Abstract
Objectives:
Meningococcal meningitis is reported as a rare condition in Mexico. There are no internationally published studies on bacterial causes of meningitis in the country based on active surveillance. This study focuses on finding the etiology of bacterial meningitis in children from nine Mexican Hospitals.
Methods:
From January 2010 to February 2013, we conducted a three years of active surveillance for meningitis in nine hospitals throughout Mexico. Active surveillance started at the emergency department for every suspected case, and microbiological studies confirmed/ruled out all potentially bacterial pathogens. We diagnosed based on routine cultures from blood and cerebrospinal fluid (not polymerase chain reaction or other molecular diagnostic tests), and both pneumococcal serotyping and meningococcal serogrouping by using standard methods.
Results:
Neisseria meningitidis was the leading cause, although 75% of cases occurred in the northwest of the country in Tijuana on the US border. Serogroup C was predominant. Streptococcus pneumoniae followed Neisseria meningitides, but was uniformly distributed throughout the country. Serotype 19A was the most incident but before universal implementation of the 13-valent pneumococcal conjugate vaccine. Other bacteria were much less common, including Enterobacteriaceae and Streptococcus agalactiae (these two affecting mostly young infants).
Conclusions:
Meningococcal meningitis is endemic in Tijuana, Mexico, and vaccination should be seriously considered in that region. Continuous universal vaccination with the 13-valent pneumococcal conjugate vaccine should be nationally performed, and polymerase chain reaction should be included for bacterial detection in all cultures – negative but presumably bacterial meningitis cases.
Keywords: active surveillance, bacterial meningitis, children, meningococcal meningitis, pneumococcal meningitis
Background
Meningococcal meningitis (MM) is considered to be a rare disease [Almeida-Gonzalez et al. 2004] in Mexico, despite proven endemicity in the northwestern part of the country and documented outbreaks [Chacon-Cruz et al. 2011, 2014a, 2015]. However, it is endemic in other Latin American countries [Safadi and Cintra, 2010], as well as in many other parts of the world [Chang et al. 2012; Baccarini et al. 2012]. Employing an active surveillance system throughout Mexico, we sought to examine the burden of MM, pneumococcal meningitis (PM) and other bacteria causing bacterial meningitis (BM) throughout Mexico. Furthermore, active surveillance for BM has never been published in Mexico, and in Latin America, is the third-published study also prospectively looking for pathogens causing BM and other invasive diseases in children [Sacchi et al. 2011; Gaensbauer et al. 2016]; all other information on pediatric BM in Mexico and Latin America we have is mostly based on passive surveillance (SIREVA report, 2012).
Incidence rates (IR) were obtained only for MM because it had been reported as a very rare disease in Mexico based on passive surveillance. Among nine hospitals included in this study, only five had a stable population who only attended that particular hospital. They were the only ones in which it was possible to estimate IR based on the following formula:
Materials and methods
The main objectives of our study were to:
Evaluate the real epidemiological situation of meningococcal disease in Mexican children.
Estimate incidence rates of MM only in hospitals where a defined denominator was well defined.
Identify all culture-positive bacterial causes of meningitis in children.
Compare the clinical and demographic pictures of MM versus PM.
Describe clinically and demographically other bacteria causing meningitis in Mexican children.
This study was initiated by physicians in charge of children with BM. The project guidelines were developed during the initial meeting and participating microbiologists from each hospital were trained in isolating and identifying bacterial pathogens that may cause BM. The study was approved by the ethics committees from all nine hospitals.
From February 2010 to January 2013, active surveillance for BM in children <16 years of age was implemented in 9 hospitals throughout Mexico: Tijuana General Hospital (northwest); Hospital Universitario of Monterrey (northeast); Children’s Hospital of Culiacan (north-Pacific); Hospital Civil of Guadalajara (central-Pacific); three hospitals in Mexico City (National Institute of Pediatrics, Hospital Picacho–PEMEX and Hospital Medica-Sur); Children’s Hospital of Morelia (southwest); and General Hospital of Tuxtla-Gutierrez (southeast).
All but two are tertiary hospitals (National Institute of Pediatrics and Hospital Civil of Guadalajara); the rest are secondary hospitals. All but one are public; the exception is a private hospital in Mexico City, Hospital Medica-Sur. These hospitals were chosen because they represent a geographical area, and by looking at their microbiological laboratories, they fulfilled the basic equipment and personnel for identification of invasive bacteria.
