)70017-5. [DOI] [PubMed] [Google Scholar]
- 49.Charpin C, et al. Fel d I allergen distribution in cat fur and skin. J Allergy Clin Immunol. 1991;88:77–82. doi: 10.1016/0091-6749(91)90303-6. [DOI] [PubMed] [Google Scholar]
- 50.De Andrade AD, et al. Fel d I levels in cat anal glands. Clin Exp Allergy. 1996;26:178–80. doi: 10.1111/j.1365-2222.1996.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 51.Custovic A, et al. Distribution, aerodynamic characteristics, and removal of the major cat allergen Fel d 1 in British homes. Thorax. 1998;53:33–8. doi: 10.1136/thx.53.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almqvist C, et al. Worsening of asthma in children allergic to cats, after indirect exposure to cat at school. Am J Respir Crit Care Med. 2001;163:694–8. doi: 10.1164/ajrccm.163.3.2006114. [DOI] [PubMed] [Google Scholar]
- 53.Smith W, et al. Fel d 4, a cat lipocalin allergen. Clin Exp Allergy. 2004;34:1732–8. doi: 10.1111/j.1365-2222.2004.02090.x. [DOI] [PubMed] [Google Scholar]
- 54.Smith W, et al. Two newly identified cat allergens: the von Ebner gland protein Fel d 7 and the latherin-like protein Fel d 8. Int Arch Allergy Immunol. 2011;156:159–70. doi: 10.1159/000322879. [DOI] [PubMed] [Google Scholar]
- 55.van RR, van Leeuwen WA, Bulder I, Bond J, Aalberse RC. Purified natural and recombinant Fel d 1 and cat albumin in in vitro diagnostics for cat allergy. J Allergy Clin Immunol. 1999;104:1223–30. doi: 10.1016/s0091-6749(99)70017-5. [DOI] [PubMed] [Google Scholar]
- 56.Konradsen JR, et al. Allergy to furry animals: new insights, diagnostic approaches, and challenges. J Allergy Clin Immunol. 2015;135:616–25. doi: 10.1016/j.jaci.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 57.Konieczny A, et al. The major dog allergens, Can f 1 and Can f 2, are salivary lipocalin proteins: cloning and immunological characterization of the recombinant forms. Immunology. 1997;92:577–86. doi: 10.1046/j.1365-2567.1997.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de GH, Goei KG, Van SP, Aalberse RC. Affinity purification of a major and a minor allergen from dog extract: serologic activity of affinity-purified Can f I and of Can f I-depleted extract. J Allergy Clin Immunol. 1991;87:1056–65. doi: 10.1016/0091-6749(91)92150-y. [DOI] [PubMed] [Google Scholar]
- 59.Nicholas C, et al. Dog characteristics and allergen levels in the home. Ann Allergy Asthma Immunol. 2010;105:228–33. doi: 10.1016/j.anai.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Polovic N, et al. Dog saliva—an important source of dog allergens. Allergy. 2013;68:585–92. doi: 10.1111/all.12130. The study describes the presence of new dog allergens in saliva not previously identified in dander. Among patients that were IgE negative to dander, but with symptoms to dog, 20% were IgE positive to saliva. The authors discuss the importance of saliva in addition to dander proteins in allergy diagnostics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramadour M, et al. Dog factor differences in Can f 1 allergen production. Allergy. 2005;60:1060–4. doi: 10.1111/j.1398-9995.2005.00824.x. [DOI] [PubMed] [Google Scholar]
- 62.Vredegoor DW, Willemse T, Chapman MD, Heederik DJ, Krop EJ. Can f 1 levels in hair and homes of different dog breeds: lack of evidence to describe any dog breed as hypoallergenic. J Allergy Clin Immunol. 2012;130:904–9. doi: 10.1016/j.jaci.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 63.Mattsson L, Lundgren T, Everberg H, Larsson H, Lidholm J. Prostatic kallikrein: a new major dog allergen. J Allergy Clin Immunol. 2009;123:362–8. doi: 10.1016/j.jaci.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 64.Hilger C, Kuehn A, Hentges F. Animal lipocalin allergens. Curr Allergy Asthma Rep. 2012;12:438–47. doi: 10.1007/s11882-012-0283-2. [DOI] [PubMed] [Google Scholar]
- 65.Polovic N, et al. Dog saliva—an important source of dog allergens. Allergy. 2013;68:585–92. doi: 10.1111/all.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saarelainen S, et al. Assessment of recombinant dog allergens Can f 1 and Can f 2 for the diagnosis of dog allergy. Clin Exp Allergy. 2004;34:1576–82. doi: 10.1111/j.1365-2222.2004.02071.x. [DOI] [PubMed] [Google Scholar]
