Abstract
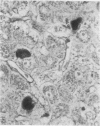
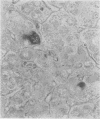
This study has shown that different supplies/batches of orcein perform differently and may fail. The "natural" forms generally performed better although the most informative results were obtained with a "synthetic" product. Orcein dye solutions can be used soon after preparation and for up to 7 days without the need for differentiation. After 10 days or so the staining properties become much less selective. Non-specific staining severely reduces contrast and upon differentiation overall contrast is reduced and the staining of elastin is reduced. Copper-associated protein positivity gradually fails and after 14 days is lost. For demonstrating HBsAg in paraffin sections of liver, it is best to use orcein dye preparations that are no older than 7 days and to test each batch or orcein against a known positive control.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns J. Immunoperoxidase localisation of hepatitis B antigen (HB) in formalin-paraffin processed liver tissue. Histochemistry. 1975 Jul 30;44(2):133–135. doi: 10.1007/BF00494074. [DOI] [PubMed] [Google Scholar]
- Clausen P. P., Thomsen P. Demonstration of hepatitis B-surface antigen in liver biopsies. A comparative investigation of immunoperoxidase and orcein staining on identical sections of formalin fixed, paraffin embedded tissue. Acta Pathol Microbiol Scand A. 1978 Sep;86A(5):383–388. [PubMed] [Google Scholar]
- Konturek S. J. Somatostatin and the gastrointestinal secretions. Scand J Gastroenterol. 1976;11(1):1–4. [PubMed] [Google Scholar]
- Portmann B., Galbraith R. M., Eddleston A. L., Zuckerman A. J., Williams R. Detection of HBSAG in fixed liver tissue - use of a modified immunofluorescent technique and comparison with histochemical methods. Gut. 1976 Jan;17(1):1–9. doi: 10.1136/gut.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata T., Uzawa T., Yoshiwara N., Akatsuka T., Yamazaki S. Staining methods of Australia antigen in paraffin section--detection of cytoplasmic inclusion bodies. Jpn J Exp Med. 1974 Feb;44(1):25–36. [PubMed] [Google Scholar]
- Shikata T., Uzawa T., Yoshiwara N., Akatsuka T., Yamazaki S. Staining methods of Australia antigen in paraffin section--detection of cytoplasmic inclusion bodies. Jpn J Exp Med. 1974 Feb;44(1):25–36. [PubMed] [Google Scholar]
- Sipponen P., Salaspuro M. P., Makkonen H. M. Orcein positive hepatocellular material in histological diagnosis of primary biliary cirrhosis. Ann Clin Res. 1975 Aug;7(4):273–277. [PubMed] [Google Scholar]