Active surveillance was as follows. At the emergency department, for every patient with suspected meningitis, lumbar punctures [cytochemical analysis including glucose, proteins and leucocytes from cerebrospinal fluid (CSF) and culture] and blood cultures were immediately performed along with other routine tests [cell blood count (CBC), serum biochemical profiles (glucose, liver and renal functional tests), erythrocyte sedimentation rate (ESR) and clotting times (prothrombin and thromboplastin times]. Diagnosis of BM was established by a positive CSF and/or blood culture, with abnormal CSF cytochemical analysis. Bacterial identification was performed using initially broth media followed by specific automatized methods for each pathogen. Standard isolation and identification of each pathogen was established during the first meeting of all investigators and microbiologists, and annual supervision by the principal investigator.
For all Neisseria meningitidis isolates, serogroup identification was performed using the Pastorex meningitis kit (Alere Ltd, Stockport, UK). For some Streptococcus pneumoniae isolates, serotype identification was performed using the Quellung reaction (Statens Serum Institute, Copenhagen, Denmark). We did not use polymerase chain reaction (PCR) or another molecular diagnostic tool due to lack of resources. For every culture-negative meningitis, a discussion took place within the main investigators on an individual basis and it was decided whether it was viral meningitis (VM) or potential bacterial meningitis (PBM); decisions were made based on CSF cytochemical analysis (leucocytes, proteins and glucose), clinical picture at admission, and clinical outcome during hospitalization. All patients with VM received no more than 2 days of antimicrobials, a second lumbar puncture revealed a normal cytochemical analysis, and were discharged with at least 3 days without antibacterials. The reasons for a second lumbar puncture in all VM cases was to confirm they were not BM, mostly due to the lack of molecular tools for identification of viruses and because there are reports of BM without CSF pleocytosis [Hase et al. 2014]. Patients with PBM had a granulocytic CSF and a second abnormal granulocytic CSF again, but isolation of a bacterial pathogen was not possible.
The principal investigator supervised once a year all nine hospitals to assure compliance.
Results
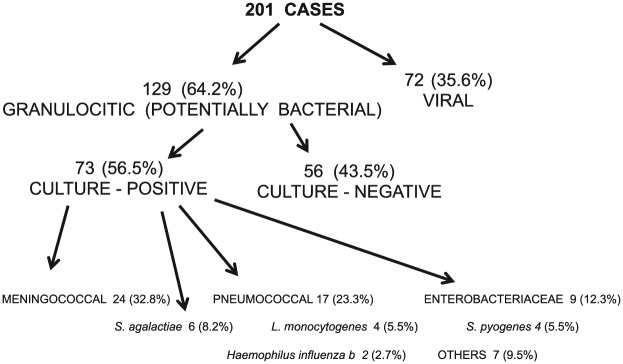
There were 201 cases of meningitis; 72 (36%) were considered to be VM and 129 (64%) were considered to be PBM. Culture-confirmed BM cases were 73 (56.5% positivity) and isolates were as follows: N. meningitidis 24 (33%); S. pneumoniae 17 (23.3%); Enterobacteriaceae 9 (12%); Streptococcus agalactiae 6 (8%); Listeria monocytogenes 4 (5.5%); Streptococcus pyogenes 4 (5.5%); Haemophilus influenzae type b 2 (2.7%); and others 7 (9.5%) (Figure 1).
Figure 1.
Pediatric meningitis surveillance in nine Mexican hospitals: number and percentage of cases by disease and agent.
Period: 1 February 2010 to 31 January 2013.
Culture-confirmed BM was from CSF only in 100% of cases and from both CSF and blood in 36% of patients.
MM incidence was significantly higher in the northwest (Tijuana, 75%); of these 41.6% were caused by serogroup C (Figure 2).
Figure 2.