- 67.Rytkonen-Nissinen M, et al. IgE reactivity of the dog lipocalin allergen Can f 4 and the development of a sandwich ELISA for its quantification. Allergy Asthma Immunol Res. 2015;7:384–92. doi: 10.4168/aair.2015.7.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeal H, Jones M. Allergy to rodents: an update. Clin Exp Allergy. 2010;40:1593–601. doi: 10.1111/j.1365-2222.2010.03609.x. [DOI] [PubMed] [Google Scholar]
- 69.Simons E, Curtin-Brosnan J, Buckley T, Breysse P, Eggleston PA. Indoor environmental differences between inner city and suburban homes of children with asthma. J Urban Health. 2007;84:577–9. doi: 10.1007/s11524-007-9205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Sharpe RA, Bearman N, Thornton CR, Husk K, Osborne NJ. Indoor fungal diversity and asthma: a meta-analysis and systematic review of risk factors. J Allergy Clin Immunol. 2015;135:110–22. doi: 10.1016/j.jaci.2014.07.002. Longitudinal studies evaluating exposure to indoor fungi before the development of asthma symptoms suggested that Penicillium, Aspergillus, and Cladosporium species pose a respiratory health risk in susceptible populations. Increased exacerbation of current asthma symptoms in children and adults were linked to increased levels of Penicillium, Aspergillus, Cladosporium, and Alternaria species. [DOI] [PubMed] [Google Scholar]
- 71.Perry T, Matsui E, Merriman B, Duong T, Eggleston P. The prevalence of rat allergen in inner-city homes and its relationship to sensitization and asthma morbidity. J Allergy Clin Immunol. 2003;112:346–52. doi: 10.1067/mai.2003.1640. [DOI] [PubMed] [Google Scholar]
- 72.Liccardi G, et al. Sensitization to rodents (mouse/rat) in an urban atopic population without occupational exposure living in Naples. Italy Eur Ann Allergy Clin Immunol. 2012;44:200–4. [PubMed] [Google Scholar]
- 73.Sedaghat AR, et al. Mouse sensitivity is an independent risk factor for rhinitis in children with asthma. J Allergy Clin Immunol Pract. 2016;4:82–8. doi: 10.1016/j.jaip.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ohman JL, Jr, et al. Distribution of airborne mouse allergen in a major mouse breeding facility. J Allergy Clin Immunol. 1994;94:810–7. doi: 10.1016/0091-6749(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 75.Krop EJ, Doekes G, Stone MJ, Aalberse RC, van der Zee JS. Spreading of occupational allergens: laboratory animal allergens on hair-covering caps and in mattress dust of laboratory animal workers. Occup Environ Med. 2007;64:267–72. doi: 10.1136/oem.2006.028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krakowiak A, Szulc B, Gorski P. Allergy to laboratory animals in children of parents occupationally exposed to mice, rats and hamsters. Eur Respir J. 1999;14:352–6. doi: 10.1034/j.1399-3003.1999.14b19.x. [DOI] [PubMed] [Google Scholar]
- 77.Sharpe RA, Bearman N, Thornton CR, Husk K, Osborne NJ. Indoor fungal diversity and asthma: a meta-analysis and systematic review of risk factors. J Allergy Clin Immunol. 2015;135:110–22. doi: 10.1016/j.jaci.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Fukutomi Y, Taniguchi M. Sensitization to fungal allergens: resolved and unresolved issues. Allergol Int. 2015;64:321–31. doi: 10.1016/j.alit.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Gergen PJ, Turkeltaub PC. The association of individual allergen reactivity with respiratory disease in a national sample: data from the second National Health and Nutrition Examination Survey, 1976–80 (NHANES II) J Allergy Clin Immunol. 1992;90:579–88. doi: 10.1016/0091-6749(92)90130-t. [DOI] [PubMed] [Google Scholar]
- 80.Kleine-Tebbe J, et al. Predominance of the major allergen (Alt a I) in Alternaria sensitized patients. Clin Exp Allergy. 1993;23:211–8. doi: 10.1111/j.1365-2222.1993.tb00884.x. [DOI] [PubMed] [Google Scholar]
- 81.Chruszcz M, et al. Alternaria alternata allergen Alt A 1: a unique beta-barrel protein dimer found exclusively in fungi. J Allergy Clin Immunol. 2012;130:241–7. doi: 10.1016/j.jaci.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Breitenbach M, Simon-Nobbe B. The allergens of Cladosporium herbarum and Alternaria alternata. Chem Immunol. 2002;81:48–72. doi: 10.1159/000058862. [DOI] [PubMed] [Google Scholar]
- 83.Bush RK, Sanchez H, Geisler D. Molecular cloning of a major Alternaria alternata allergen, rAlt a 2. J Allergy Clin Immunol. 1999;104:665–71. doi: 10.1016/s0091-6749(99)70340-4. [DOI] [PubMed] [Google Scholar]
- 84.Vijay HM, Kurup VP. Fungal allergens. Clin Allergy Immunol. 2008;21:141–60. [PubMed] [Google Scholar]