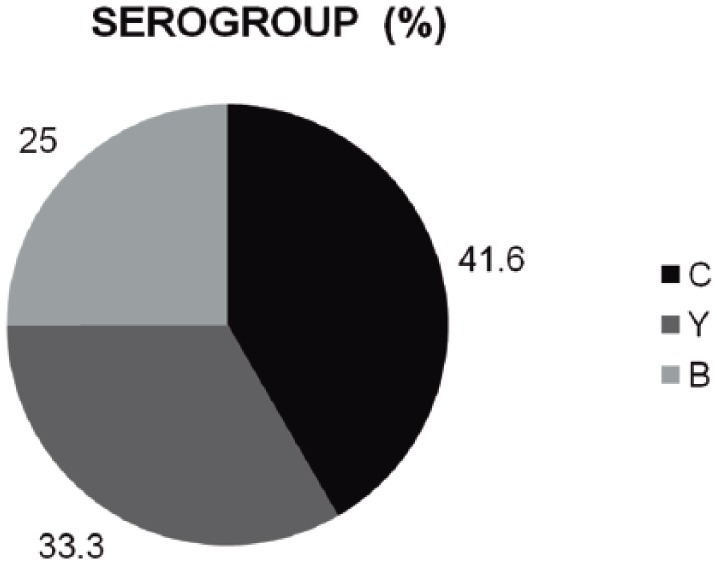
Pediatric meningitis surveillance in nine Mexican hospitals: meningitis due to Neisseria meningitidis by serogroup (n = 24)
Period: 1 February 2010 to 31 January 2013.
The median age of presentation for MM was 1 year (21 days to 15 years), 71% were <2 years old. Purpura and thrombocytopenia were present in 46% and 41.6%, respectively. A total of 6 children died (25%, between 1 and 3 days following hospitalization). Among survivors, 4 (22.2%) developed neurologic sequelae (motor and speech impairment) at discharge from the hospital. It was possible to estimate the IR of MM from five hospitals. The vast majority of cases were found in Tijuana, with annual rates in children <5 years old of 25/100,000, while the overall rate in that same age group was of 5/100,000 (Table 1).
Table 1.
Pediatric meningitis surveillance in nine Mexican hospitals.
| Geographical zone |
|||
|---|---|---|---|
| North | Central | South | |
| Number of hospitals | 3 | 4 | 2 |
| Meningococcal/pneumococcal | 20/7 | 4/8 | 0/2 |
Meningococcal and pneumococcal meningitis in children: regional distribution in Mexico from 1 February 2010 to 31 January 2013.
PM was more equally distributed throughout the country (Table 1). PM median age of presentation was 2.5 years (3 months to 16 years) and 47% were <2 years old. Purpura was not present in any case, thrombocytopenia was present in 11.7% of patients, and overall PM mortality was 5 (29.4%, between 1 and 8 days following hospitalization). Among survivors, 3 (25%) developed neurologic sequelae (motor and speech impairment) at discharge from the hospital. Pneumococcal serotype identification was obtained in seven patients, six of which were serotype 19A.
Among patients with PM, pneumococcal vaccination status was of at least 2 doses of 7-valent pneumococcal conjugate vaccine in 15 from 17 patients (88.2%); 2 patients were not vaccinated at all.
Other bacteria were much less common, with Enterobacteriaceae and S. agalactiae affecting mostly children less than 3 months old. Enterobacteriaceae caused three deaths and all patients were discharged with neurologic sequelae. Patients with meningitis due to S. agalactiae developed well, with no mortality and no apparent sequelae at discharge.
There were 4 cases due to S. pyogenes, affecting mostly older children (4–8 years of age) and 4 cases of L. monocytogenes affecting mostly young infants with a good clinical response. There were two cases of Haemophilus influenzae type b, both of which were unvaccinated children, one the son of parents belonging to anti-vaccine groups (this patient died).
All patients did not have any apparent immunological disorder, none had any kind of bacterial invasive disease in the past and all were human immunodeficiency virus (HIV) negative. No further immunological studies were performed.
Discussion
This is the first internationally published study performed in Mexico based on active surveillance (not retrospectively) looking for etiological bacterial pathogens of BM. Even though MM is a notifiable condition, the overall belief that MM is a rare disease persists. This assumption, however, is based only on observation. Nevertheless, MM was mostly present in Tijuana (75%), with indeed an outbreak due to serogroup C, MTLS ST-11 – an outbreak that occurred 1 month after this surveillance was finished [Chacon-Cruz et al. 2011, 2014, 2015]. We believe that meningococcal vaccination should be seriously evaluated in that region. In other areas than Tijuana, Mexico, we do not have enough information to recommend universal vaccination. However, we should encourage active, national surveillance.
PM presence was equally notable throughout all nine hospitals. Among the seven serotyped strains, six were caused by serotype 19A. This study was mostly conducted before initiation (May–June 2012) of the 13-valent pneumococcal conjugate vaccine (PCV-13). Currently, following PCV-13 universal introduction, serotype 19A and PM have dropped significantly in Tijuana [Chacon-Cruz et al. 2014b].
Other bacteria were much less common, with Enterobacteriaceae and S. agalactiae affecting mostly children <3 months old; there were also 4 cases each of L. monocytogenes and S. pyogenes and 2 children with Haemophilus influenzae type b meningitis who were not vaccinated.
The main limitation of our study is that we were unable to perform PCR for any PBM case due to lack of resources. We believe our results will bring more resources in the near future to continue active surveillance and to encourage the use of PCR as a common tool for all PBM cases as proven in many other studies [Salgado et al. 2013; Sacchi et al. 2011; Ubukata 2013; Diggle and Clarke, 2006; Mothershed and Whitney, 2006; Bennet and Cafferkey, 2006].
Conclusion
Meningococcal meningitis is among the leading causes of BM in Mexico. However, it is predominant only in the northwest of Mexico (Baja-California–California border) and universal vaccination should be seriously evaluated in that region [World Health Organization, 2011].
PM is the most frequent bacterial pathogen found, but cases are more equally distributed throughout the country. Serotype identification was performed only in 41% of cases, all of which were 19A, suggesting that PCV-13 should be continued as a universal antipneumococcal vaccination in Mexican children.
PCR or other molecular diagnostic tools should be included to increase the yield of diagnosis of all cases of BM.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Novartis-Vaccines® funded all annual related expenses (travel, food and accommodation) for investigators’ meetings and for the main investigator’s visits to each hospital. All other expenses were paid by every hospital included in the study.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Enrique Chacon-Cruz, Hospital General de Tijuana, Paseo Centario S/N, Zona del Rio, Tijuana, 22010, Mexico.
Cesar Adrian Martinez-Longoria, Hospital Universitario ‘Dr. Jose Eleuterio Gonzalez,’ Monterrey, Nuevo Leon, Mexico.
Eduardo Llausas-Magana, Hospital Pediatrico de Sinaloa ‘Dr. Rigoberto Aguilar Pico,’ Culiacan, Sinaloa, Culiacan, Mexico.
Antonio Luevanos-Velazquez, Hospital Civil de Guadalajara ‘Fray Antonio Alcalde,’ Guadalajara, Jalisco, Guadalajara, Mexico.
Jorge Alejandro Vazquez-Narvaez, Hospital Infantil de Morelia ‘Eva Samano de Lopez Mateos,’ Morelia, Michoacan, Morelia, Mexico.
Sandra Beltran, Hospital General ‘Dr. Rafael Pascacio Gamboa,’ Tuxtla Gutierrez Chiapas, Tuxtla Gutierrez, Mexico.
Ana Elena Limon-Rojas, Hospital de Pemex Picacho, Mexico City, Mexico City, Mexico.
Fernando Urtiz-Jeronimo, Hospital para el Niño de Toluca, Edo. Mexico, Toluca, Mexico.
Jose Luis Castaneda-Narvaez, Instituto Nacional de Pediatria, Mexico City, Mexico.
Francisco Otero-Mendoza, Instituto Nacional de Pediatria, Mexico City, Mexico.
Fernando Aguilar-Del Real, Hospital Médica Sur, Mexico City, Mexico.
Jesus Rodriguez-Chagoyan, Hospital Civil de Guadalajara ‘Fray Antonio Alcalde,’ Guadalajara, Jalisco, Guadalajara, Mexico.
Rosa Maria Rivas-Landeros, Hospital General de Tijuana, Tijuana, Mexico.
Maria Luisa Volker-Soberanes, Hospital General de Tijuana, Tijuana, Mexico.
Rosa Maria Hinojosa-Robles, Hospital Universitario ‘Dr. Jose Eleuterio Gonzalez,’ Monterrey, Nuevo Leon, Mexico.
Patricia Arzate-Barbosa, Instituto Nacional de Pediatria, Mexico City, Mexico.
Laura Karina Aviles-Benitez, Hospital Infantil de Morelia ‘Eva Samano de Lopez Mateos,’ Morelia, Michoacan, Morelia, Mexico.
Fernando Ivan Elenes-Zamora, Hospital Pediatrico de Sinaloa ‘Dr. Rigoberto Aguilar Pico,’ Culiacan, Sinaloa, Culiacan, Mexico.