- 85.Achatz G, et al. Molecular cloning of major and minor allergens of Alternaria alternata and Cladosporium herbarum. Mol Immunol. 1995;32:213–27. doi: 10.1016/0161-5890(94)00108-d. [DOI] [PubMed] [Google Scholar]
- 86.Zureik M, et al. Sensitisation to airborne moulds and severity of asthma: cross sectional study from European Community respiratory health survey. BMJ. 2002;325:411–4. doi: 10.1136/bmj.325.7361.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Breitenbach M, et al. Enolases are highly conserved fungal allergens. Int Arch Allergy Immunol. 1997;113:114–7. doi: 10.1159/000237521. [DOI] [PubMed] [Google Scholar]
- 88.Knutsen AP, et al. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol. 2012;129:280–91. doi: 10.1016/j.jaci.2011.12.970. [DOI] [PubMed] [Google Scholar]
- 89.Kurup VP, et al. Selected recombinant Aspergillus fumigatus allergens bind specifically to IgE in ABPA. Clin Exp Allergy. 2000;30:988–93. doi: 10.1046/j.1365-2222.2000.00837.x. [DOI] [PubMed] [Google Scholar]
- 90.Arruda LK, Platts-Mills TA, Fox JW, Chapman MD. Aspergillus fumigatus allergen I, a major IgE-binding protein, is a member of the mitogillin family of cytotoxins. J Exp Med. 1990;172:1529–32. doi: 10.1084/jem.172.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shen HD, et al. Characterization of allergens from Penicillium oxalicum and P. notatum by immunoblotting and N-terminal amino acid sequence analysis. Clin Exp Allergy. 1999;29:642–51. doi: 10.1046/j.1365-2222.1999.00509.x. [DOI] [PubMed] [Google Scholar]
- 92.Chow LP, Chiou SH, Hsiao MC, Yu CJ, Chiang BL. Characterization of Pen n 13, a major allergen from the mold Penicillium notatum. Biochem Biophys Res Commun. 2000;269:14–20. doi: 10.1006/bbrc.2000.2253. [DOI] [PubMed] [Google Scholar]
- 93.Shen HD, et al. Complementary DNA cloning and immunologic characterization of a new Penicillium citrinum allergen (Pen c 3) J Allergy Clin Immunol. 2000;105:827–33. doi: 10.1067/mai.2000.105220. [DOI] [PubMed] [Google Scholar]
- 94.Becker S, Groger M, Canis M, Pfrogner E, Kramer MF. Tropomyosin sensitization in house dust mite allergic patients. Eur Arch Otorhinolaryngol. 2012;269:1291–6. doi: 10.1007/s00405-011-1826-1. [DOI] [PubMed] [Google Scholar]
- 95.Ayuso R, Lehrer SB, Reese G. Identification of continuous, allergenic regions of the major shrimp allergen Pen a 1 (tropomyosin) Int Arch Allergy Immunol. 2002;127:27–37. doi: 10.1159/000048166. [DOI] [PubMed] [Google Scholar]
- 96.Spitzauer S. Allergy to mammalian proteins: at the borderline between foreign and self? Int Arch Allergy Immunol. 1999;120:259–69. doi: 10.1159/000024278. [DOI] [PubMed] [Google Scholar]
- 97.Quirce S, et al. Chicken serum albumin (Gal d 5*) is a partially heat-labile inhalant and food allergen implicated in the bird-egg syndrome. Allergy. 2001;56:754–62. doi: 10.1034/j.1398-9995.2001.056008754.x. [DOI] [PubMed] [Google Scholar]
- 98.Posthumus J, et al. Initial description of pork-cat syndrome in the United States. J Allergy Clin Immunol. 2013;131:923–5. doi: 10.1016/j.jaci.2012.12.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zimmer J, Vieths S, Kaul S. Standardization and regulation of allergen products in the European Union. Curr Allergy Asthma Rep. 2016;16:21. doi: 10.1007/s11882-016-0599-4. [DOI] [PubMed] [Google Scholar]
- 100.van Ree R, et al. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008;63:310–26. doi: 10.1111/j.1398-9995.2007.01612.x. [DOI] [PubMed] [Google Scholar]
- 101.Himly M, et al. Standardization of allergen products: 2. Detailed characterization of GMP-produced recombinant Phl p 5.0109 as European Pharmacopoeia reference standard. Allergy. 2015 doi: 10.1111/all.12824. [DOI] [PubMed] [Google Scholar]
- 102.Asarnoj A, et al. Sensitization to cat and dog allergen molecules in childhood and prediction of symptoms of cat and dog allergy in adolescence: a BAMSE/MeDALL study. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arruda LK, et al. Recombinant allergens for diagnosis of cockroach allergy. Curr Allergy Asthma Rep. 2014;14:428. doi: 10.1007/s11882-014-0428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Resch Y, et al. Different IgE recognition of mite allergen components in asthmatic and nonasthmatic children. J Allergy Clin Immunol. 2015;136:1083–91. doi: 10.1016/j.jaci.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.King EM, et al. A multi-center ring trial of allergen analysis using fluorescent multiplex array technology. J Immunol Methods. 2012 doi: 10.1016/j.jim.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Filep S, et al. A multi-allergen standard for the calibration of immunoassays: CREATE principles applied to eight purified allergens. Allergy. 2012;67:235–41. doi: 10.1111/j.1398-9995.2011.02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chapman MD, Briza P. Molecular approaches to allergen standardization. Curr Allergy Asthma Rep. 2012;12:478–84. doi: 10.1007/s11882-012-0282-3. [DOI] [PubMed] [Google Scholar]
- 108.Phipatanakul W, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375–87. doi: 10.1016/j.anai.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Portnoy J, et al. Environmental assessment and exposure control: a practice parameter—furry animals. Ann Allergy Asthma Immunol. 2012;108:223–15. doi: 10.1016/j.anai.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Portnoy J, et al. Environmental assessment and exposure control of dust mites: a practice parameter. Ann Allergy Asthma Immunol. 2013;111:465–507. doi: 10.1016/j.anai.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Portnoy J, et al. Environmental assessment and exposure reduction of cockroaches: a practice parameter. J Allergy Clin Immunol. 2013;132:802–8. doi: 10.1016/j.jaci.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Calderon MA, Casale TB, Nelson HS, Demoly P. An evidence-based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol. 2013;132:1322–36. doi: 10.1016/j.jaci.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 113.Wood RA, et al. Development of cockroach immunotherapy by the Inner-City Asthma Consortium. J Allergy Clin Immunol. 2014;133:846–52. doi: 10.1016/j.jaci.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cromwell O, Hafner D, Nandy A. Recombinant allergens for specific immunotherapy. J Allergy Clin Immunol. 2011;127:865–72. doi: 10.1016/j.jaci.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 115.Passalacqua G, et al. Randomized double-blind controlled study with sublingual carbamylated allergoid immunotherapy in mild rhinitis due to mites. Allergy. 2006;61:849–54. doi: 10.1111/j.1398-9995.2006.01095.x. [DOI] [PubMed] [Google Scholar]
- 116.Bussmann C, et al. Clinical improvement and immunological changes in atopic dermatitis patients undergoing subcutaneous immunotherapy with a house dust mite allergoid: a pilot study. Clin Exp Allergy. 2007;37:1277–85. doi: 10.1111/j.1365-2222.2007.02783.x. [DOI] [PubMed] [Google Scholar]
- 117.Urry ZL, et al. Depigmented-polymerised allergoids favour regulatory over effector T cells: enhancement by 1alpha, 25-dihydroxyvitamin D3. BMC Immunol. 2014;15:21. doi: 10.1186/1471-2172-15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patel D, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2013;131:103–9. doi: 10.1016/j.jaci.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 119.Couroux P, Patel D, Armstrong K, Larche M, Hafner RP. Fel d 1-derived synthetic peptide immuno-regulatory epitopes show a long-term treatment effect in cat allergic subjects. Clin Exp Allergy. 2015;45:974–81. doi: 10.1111/cea.12488. [DOI] [PubMed] [Google Scholar]
- 120.Asturias JA, et al. Engineering of major house dust mite allergens Der p 1 and Der p 2 for allergen-specific immunotherapy. Clin Exp Allergy. 2009;39:1088–98. doi: 10.1111/j.1365-2222.2009.03264.x. [DOI] [PubMed] [Google Scholar]
- 121.Chen KW, et al. Hypoallergenic Der p 1/Der p 2 combination vaccines for immunotherapy of house dust mite allergy. J Allergy Clin Immunol. 2012;130:435–43. doi: 10.1016/j.jaci.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen KW, et al. Reduction of the in vivo allergenicity of Der p 2, the major house-dust mite allergen, by genetic engineering. Mol Immunol. 2008;45:2486–98. doi: 10.1016/j.molimm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 123.Pomés A, et al. Antigenic determinants on Der p 1 identified by mutagenesis analysis based on the structure of allergen-antibody complexes. J Allergy Clin Immunol. 2014;133:AB164. [Google Scholar]
- 124.Li M, et al. Carbohydrates contribute to the interactions between cockroach allergen Bla g 2 and a monoclonal antibody. J Immunol. 2011;186:333–40. doi: 10.4049/jimmunol.1002318. [DOI] [PMC free article] [PubMed] [Google Scholar]