Chandra M. Becka, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana, New Orleans, LA, USA
Ricardo Ruttimann, FIDEC, University of Miami, Miami, FL, USA.
References
- Almeida-Gonzalez L., Franco-Paredes C., Perez L., Santos-Preciado J. (2004) Meningococcal disease caused by Neisseria meningitidis: epidemiological, clinical, and preventive perspectives. Salud Publica Mex 46: 438–450. [DOI] [PubMed] [Google Scholar]
- Baccarini C., Ternouth A., Wieffer H., Vyse A. (2012) The changing epidemiology of meningococcal disease in North America 1945–2010. Hum Vaccin Immunother 9: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet D., Cafferkey M. (2006) Consecutive use of two multiplex PCR-based assays for simultaneous identification and determination of capsular status of nine common Neisseria meningitidis serogroups associated with invasive disease. J Clin Microbiol 44: 1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon-Cruz E., Espinosa-De Los Monteros L., Navarro-Alvarez S., Aranda-Lozano J., Volker-Soberanes M., Rivas-Landeros R., et al. (2014a) An outbreak of serogroup C (ST-11) meningococcal disease in Tijuana, Mexico. Ther Adv Vaccines 2: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon-Cruz E., Rivas-Landeros R., Volker-Soberanes M. (2014b) Early trends in invasive pneumococcal disease in children following the introduction of 13-valent pneumococcal conjugate vaccine: results from eight years of active surveillance in a Mexican hospital. Ther Adv Vaccines 2: 155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon-Cruz E., Rivas-Landeros R., Volker-Soberanes M. (2015), Pediatric meningococcal disease in a northern Mexican hospital: results of nine years of active surveillance. Open Forum Infect Dis 2(Suppl. 1): abstract 1755. [Google Scholar]
- Chacon-Cruz E., Sugerman D., Ginsberg M., Hopkins J., Hurtado-Montalvo J., Lopez-Viera J., et al. (2011) Surveillance for meningococcal disease US–Mexico border, 2005–2008. Emerg Infect Dis 17: 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q., Tzeng Y., Stephens D. (2012) Meningococcal disease: changes in epidemiology and prevention. Clin Epidemiol 4: 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle M, Clarke S. (2006) Molecular methods for the detection and characterization of Neisseria meningitidis. Expert Rev Mol Diagn 6: 79–87. [DOI] [PubMed] [Google Scholar]
- Gaensbauer J., Asturias E., Soto M., Holt E., Olson D., Halsey N., et al. (2016) Pediatric invasive pneumococcal disease in Guatemala City: Importance of serotype 2. Pediatr Infect Dis J 35: e139–e143. [DOI] [PubMed] [Google Scholar]
- Hase R., Hosokawa N., Yaegashi M., Muranaka K. (2014) Bacterial meningitis in the absence of cerebrospinal fluid pleocytosis: a case report and review of the literature. Can J Infect Dis Med Microbiol 25: 249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothershed E., Whitney A. (2006) Nucleic acid-based methods for the detection of bacterial pathogens: present and future considerations for the clinical laboratory. Clin Chim Acta 363: 206–220. [DOI] [PubMed] [Google Scholar]
- Sacchi C., Fukasawa L., Goncalves M., Salgado M., Shut K., Carvalhanas T., et al. (2011) Incorporation of real-time PCR into routine public health surveillance of culture negative bacterial meningitis in São Paulo, Brazil. PLoS One 6: e20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safadi M., Cintra O. (2010) Epidemiology of meningococcal disease in Latin America: current situation and opportunities for prevention. Neurol Res 32: 263–271. [DOI] [PubMed] [Google Scholar]
- Salgado M., Gonçalves M., Fukasawa L., Higa F., Paulino J., Sacchi C. (2013) Evolution of bacterial meningitis diagnosis in São Paulo State-Brazil and future challenges. Arq Neuropsiquiatr 71: 672–676. [DOI] [PubMed] [Google Scholar]
- SIREVA REPORT (2012) http://www.paho.org/index.php?option=com_docman&task=doc_download&gid=22372<emid=270&lang=es
- Ubukata K. (2013) Rapid identification of meningitis due to bacterial pathogens. Rinsho Shinkeigaku 53: 1184–1186. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2011) Meningococcal vaccines: WHO position paper, November 2011. Wkly Epidemiol Rec 86: 521–540. [PubMed] [Google Scholar]